Effects of Bi2O3 Doping on the Mechanical Properties of PbO Ceramic Pellets Used in Lead-Cooled Fast Reactors
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Mechanical Properties of Bi2O3-PbO Ceramic Pellets
3.2. Flushing Test
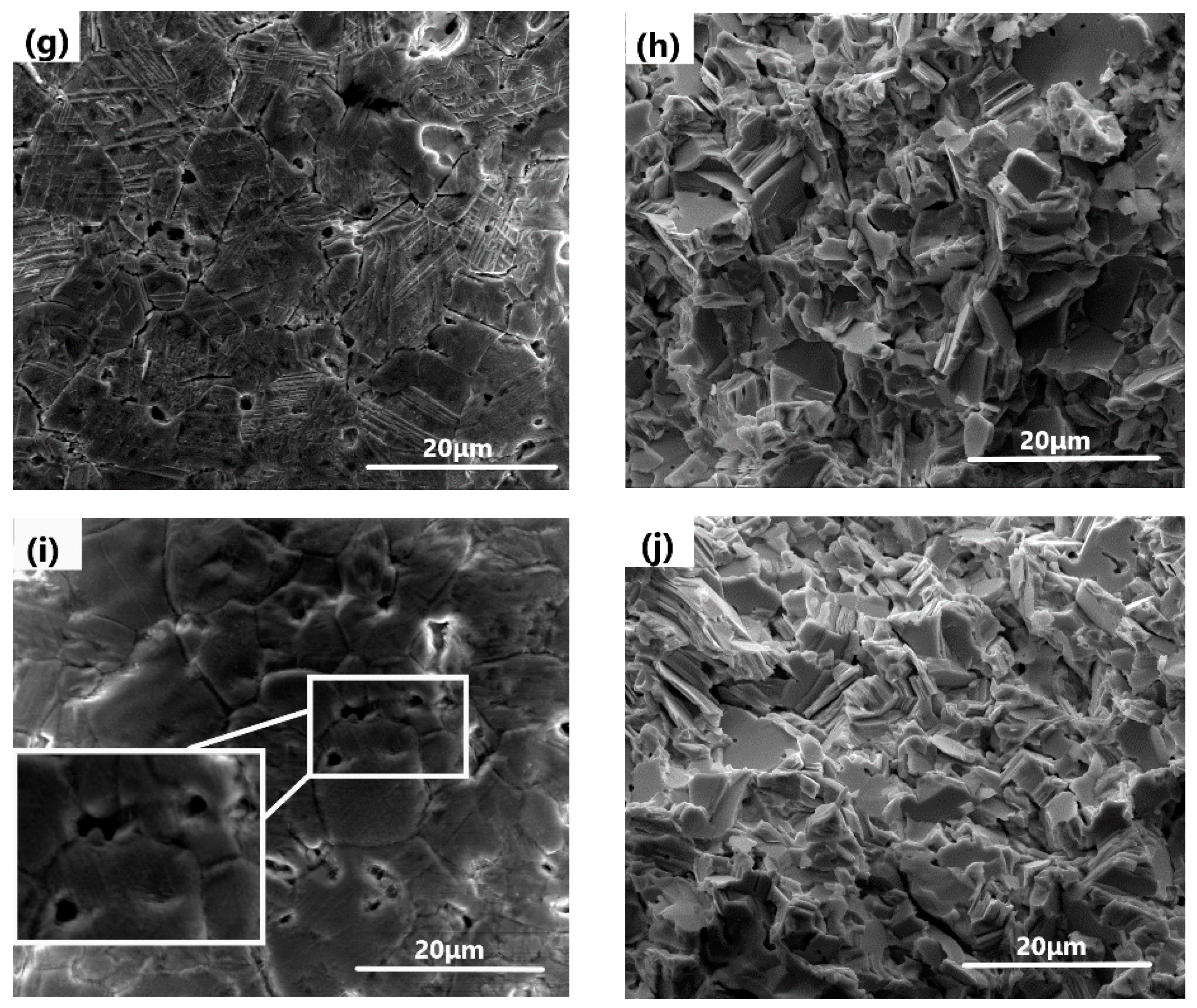
3.3. Microstructures of Bi2O3-PbO Ceramic Pellets
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gromov, B.F.; Belomitcev, Y.S.; Yefimov, E.I.; Leonchuk, M.P.; Martinov, P.N.; Orlov, Y.I.; Pankratov, D.V.; Pashkin, Y.G.; Toshinsky, G.I.; Chekunov, V.V.; et al. Use of lead-bismuth coolant in nuclear reactors and accelerator-driven systems. Nucl. Eng. Des. 1997, 173, 207–217. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N. Review of the studies on fundamental issues in LBE corrosion. J. Nucl. Mater. 2008, 373, 351–377. [Google Scholar] [CrossRef]
- Handbook on Lead–bismuth Eutectic Alloy and Lead Properties, Materials Compatibility, Thermal-hydraulics and Technologies. Available online: http://www.oecd-nea.org/science/reports/2007/nea6195-handbook.html. (accessed on 12 September 2018).
- Martynov, P.N.; Askhadullin, R.S.; Simakov, A.A.; Chaban, A.Y.; Legkikh, A.Y. Designing mass exchangers for control of oxygen content in Pb-Bi (Pb) coolants in various research facilities. In Proceedings of the International Conference on Nuclear Engineering, Brussels, Belgium, 12–16 July 2009; Volume 1, pp. 555–561. [Google Scholar]
- Marino, A.; Lim, J.; Keijers, S.; Vanmaercke, S.; Aerts, A.; Rosseel, K.; Deconinck, J.; Van den Bosch, J. A mass transfer correlation for packed bed of lead oxide spheres in flowing lead bismuth eutectic at high Péclet numbers. Int. J. Heat Mass Tran. 2015, 80, 737–747. [Google Scholar] [CrossRef]
- Kondo, M.; Takahashi, M.; Miura, K.; Onizawa, T. Study on control of oxygen concentration in lead–bismuth flow using lead oxide particles. J. Nucl. Mater. 2006, 357, 97–104. [Google Scholar] [CrossRef]
- Brissonneau, L.; Beauchamp, F.; Morier, O.; Schroer, C.; Konys, J.; Kobzova, A.; Di Gabriele, F.; Courouau, J.L. Oxygen control systems and impurity purification in LBE: Learning from DEMETRA project. J. Nucl. Mater. 2011, 415, 348–360. [Google Scholar] [CrossRef]
- Zhang, S.Y. Sintering and performance of lead oxide ceramics used for solid phase oxygen control system in LBE (in Chinese). Master’s Degree, North China Electric Power University, Beijing, China, April 2016. [Google Scholar]
- Ma, Y.; Zhang, S.Y.; Xu, Y.Z.; Lu, C.L. Sintering behavior of lead oxide ceramic prepared by microwave sintering. Adv. Mater. Res. 2014, 941, 525–528. [Google Scholar] [CrossRef]
- Ramana, M.V.; Kiran, S.R.; Reddy, N.R.; Kumar, K.S.; Murthy, V.R.K.; Murty, B.S. Investigation and characterization of Pb(Zr0.52Ti0.48)O3 nanocrystalline ferroelectric ceramics: By conventional and microwave sintering methods. Mater. Chem. Phys. 2011, 126, 295–300. [Google Scholar] [CrossRef]
- Kingon, A.I.; Clark, J.B. Sintering of PZT Ceramics: I, atmosphere control, II, effect of PbO content on densification kinetics. J. Am. Ceram. Soc. 1983, 66, 253–256. [Google Scholar] [CrossRef]
- ASTM C1327-15. Standard Test Method for Vickers Indentation Hardness of Advanced Ceramics; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Ostrowski, T.; Ziegler, A.; Bordia, R.K.; Rödel, J. Evolution of young’s modulus, strength, and microstructure during liquid-phase sintering. J. Am. Ceram. Soc. 1998, 81, 195–200. [Google Scholar] [CrossRef]
- Guo, S.G. Powder Sintering Theory (in Chinese), 1st ed.; Metallurgical Industry Press: Beijing, China, 1998; pp. 292–295. [Google Scholar]
- Liu, N.; Xu, Y.; Li, Z.; Chen, M.; Li, G.; Zhang, L. Influence of molybdenum addition on the microstructure and mechanical properties of TiC-based cermets with nano-TiN modification. Ceram. Int. 2003, 29, 919–925. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Yang, A.; Zhu, H.; Muhammad, A.; Yang, P.; Huang, B.; Niu, F. Effects of Bi2O3 Doping on the Mechanical Properties of PbO Ceramic Pellets Used in Lead-Cooled Fast Reactors. Materials 2019, 12, 1948. https://doi.org/10.3390/ma12121948
Ma Y, Yang A, Zhu H, Muhammad A, Yang P, Huang B, Niu F. Effects of Bi2O3 Doping on the Mechanical Properties of PbO Ceramic Pellets Used in Lead-Cooled Fast Reactors. Materials. 2019; 12(12):1948. https://doi.org/10.3390/ma12121948
Chicago/Turabian StyleMa, Yan, Anxia Yang, Huiping Zhu, Arslan Muhammad, Pengwei Yang, Bokun Huang, and Fenglei Niu. 2019. "Effects of Bi2O3 Doping on the Mechanical Properties of PbO Ceramic Pellets Used in Lead-Cooled Fast Reactors" Materials 12, no. 12: 1948. https://doi.org/10.3390/ma12121948



