In-Situ High Resolution Dynamic X-ray Microtomographic Imaging of Olive Oil Removal in Kitchen Sponges by Squeezing and Rinsing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Material
2.2. Scanner System
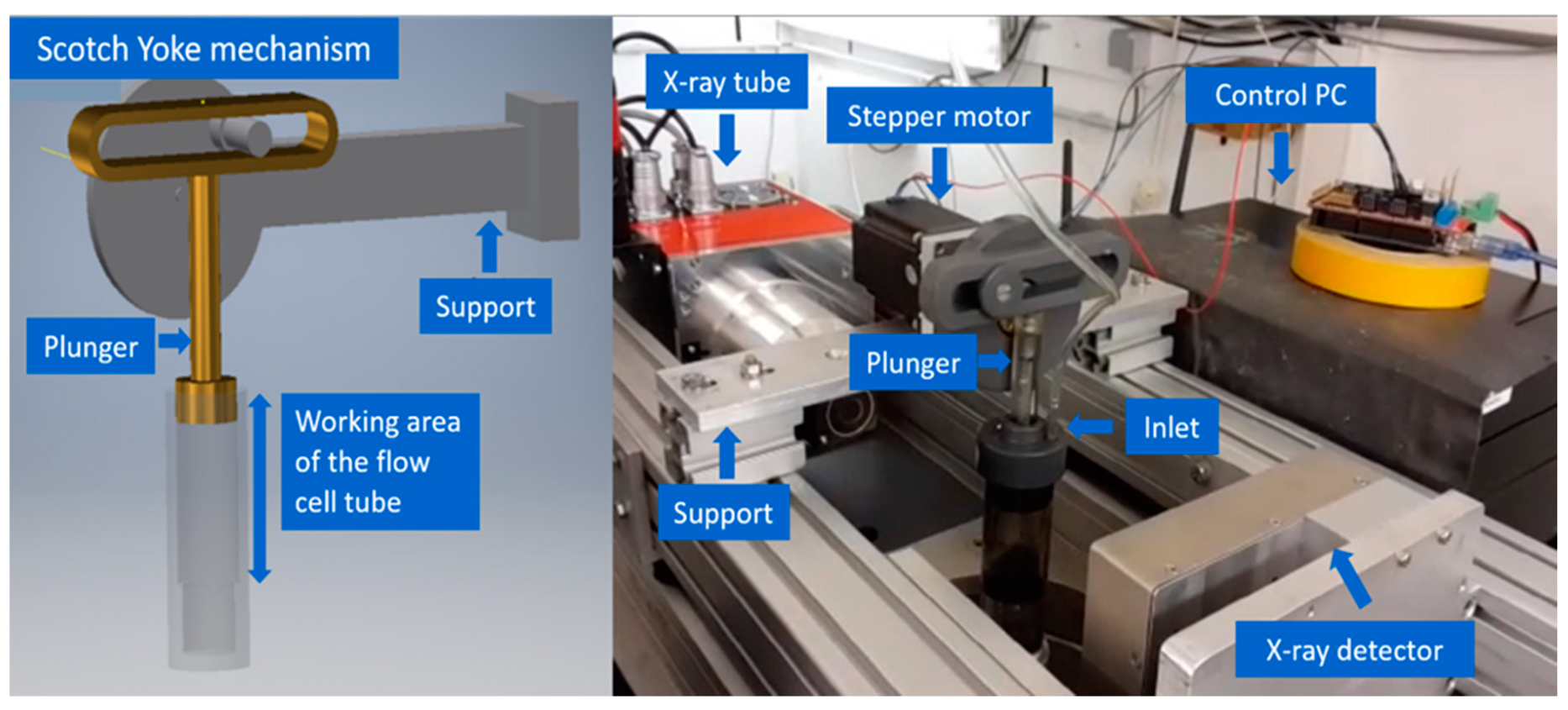
2.3. Flow Cell and Its Automation
2.4. Experimental Design
- Section 2.5 describes two preliminary experiments performed to characterize the samples in more detail. In the first experiment the sponge sample was placed inside the flow cell and fluids (olive oil and water) were added on to the sponge. This system was scanned using X-ray μCT to visualize the microstructure of the sponge and to spot the fluids (water and olive oil) considering their attenuation coefficient values. In the second experiment each of the fluids (olive oil, water and detergent) were scanned separately to determine and note the difference in their attenuation coefficient values.
- Section 2.7 elaborates on the third experiment where the contrasted olive oil was scanned using X-ray μCT to estimate the attenuation coefficient enhancement of olive oil due to the application of contrast agents. A fourth experiment is also described in which the developed experimental protocol was followed, aiming to demonstrate the cleaning capabilities of the custom-built flow cell and quantification of the contrasted olive oil present in the sponge before and after cleaning (i.e., removal of contrasted olive oil from the sponge) with detergents.
2.5. Sample Characterisation
2.6. Contrast Agents
2.7. Application of Contrast Agents on Sponges
- Process 1:
- The flow cell was connected to an inlet and outlet channel for continuous flow of water through the sponge sample.
- Process 2:
- The sponge was compressed using the plunger for one minute at 10 cycles/min to disperse the contrasted olive oil with continuous flow of water.
- Process 3:
- 5 mL of detergent was added to the dirty sponge and squeezed for one minute at 10 cycles/min inside the flow cell without water supply.
- Process 4:
- At Stage 1 of cleaning process, with live water feed 10 min of squeezing was performed at 10 cycles/min.
- Process 5:
- For the Stage 2 of cleaning process another 5 mL of detergent was added onto the same sponge and again 10 min of squeezing with continuous water feed was performed at 10 cycles/min.
- Process 6:
- In the end the sponge was removed and air dried for a day at room temperature.
2.8. Criteria for Cleaning
3. Results
3.1. Characterization of Materials
3.2. Assessment of Contrast Agent Specificity
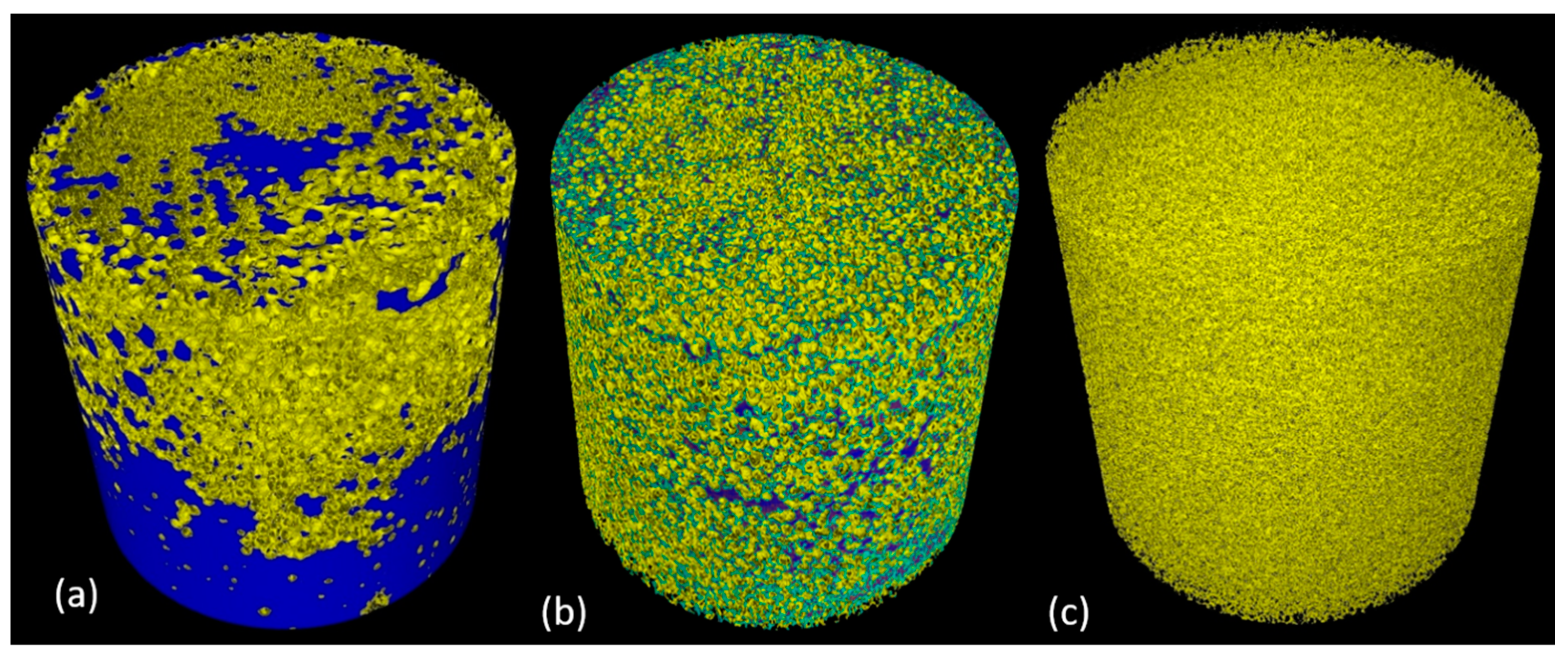
3.3. Experiments of Contrasted Olive Oil on Sponges: Cleaning Assessment of the Custom-Built Device and Quantification of Contrasted Olive Oil in the Sponge
3.4. Experiments: Dynamics of Soil Removal from Sponges under Loading
4. Discussion
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Landrock, A.H. Handbook of Plastic Foams: Types, Properties, Manufacture and Applications; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Menges, G.; Knipschild, F. Estimation of mechanical properties for rigid polyurethane foams. Polym. Eng. Sci. 1975, 15, 623–627. [Google Scholar] [CrossRef]
- WorldBioProducts. The Polyurethane Advantage. Available online: http://www.worldbioproducts.com/polyurethane.html (accessed on 16 August 2018).
- Fort Richard Laboratories. White Paper on Cellulose and Polyurethane Sponges for Surface Sampling. Available online: http://www.fortrichard.com/uploads/resources/Environmental%20Monitoring/White%20Paper%20on%20Use%20of%20Cellulose%20and%20Polyurethane%20Sponge%20for%20EZ%20Reach%20Samplers%20(Updated%2010-25-11).pdf (accessed on 16 August 2018).
- Services, T.F. Some Properties of Polyurethane Foams. Available online: https://www.technicalfoamservices.co.uk/blog/some-properties-of-polyurethane-foams/ (accessed on 16 August 2018).
- Cnudde, V.; Boone, M.N. High-resolution X-ray computed tomography in geosciences: A review of the current technology and applications. Earth Sci. Rev. 2013, 123, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Maire, E.; Withers, P.J. Quantitative X-ray tomography. Int. Mater. Rev. 2014, 59, 1–43. [Google Scholar] [CrossRef]
- Long, H.; Swennen, R.; Foubert, A.; Dierick, M.; Jacobs, P. 3D quantification of mineral components and porosity distribution in westphalian c sandstone by microfocus X-ray computed tomography. Sediment. Geol. 2009, 220, 116–125. [Google Scholar] [CrossRef]
- Pardo-Alonso, S.; Solórzano, E.; Brabant, L.; Vanderniepen, P.; Dierick, M.; Van Hoorebeke, L. 3D analysis of the progressive modification of the cellular architecture in polyurethane nanocomposite foams via X-ray microtomography. Eur. Polym. J. 2013, 49, 999–1006. [Google Scholar] [CrossRef]
- Brabant, L.; Vlassenbroeck, J.; De Witte, Y.; Cnudde, V.; Boone, M.N.; Dewanckele, J.; Van Hoorebeke, L. Three-dimensional analysis of high-resolution X-ray computed tomography data with morpho+. Microsc. Microanal. 2011, 17, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Koloushani, M.; Hedayati, R.; Sadighi, M.; Mohammadi-Aghdam, M. CT-based micro-mechanical approach to predict response of closed-cell porous biomaterials to low-velocity impact. J. Imaging 2018, 4, 49. [Google Scholar] [CrossRef]
- Soltani, P.; Zarrebini, M.; Laghaei, R.; Hassanpour, A. Prediction of permeability of realistic and virtual layered nonwovens using combined application of X-ray μct and computer simulation. Chem. Eng. Res. Des. 2017, 124, 299–312. [Google Scholar] [CrossRef]
- Chen, X.; Verma, R.; Espinoza, D.N.; Prodanović, M. Pore-scale determination of gas relative permeability in hydrate-bearing sediments using X-ray computed micro-tomography and lattice boltzmann method. Water Resour. Res. 2018, 54, 600–608. [Google Scholar] [CrossRef]
- Bultreys, T.; Boone, M.A.; Boone, M.N.; De Schryver, T.; Masschaele, B.; Van Hoorebeke, L.; Cnudde, V. Fast laboratory-based micro-computed tomography for pore-scale research: Illustrative experiments and perspectives on the future. Adv. Water Resour. 2016, 95, 341–351. [Google Scholar] [CrossRef]
- Bultreys, T.; Stappen, J.V.; Kock, T.D.; Boever, W.D.; Boone, M.A.; Hoorebeke, L.V.; Cnudde, V. Investigating the relative permeability behavior of microporosity-rich carbonates and tight sandstones with multiscale pore network models. J. Geophys. Res. B Solid Earth 2016, 121, 7929–7945. [Google Scholar] [CrossRef]
- Von der Schulenburg, D.G.; Paterson-Beedle, M.; Macaskie, L.; Gladden, L.; Johns, M. Flow through an evolving porous media—Compressed foam. J. Mater. Sci. 2007, 42, 6541–6548. [Google Scholar] [CrossRef]
- Moser, S.; Nau, S.; Salk, M.; Thoma, K. In situ flash X-ray high-speed computed tomography for the quantitative analysis of highly dynamic processes. Meas. Sci. Technol. 2014, 25, 025009. [Google Scholar] [CrossRef]
- De Kock, T.; Boone, M.A.; De Schryver, T.; Van Stappen, J.; Derluyn, H.; Masschaele, B.; De Schutter, G.; Cnudde, V. A pore-scale study of fracture dynamics in rock using X-ray micro-CT under ambient freeze–thaw cycling. Environ. Sci. Technol. 2015, 49, 2867–2874. [Google Scholar] [CrossRef] [PubMed]
- Van Stappen, J.F.; Meftah, R.; Boone, M.A.; Bultreys, T.; De Kock, T.; Blykers, B.K.; Senger, K.; Olaussen, S.; Cnudde, V. In situ triaxial testing to determine fracture permeability and aperture distribution for CO2 sequestration in Svalbard, Norway. Environ. Sci. Technol. 2018, 52, 4546–4554. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.; De Kock, T.; Dewanckele, J.; Cnudde, V.; Van Loo, D.; Van de Casteele, E.; De Schutter, G.; Jacobs, P. Four-Dimensional Monitoring of Freeze-Thaw Cycles in Limestone with X-ray Computed Microtomography (micro-CT). In Proceedings of the 12th Euroseminar on Microscopy Applied on Natural Building Stones, Dortmund, Germany, 15–19 September 2009; Available online: https://biblio.ugent.be/publication/752070 (accessed on 19 December 2016).
- De Schryver, T.; Dierick, M.; Heyndrickx, M.; Van Stappen, J.; Boone, M.A.; Van Hoorebeke, L.; Boone, M.N. Motion compensated micro-CT reconstruction for in-situ analysis of dynamic processes. Sci. Rep. 2018, 8, 7655. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Schlüter, S.; Sheppard, A.; Wildenschild, D. On the challenges of measuring interfacial characteristics of three-phase fluid flow with X-ray microtomography. J. Microsc. 2014, 253, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Garrett, P.R.; Meshkov, S.; Perlmutter, G. Oral contrast agents in CT of the abdomen. Radiology 1984, 153, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Descamps, E.; Sochacka, A.; De Kegel, B.; Van Loo, D.; Van Hoorebeke, L.; Adriaens, D. Soft tissue discrimination with contrast agents using micro-CT scanning. Belg. J. Zool. 2014, 144, 20–40. [Google Scholar]
- Pauwels, E.; Van Loo, D.; Cornillie, P.; Brabant, L.; Van Hoorebeke, L. An exploratory study of contrast agents for soft tissue visualization by means of high resolution X-ray computed tomography imaging. J. Microsc. 2013, 250, 21–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lusic, H.; Grinstaff, M.W. X-ray-computed tomography contrast agents. Chem. Rev. 2012, 113, 1641–1666. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Jarzyna, P.A.; Mulder, W.J.; Fayad, Z.A. Modified natural nanoparticles as contrast agents for medical imaging. Adv. Drug Deliv. Rev. 2010, 62, 329–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Hannula, M.; Misra, S.; Feng, H.; Labrador, R.H.; Aula, A.S.; Hyttinen, J.; Pyykkö, I. Micro CT visualization of silver nanoparticles in the middle and inner ear of rat and transportation pathway after transtympanic injection. J. Nanobiotechnol. 2015, 13, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellington, S.L.; Vinegar, H.J. X-ray computerized-tomography. J. Pet. Technol. 1987, 39, 885–898. [Google Scholar] [CrossRef]
- Van Loo, D.; Bouckaert, L.; Leroux, O.; Pauwels, E.; Dierick, M.; Van Hoorebeke, L.; Cnudde, V.; De Neve, S.; Sleutel, S. Contrast agents for soil investigation with X-ray computed tomography. Geoderma 2014, 213, 485–491. [Google Scholar] [CrossRef]
- Smith, R.W.; Bryg, V. Staining polymers for microscopical examination. Rubber Chem. Technol. 2006, 79, 520–540. [Google Scholar] [CrossRef]
- Allan-Wojtas, P.; Hildebrand, P.; Braun, P.; Smith-King, H.; Carbyn, S.; Renderos, W. Low temperature and anhydrous electron microscopy techniques to observe the infection process of the bacterial pathogen xanthomonas fragariae on strawberry leaves. J. Microsc. 2010, 239, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Priester, J.H.; Horst, A.M.; Van De Werfhorst, L.C.; Saleta, J.L.; Mertes, L.A.; Holden, P.A. Enhanced visualization of microbial biofilms by staining and environmental scanning electron microscopy. J. Microbiol. Methods 2007, 68, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S. Reduction of phosphomolybdic acid by compounds possessing conjugated double bonds. Anal. Chem. 1953, 25, 422–424. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Rahman, M.Z.A.; Amin, J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabherr, S.; Gygax, E. X ray Contrasting Agent for Post-Mortem, Experimental and Diagnostic Angiography. European Patent EP2526973A2, 12 September 2007. [Google Scholar]
- Buffiere, J.Y.; Maire, E.; Adrien, J.; Masse, J.P.; Boller, E. In situ experiments with X ray tomography: An attractive tool for experimental mechanics. Exp. Mech. 2010, 50, 289–305. [Google Scholar] [CrossRef]
- Kyrieleis, A.; Titarenko, V.; Ibison, M.; Connolley, T.; Withers, P. Region-of-interest tomography using filtered backprojection: Assessing the practical limits. J. Microsc. 2011, 241, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Masschaele, B.; Dierick, M.; Loo, D.V.; Boone, M.N.; Brabant, L.; Pauwels, E.; Cnudde, V.; Hoorebeke, L.V. Hector: A 240 kv micro-ct setup optimized for research. J. Phys. Conf. Ser. 2013, 463, 012012. [Google Scholar] [CrossRef]
- Dierick, M.; Van Loo, D.; Masschaele, B.; Van den Bulcke, J.; Van Acker, J.; Cnudde, V.; Van Hoorebeke, L. Recent micro-ct scanner developments at ugct. Nucl. Instrum. Methods Phys. Res. B 2014, 324, 35–40. [Google Scholar] [CrossRef]
- Vlassenbroeck, J.; Dierick, M.; Masschaele, B.; Cnudde, V.; Van Hoorebeke, L.; Jacobs, P. Software tools for quantification of X-ray microtomography. Nucl. Instrum. Methods Phys. Res. A 2007, 580, 442–445. [Google Scholar] [CrossRef]
- Boone, M.; Bultreys, T.; Masschaele, B.; Van Loo, D.; Van Hoorebeke, L.; Cnudde, V. In-Situ, Real Time Micro-CT Imaging of Pore Scale Processes, the Next Frontier for Laboratory Based Micro-CT Scanning. In Proceedings of the 30th International Symposium of the Society of Core Analysts, Snowmass, CO, USA, 21–26 August 2016; Available online: https://biblio.ugent.be/publication/8130807 (accessed on 21 December 2016).
- Taufiq, A.; Saputro, R.; Hidayat, N.; Hidayat, A.; Mufti, N.; Diantoro, M.; Patriati, A.; Putra, E.; Nur, H. Fabrication of magnetite nanoparticles dispersed in olive oil and their structural and magnetic investigations. In Proceedings of the IOP Conference Series: Materials Science and Engineering, 2017; IOP Publishing: Bristol, UK, 2017; p. 012008. [Google Scholar]
- Moura, M.; Fiorentino, E.A.; Måløy, K.J.; Schäfer, G.; Toussaint, R. Impact of sample geometry on the measurement of pressure—Saturation curves: Experiments and simulations. Water Resour. Res. 2015, 51, 8900–8926. [Google Scholar] [CrossRef]
- Rapp, B.E. Microfluidics: Modeling, Mechanics and Mathematics; William Andrew: Norwich, NY, USA, 2016. [Google Scholar]



| Solvents | μ (cm−1) | σ (μ) (cm−1) |
|---|---|---|
| Olive oil | 0.25 | 0.0158 |
| Water | 0.33 | 0.0261 |
| Detergent (Dish washing liquid) | 0.35 | 0.0297 |
| Magnetite powder dispersed olive oil | 0.45 | 0.0421 |
| Brominated vegetable oil with olive oil | 0.62 | 0.0775 |
| Contrasted Olive Oil | Actual Volume of Contrasted Olive Oil | Uncleaned Sponge | Stage 1 | Stage 2 |
|---|---|---|---|---|
| Volume of magnetite dispersed olive oil (mL) | 5 | 3.6 | 0.8 | 0.79 |
| Volume of brominated vegetable oil with olive oil (mL) | 5 | 1.2 | 0.08 | 0.01 |
| Sponge Samples | Stage 1 of Cleaning | Stage 2 of Cleaning |
|---|---|---|
| Sponge 1 | 77.2% | 77.5% |
| Sponge 2 | 92.5% | 98.8% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shastry, A.; Palacio-Mancheno, P.E.; Braeckman, K.; Vanheule, S.; Josipovic, I.; Van Assche, F.; Robles, E.; Cnudde, V.; Van Hoorebeke, L.; Boone, M.N. In-Situ High Resolution Dynamic X-ray Microtomographic Imaging of Olive Oil Removal in Kitchen Sponges by Squeezing and Rinsing. Materials 2018, 11, 1482. https://doi.org/10.3390/ma11081482
Shastry A, Palacio-Mancheno PE, Braeckman K, Vanheule S, Josipovic I, Van Assche F, Robles E, Cnudde V, Van Hoorebeke L, Boone MN. In-Situ High Resolution Dynamic X-ray Microtomographic Imaging of Olive Oil Removal in Kitchen Sponges by Squeezing and Rinsing. Materials. 2018; 11(8):1482. https://doi.org/10.3390/ma11081482
Chicago/Turabian StyleShastry, Abhishek, Paolo E. Palacio-Mancheno, Karl Braeckman, Sander Vanheule, Ivan Josipovic, Frederic Van Assche, Eric Robles, Veerle Cnudde, Luc Van Hoorebeke, and Matthieu N. Boone. 2018. "In-Situ High Resolution Dynamic X-ray Microtomographic Imaging of Olive Oil Removal in Kitchen Sponges by Squeezing and Rinsing" Materials 11, no. 8: 1482. https://doi.org/10.3390/ma11081482






