Biodegradable Cellulose-based Hydrogels: Design and Applications
Abstract
:1. Introduction
2. Cellulose and Cellulose-based Hydrogels
2.1. Cellulose Structure and Biodegradability
2.2. Water-soluble Cellulose Derivatives
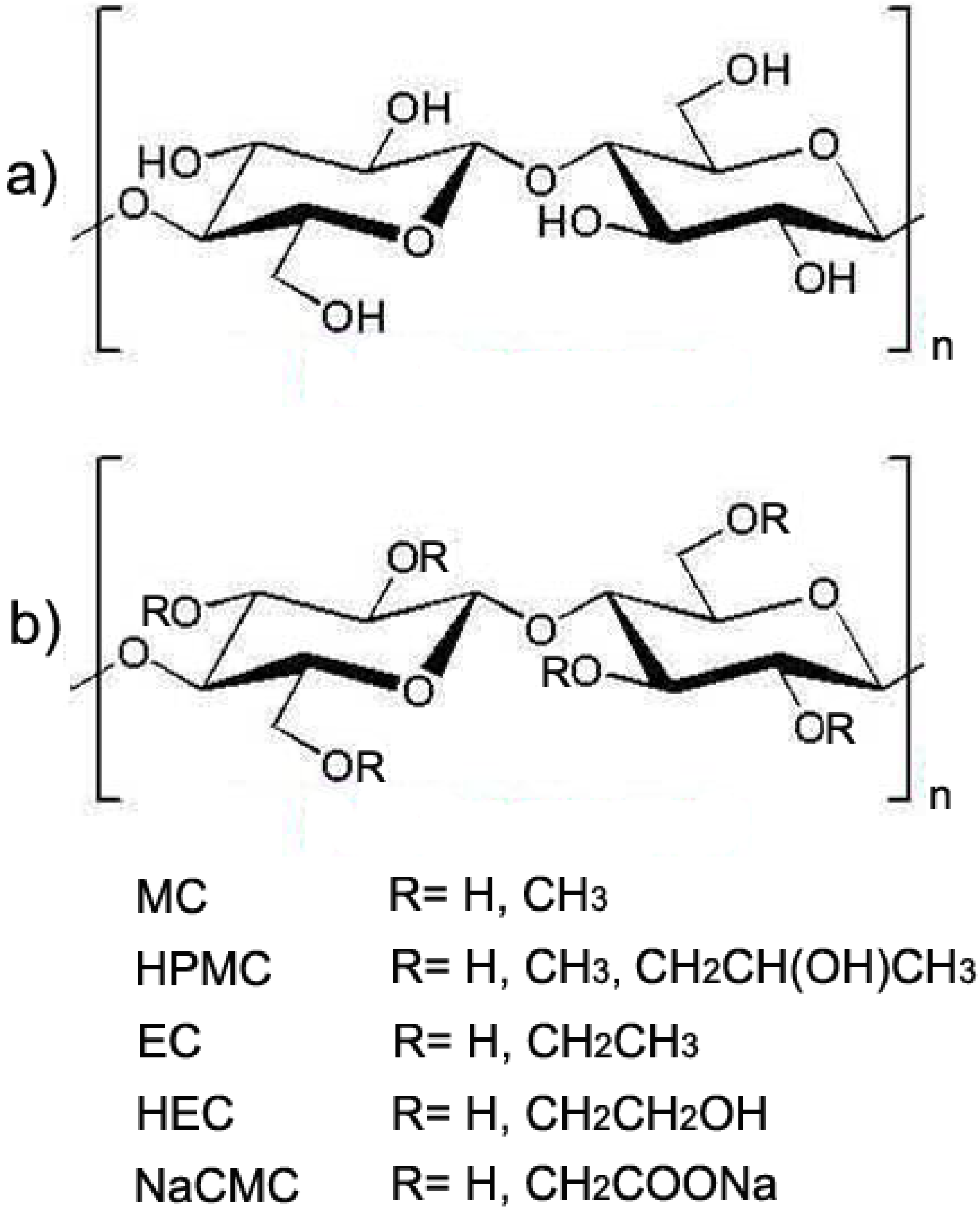
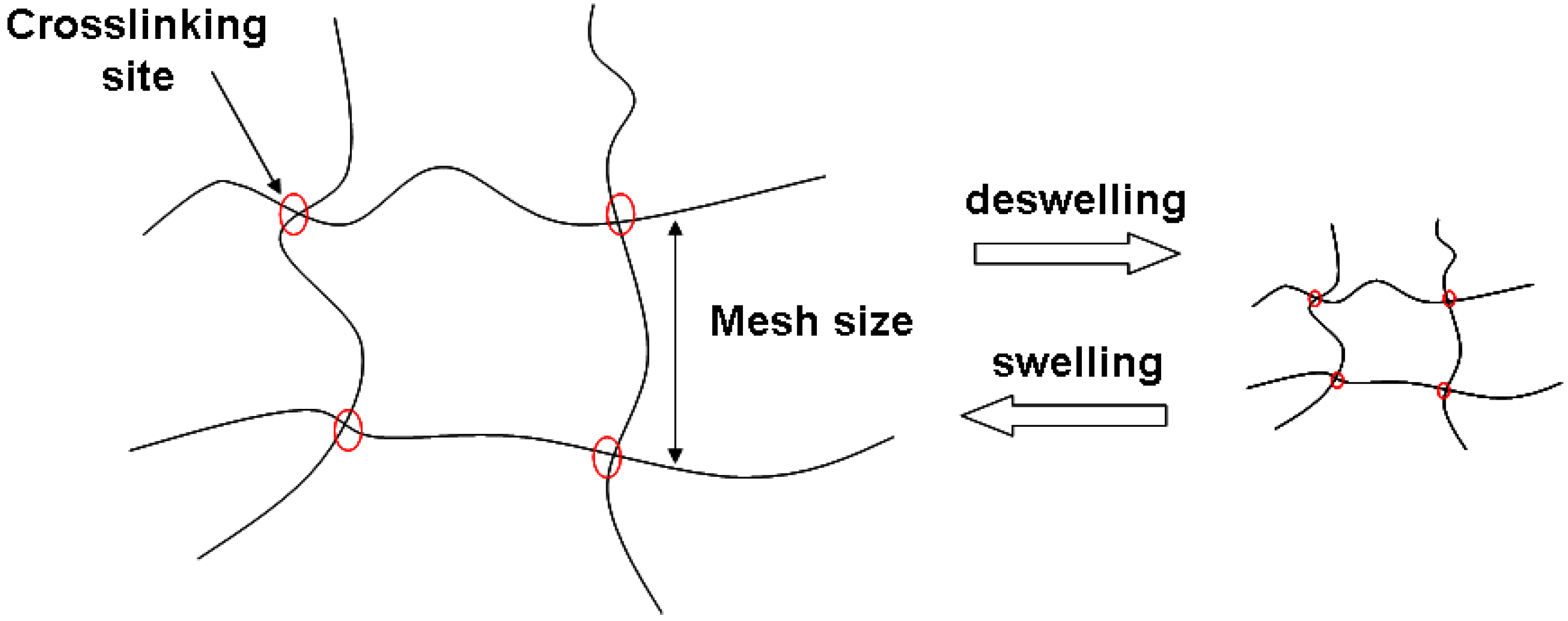
2.3. Cellulose-based Hydrogels and Crosslinking Strategies
3. Applications of Cellulose-based Hydrogels
3.1. Superabsorbents for Personal Hygiene Products
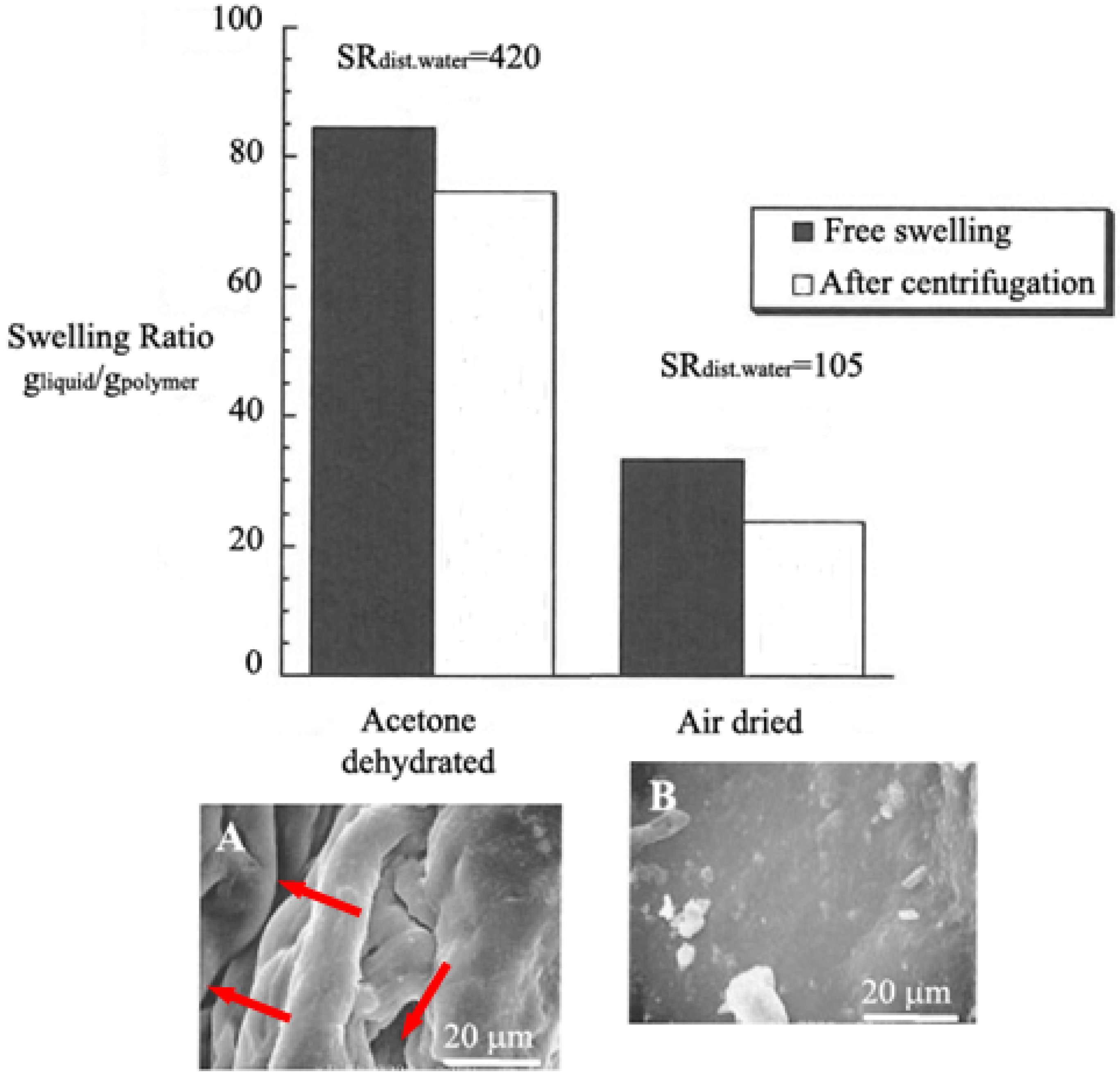
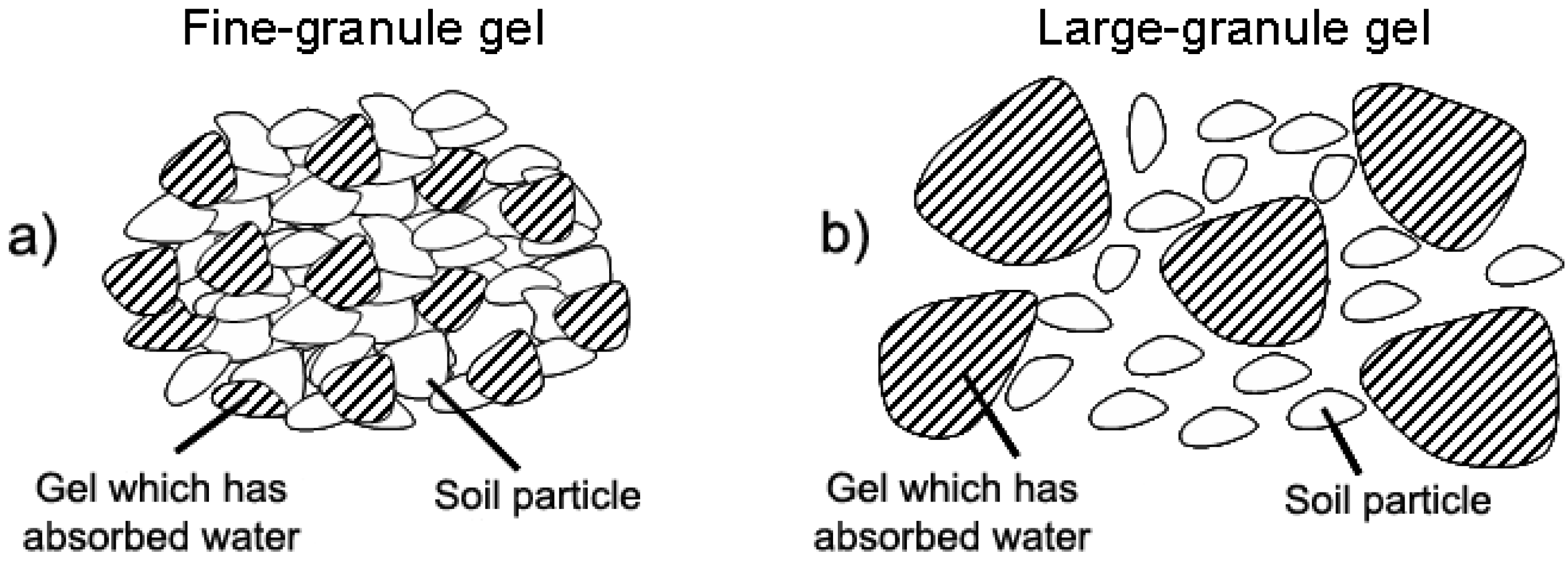
3.2. Water Reservoirs in Agriculture
3.3. Body Water Retainers
3.4. Stomach Bulking Agents
3.5. Devices for Controlled Drug Delivery
3.6. Scaffolds for Regenerative Medicine

3.7. Wound Dressings
| Hydrogel wound dressing (Producer) | Composition |
|---|---|
| IntraSiteTM Gel (Smith and Nephew) | Water |
| Propylene glycol | |
| NaCMC | |
| GranuGelTM (ConvaTec) | Water |
| NaCMC | |
| Propylene glycol | |
| Pectin | |
| Purilon GelTM (ColoPlast) | Water |
| CMC | |
| Calcium alginate | |
| Aquacel AgTM | NaCMC |
| (ConvaTec) | Silver ions (1.2%) |
| SilvercelTM (Johnson & Johnson) | Calcium alginate |
| CMC | |
| Silver ions (8%) |
4. Conclusions
Acknowledgements
References and Notes
- Tanaka, T. Gels. Sci. Am. 1981, 244(1), 124–136, 138. [Google Scholar] [PubMed]
- Richter, A.; Howitz, S.; Kuckling, D.; Arndt, K.F. Influence of volume phase transition phenomena on the behavior of hydrogel-based valves. Sens. Actuat. B 2004, 99(2-3), 451–458. [Google Scholar]
- Mao, L.; Hu, Y.; Piao, Y.; Chen, X.; Xian, W.; Piao, D. Structure and character of artificial muscle model constructed from fibrous hydrogel. Curr. Appl. Phys. 2005, 5(5), 426–428. [Google Scholar] [CrossRef]
- Peppas, N.A. Hydrogels and drug delivery. Curr. Opin. Colloid Interface Sci. 1997, 2(5), 531–537. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Delivery Rev. 2001, 53(3), 321–339. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185(4706), 117–118. [Google Scholar] [CrossRef]
- Chambers, D.R.; Fowler, H.H.; Fujiura, Y.; Masuda, F. Super-absorbent polymer having improved absorbency properties. US Patent 5145906, 1992. [Google Scholar]
- Smith, M.J.; Flowers, T.H.; Cowling, M.J.; Duncan, H.J. Release studies of benzalkonium chloride from hydrogel in a freshwater environment. J. Environ. Monit. 2003, 5(2), 359–362. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.S. The effects of gel-forming polyacrylamides on moisture storage in sandy soils. J. Sci. Food Agric. 1984, 35(11), 1063–1066. [Google Scholar] [CrossRef]
- Bakass, M.; Mokhlisse, A.; Lallemant, M. Absorption and desorption of liquid water by a superabsorbent polymer: effect of polymer in the drying of the soil and the quality of certain plants. J. Appl. Polym. Sci. 2002, 83, 234–243. [Google Scholar] [CrossRef]
- Francois, P.; Vaudaux, P.; Nurdin, P.; Descouts, P. Physical and biological effects of a hydrophilic coating on polyurethane catheters. Biomaterials 1996, 17(7), 667–676. [Google Scholar] [CrossRef] [PubMed]
- Demitri, C.; Sannino, A.; Conversano, F.; Casciaro, S.; Distante, A.; Maffezzoli, A. Hydrogel based tissue mimicking phantom for in-vitro ultrasound contrast agents studies. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2008, 87(2), 338–345. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.R.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26(32), 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24(24), 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Seliktar, D. Extracellular stimulation in tissue engineering. Ann. N. Y. Acad. Sci. 2005, 1047(1), 386–394. [Google Scholar] [CrossRef] [PubMed]
- Jen, A.C.; Conley Wake, M.; Mikos, A.G. Hydrogels for cell immobilization. Biotechnol. Bioeng. 1996, 50(4), 357–364. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991, 55(1), 35–58. [Google Scholar] [PubMed]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Malcolm Brown, R. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8(1), 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tomsic, B.; Simoncic, B.; Orel, B.; Vilcnik, A.; Spreizer, H. Biodegradability of cellulose fabric modified by imidazolidinone. Carbohydr. Polym. 2007, 69(3), 478–488. [Google Scholar] [CrossRef]
- Miyamoto, T.; Takahashi, S.; Ito, H.; Inagaki, H.; Nioshiki, Y. Tissue biocompatibility of cellulose and its derivatives. J. Biomed. Mater. Res. 1989, 23(1), 125–133. [Google Scholar] [CrossRef] [PubMed]
- Entcheva, E.; Bien, H.; Yin, L.; Chung, C.Y.; Farrell, M.; Kostov, Y. Functional cardiac cell constructs on cellulose-based scaffolding. Biomaterials 2004, 25(26), 5753–5762. [Google Scholar] [CrossRef] [PubMed]
- Martson, M.; Viljanto, J.; Hurme, T.; Laippala, P.; Saukko, P. Is cellulose sponge degradable or stable as implantation material? An in vivo subcutaneous study in the rat. Biomaterials 1999, 20(21), 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Sun, B. New tissue engineering material copolymers of derivatives of cellulose and lactide: their synthesis and characterization. Mater. Sci. Eng. C 2000, 11(1), 57–60. [Google Scholar] [CrossRef]
- Sannino, A.; Madaghiele, M.; Conversano, F.; Mele, G.; Maffezzoli, A.; Netti, P.A.; Ambrosio, L.; Nicolais, L. Cellulose derivative-hyaluronic acid-based microporous hydrogels cross-linked through divinyl sulfone (DVS) to modulate equilibrium sorption capacity and network stability. Biomacromolecules 2004, 5(1), 92–96. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Pappadà, S.; Madaghiele, M.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Crosslinking of cellulose derivatives and hyaluronic acid with water-soluble carbodiimide. Polymer 2005, 46(25), 11206–11212. [Google Scholar] [CrossRef]
- Ito, T.; Yeo, Y.; Highley, C.B.; Bellas, E.; Benitez, C.A.; Kohane, D.S. The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials 2007, 28(6), 975–983. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Principles of polymer chemistry; Cornell University Press: Ithaca, New York, USA, 1953. [Google Scholar]
- Esposito, F.; Del Nobile, M.A.; Mensitieri, M.; Nicolais, L. Water sorption in cellulose-based hydrogels. J. Appl. Polym. Sci. 1996, 60(13), 2403–2407. [Google Scholar] [CrossRef]
- Sannino, A.; Esposito, A.; Nicolais, L.; Del Nobile, M.A.; Giovane, A.; Balestrieri, C.; Esposito, R.; Agresti, M. Cellulose-based hydrogels as body water retainers. J. Mater. Sci. - Mater. Med. 2000, 11(4), 247–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, M. Novel thermally sensitive pH-dependent chitosan/carboxymethyl cellulose hydrogels. J. Bioact. Compat. Polym. 2008, 23(1), 38–48. [Google Scholar] [CrossRef]
- Chang, C.; Lue, A.; Zhang, L. Effects of crosslinking methods on structure and properties of cellulose/PVA hydrogels. Macromol. Chem. Phys. 2008, 209(12), 1266–1273. [Google Scholar] [CrossRef]
- Sarkar, N. Thermal gelation properties of methyl and hydroxypropyl methylcellulose. J. Appl. Polym. Sci. 1979, 24(4), 1073–1087. [Google Scholar] [CrossRef]
- Tate, M.C.; Shear, D.A.; Hoffman, S.W.; Stein, D.G.; LaPlaca, M.C. Biocompatibility of methylcellulose-based constructs designed for intracerebral gelation following experimental traumatic brain injury. Biomaterials 2001, 22(10), 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tsai, C.; Chen, W.; Mi, F.; Liang, H.; Chen, S.; Sung, H. Novel living cell sheet harvest system composed of thermoreversible methylcellulose hydrogels. Biomacromolecules 2006, 7(3), 736–743. [Google Scholar] [CrossRef] [PubMed]
- Stabenfeldt, S.E.; Garcia, A.J.; LaPlaca, M.C. Thermoreversible laminin-functionalized hydrogel for neural tissue engineering. J. Biomed. Mater. Res. A 2006, 77(4), 718–725. [Google Scholar] [CrossRef] [PubMed]
- Te Nijenhuis, K. On the nature of crosslinks in thermoreversible gels. Polym. Bull. 2007, 58(1), 27–42. [Google Scholar] [CrossRef]
- Vinatier, C.; Magne, D.; Weiss, P.; Trojani, C.; Rochet, N.; Carle, G.F.; Vignes-Colombeix, C.; Chadjichristos, C.; Galera, P.; Daculsi, G.; Guicheux, J. A silanized hydroxypropyl methylcellulose hydrogel for the three-dimensional culture of chondrocytes. Biomaterials 2005, 26(33), 6643–6651. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, C.; Magne, D.; Moreau, A.; Gauthier, O.; Malard, O.; Vignes-Colombeix, C.; Daculsi, G.; Weiss, P.; Guicheux, J. Engineering cartilage with human nasal chondrocytes and a silanized hydroxypropyl methylcellulose hydrogel. J. Biomed. Mater. Res. A 2007, 80(1), 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ogushi, Y.; Sakai, S.; Kawakami, K. Synthesis of enzimatically-gellable carboxymethylcellulose for biomedical applications. J. Biosci. Bioeng. 2007, 104(1), 30–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, C. Physical properties of the crosslinked cellulose catalyzed with nanotitanium dioxide under UV irradiation and electronic field. Appl. Catal., A 2005, 293(2B), 171–179. [Google Scholar] [CrossRef]
- Coma, V.; Sebti, I.; Pardon, P.; Pichavant, F.H.; Deschamps, A. Film properties from crosslinking of cellulosic derivatives with a polyfunctional carboxylic acid. Carbohydr. Polym. 2003, 51(3), 265–271. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Q.; Cui, S.W. Studies on the granular structure of resistant starches (type 4) from normal, high amylose and waxy corn starch citrates. Food Res. Int. 2006, 39(3), 332–341. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110(4), 2453–2460. [Google Scholar] [CrossRef]
- Charlesby, A. The degradation of cellulose by ionizing radiation. J. Polym. Sci. 1955, 15(79), 263–270. [Google Scholar] [CrossRef]
- Wach, R.A.; Mitomo, H.; Nagasawa, N.; Yoshii, F. Radiation crosslinking of methylcellulose and hydroxyethylcellulose in concentrated aqueous solutions. Nucl. Instrum. Methods Phys. Res., Sect. B 2003, 211(4), 533–544. [Google Scholar] [CrossRef]
- Pekel, N.; Yoshii, F.; Kume, T.; Guven, O. Radiation crosslinking of biodegradable hydroxypropylmethylcellulose. Carbohydr. Polym. 2004, 55(2), 139–147. [Google Scholar] [CrossRef]
- Liu, P.; Peng, J.; Li, J.; Wu, J. Radiation crosslinking of CMC-Na at low dose and its application as substitute for hydrogels. Rad. Phys. Chem. 2005, 72(5), 635–638. [Google Scholar] [CrossRef]
- Noonan, C.; Quigley, S.; Curley, M.A.Q. Skin integrity in hospitalized infants and children: a prevalence survey. J. Pediatr. Nurs. 2006, 21(6), 445–453. [Google Scholar] [CrossRef] [PubMed]
- Akin, F.; Spraker, M.; Aly, R.; Leyden, J.; Raynor, W.; Landin, W. Effects of breathable disposable diapers: reduced prevalence of Candida and common diaper dermatitis. Pediatr. Dermatol. 2001, 18(4), 282–290. [Google Scholar] [CrossRef] [PubMed]
- Holaday, B.; Waugh, G.; Moukaddem, V.E.; West, J.; Harshman, S. Diaper type and fecal contamination in child day care. J. Pediatr. Health Care 1995, 9(2), 67–74. [Google Scholar] [CrossRef] [PubMed]
- Adalat, S.; Wall, D.; Goodyear, H. Diaper dermatitis-frequency and contributory factors in hospital attending children. Pediatr. Dermatol. 2007, 24(5), 483–488. [Google Scholar] [CrossRef] [PubMed]
- Harper, B.G.; Bashaw, R.N.; Atkins, B.L. Absorbent product containing a hydrocolloidal composition. U.S. Patent 3669103, 1972. [Google Scholar]
- Harmon, C. Absorbent product containing a hydrocolloidal composition. US Patent 3670731, 1972. [Google Scholar]
- Davis, J.A.; Leyden, J.J.; Grove, G.L.; Raynor, W.J. Comparison of disposable diapers with fluff absorbent and fluff plus absorbent polymers: effects on skin hydration, skin pH, and diaper dermatitis. Pediatr. Dermatol. 1989, 6(2), 102–108. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, B.L. Disposable diaper recycling process. US Patent 5292075, 1994. [Google Scholar]
- Sannino, A.; Mensitieri, G.; Nicolais, L. Water and synthetic urine sorption capacity of cellulose-based hydrogels under a compressive stress field. J. Appl. Polym. Sci. 2004, 91(6), 3791–3796. [Google Scholar] [CrossRef]
- Marcì, G.; Mele, G.; Palmisano, L.; Pulito, P.; Sannino, A. Environmentally sustainable production of cellulose-based superabsorbent hydrogels. Green Chem. 2006, 8(5), 439–444. [Google Scholar] [CrossRef]
- Sarvas, M.; Pavlenda, P.; Takacova, E. Effect of hydrogel application on survival and growth of pine seedlings in reclamations. J. Forest Sci. 2007, 53(5), 204–209. [Google Scholar]
- Lenzi, F.; Sannino, A.; Borriello, A.; Porro, F.; Capitani, D.; Mensitieri, G. Probing the degree of crosslinking of a cellulose based superabsorbing hydrogel through traditional and NMR techniques. Polymer 2003, 44(5), 1577–1588. [Google Scholar] [CrossRef]
- Sannino, A.; Esposito, A.; De Rosa, A.; Cozzolino, A.; Ambrosio, L.; Nicolais, L. Biomedical application of a superabsorbent hydrogel for body water elimination in the treatment of edemas. J. Biomed. Mater. Res. A 2003, 67(3), 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Sannino, A.; Cozzolino, A.; Quintiliano, S.N.; Lamberti, M.; Ambrosio, L.; Nicolais, L. Response of intestinal cells and macrophages to an orally administered cellulose-PEG based polymer as a potential treatment for intractable edemas. Biomaterials 2005, 26(19), 4101–4110. [Google Scholar] [CrossRef] [PubMed]
- Schachter, M.; Purcell, H.; Daly, C.; Sheppard, M. Management of overweight and obesity in patients with cardiovascular disease. Br. J. Cardiol. 2002, 9(1), 42–46. [Google Scholar]
- Sowers, J.R. Obesity as a cardiovascular risk factor. Am. J. Med. 2003, 115 (Suppl 8A), 37S–41S. [Google Scholar] [CrossRef] [PubMed]
- James, P.T. Obesity: the worldwide epidemic. Clin. Dermatol. 2004, 22(4), 276–280. [Google Scholar] [CrossRef] [PubMed]
- Pittler, M.H.; Ernst, E. Dietary supplements for body-weight reduction: a systematic review. Am. J. Clin. Nutr. 2004, 79(4), 529–536. [Google Scholar] [PubMed]
- Saper, R.B.; Eisenberg, D.M.; Phillips, R.S. Common dietary supplements for weight loss. Am. Fam. Physician 2004, 70(9), 1731–1738. [Google Scholar] [PubMed]
- Lewis, J.H. Esophageal and small bowel obstruction from guar gum-containing “diet pills”: analysis of 26 cases reported to the Food and Drug Administration. Am. J. Gastroenterol. 1992, 87(10), 1424–1428. [Google Scholar] [PubMed]
- Chen, J.; Park, H.; Park, K. Synthesis of superporous hydrogels: hydrogels with fast swelling and superabsorbent properties. J. Biomed. Mater. Res. 1999, 44(1), 53–62. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Madaghiele, M.; Lionetto, M.G.; Schettino, T.; Maffezzoli, A. A cellulose-based hydrogel as a potential bulking agent for hypocaloric diets: an in vitro biocompatibility study on rat intestine. J. Appl. Polym. Sci. 2006, 102(2), 1524–1530. [Google Scholar] [CrossRef]
- Baumgartner, S.; Kristl, J.; Peppas, N.A. Network structure of cellulose ethers used in pharmaceutical applications during swelling and at equilibrium. Pharm. Res. 2002, 19(8), 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Metters, A.T. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv. Drug. Delivery Rev. 2006, 58(12-13), 1379–1408. [Google Scholar] [CrossRef]
- El-Hag Ali, A.; Abd El-Rehim, H.; Kamal, H.; Hegazy, D. Synthesis of carboxymethyl cellulose based drug carrier hydrogel using ionizing radiation for possible use as specific delivery system. J. Macromol. Sci. Pure Appl. Chem. 2008, 45(8), 628–634. [Google Scholar] [CrossRef]
- Trojani, C.; Weiss, P.; Michiels, J.F.; Vinatier, C.; Guicheux, J.; Daculsi, G.; Gaudray, P.; Carle, G.F.; Rochet, N. Three-dimensional culture and differentiation of human osteogenic cells in an injectable hydroxypropylmethylcellulose hydrogel. Biomaterials 2005, 26(27), 5509–5517. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23(22), 4307–4314. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26(4), 419–431. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.A.; Muller, L.; Hoffman, I.; Greil, P.; Wenzel, M.M.; Staudenmaier, R. Cellulose-based scaffold materials for cartilage tissue engineering. Biomaterials 2006, 27(21), 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Backdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27(9), 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Fellah, B.H.; Weiss, P.; Gauthier, O.; Rouillon, T.; Pilet, P.; Daculsi, G.; Layrolle, P. Bone repair using a new injectable self-crosslinkable bone substitute. J. Orthop. Res. 2006, 24(4), 628–635. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.; Fini, M.; Torricelli, P.; Giardino, R.; Barbucci, R. An amidated carboxymethylcellulose hydrogel for cartilage regeneration. J. Mater. Sci. - Mater. Med. 2008, 19(8), 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Cromme, P.; Zollfrank, C.; Muller, L.; Muller, F.A.; Greil, P. Biomimetic mineralisation of apatites on Ca2+ activated cellulose templates. Mat. Sci.Eng. C 2007, 27(1), 1–7. [Google Scholar] [CrossRef]
- Ko, I.K.; Iwata, H. An approach to constructing three-dimensional tissue. Ann. N. Y. Acad. Sci. 2001, 944, (Bioartificial Organs III: tissue sourcing, immunoisolation, and clinical trials). 443–455. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Z.; Hong, L.; Jia, S.R.; Huang, Y.; Zhu, Y.; Wang, Y.L.; Jiang, H.J. Synthesis and characterization of hydroxyapatite-bacterial cellulose nanocomposites. Comp. Sci. Technol. 2006, 66(11-12), 1825–1832. [Google Scholar] [CrossRef]
- Tomihata, K.; Ikada, Y. Crosslinking of hyaluronic acid with water-soluble carbodiimide. J. Biomed. Mater. Res. 1997, 37(2), 243–251. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, N.; Ikada, Y. Mechanism of amide formation by carbodiimide for bioconjugation in aqueous media. Bioconjugate Chem. 1995, 6(1), 123–130. [Google Scholar] [CrossRef]
- Olde Damink, L.H.; Dijkstra, P.J.; van Luyn, M.J.; van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 1996, 17(8), 765–773. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, A.N.; Bershteyn, A.; Tzatzalos, E.; Irvine, D.J. Bioactive hydrogels with an ordered cellular structure combine interconnected macroporosity and robust mechanical properties. Adv. Mater. 2005, 17(4), 399–403. [Google Scholar] [CrossRef]
- Lee, W.K.; Ichi, T.; Ooya, T.; Yamamoto, T.; Katoh, M.; Yui, N. Novel poly(ethylene glycol) scaffolds crosslinked by hydrolyzable polyrotaxane for cartilage tissue engineering. J. Biomed. Mater. Res. A 2003, 67(4), 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.C.; Bertram, J.P.; Hynes, S.R.; Michaud, M.; Li, Q.; Young, M.; Segal, S.S.; Madri, J.A.; Lavik, E.B. A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo. Proc. Nat. Acad. Sci. USA 2006, 103(8), 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Cuy, J.L.; Hauch, K.D.; Ratner, B.D. Photo-patterning of porous hydrogels for tissue engineering. Biomaterials 2007, 28(19), 2978–2986. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962, 193(4812), 293–294. [Google Scholar] [CrossRef] [PubMed]
- Sinko, P.J.; Stein, S.; Menjoge, A.R.; Gunaseelan, S.; Anumolu, S.N.; Navath, R. Dressing compositions and methods. Patent WO2008133918, 2008. [Google Scholar]
- St. John, J.V.; Moro, D.G. Hydrogel wound dressing and biomaterials formed in situ and their uses. Patent WO2008070270(A2), 2008. [Google Scholar]
- Zhanshan, Y.; Zhigao, R.; Ling, Y.; Shuqin, Y.; Nankang, Z. Medical hydrogel wound dressing and preparation method thereof. Patent CN101293110(A), 2008. [Google Scholar]
- Burd, A.; Tsang, M.W. Wound healing dressings and methods of manufacturing the same. Patent WO2008101417(A1), 2008. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353-373. https://doi.org/10.3390/ma2020353
Sannino A, Demitri C, Madaghiele M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials. 2009; 2(2):353-373. https://doi.org/10.3390/ma2020353
Chicago/Turabian StyleSannino, Alessandro, Christian Demitri, and Marta Madaghiele. 2009. "Biodegradable Cellulose-based Hydrogels: Design and Applications" Materials 2, no. 2: 353-373. https://doi.org/10.3390/ma2020353





