Optimization of Optical Properties of Polycarbonate Film with Thiol Gold-Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Rayleigh spectroscopy
2.3. Atomic Force Microscopy (AFM)
2.4. Spectrophotometric analysis
3. Experimental Section
3.1. Preparation of composites
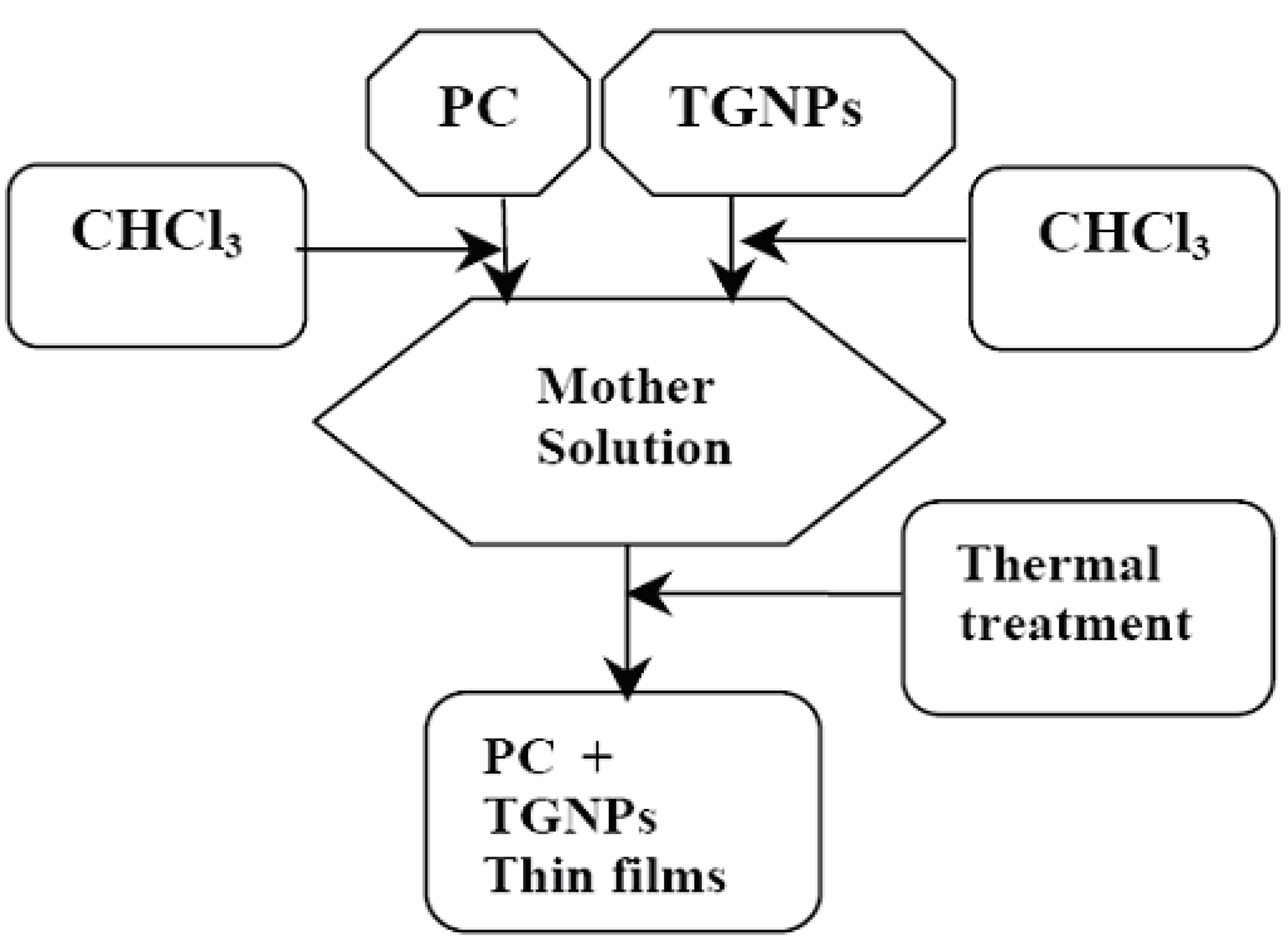
3.2. UV-vis measurement
4. Results
4.1. Rayleigh scattering
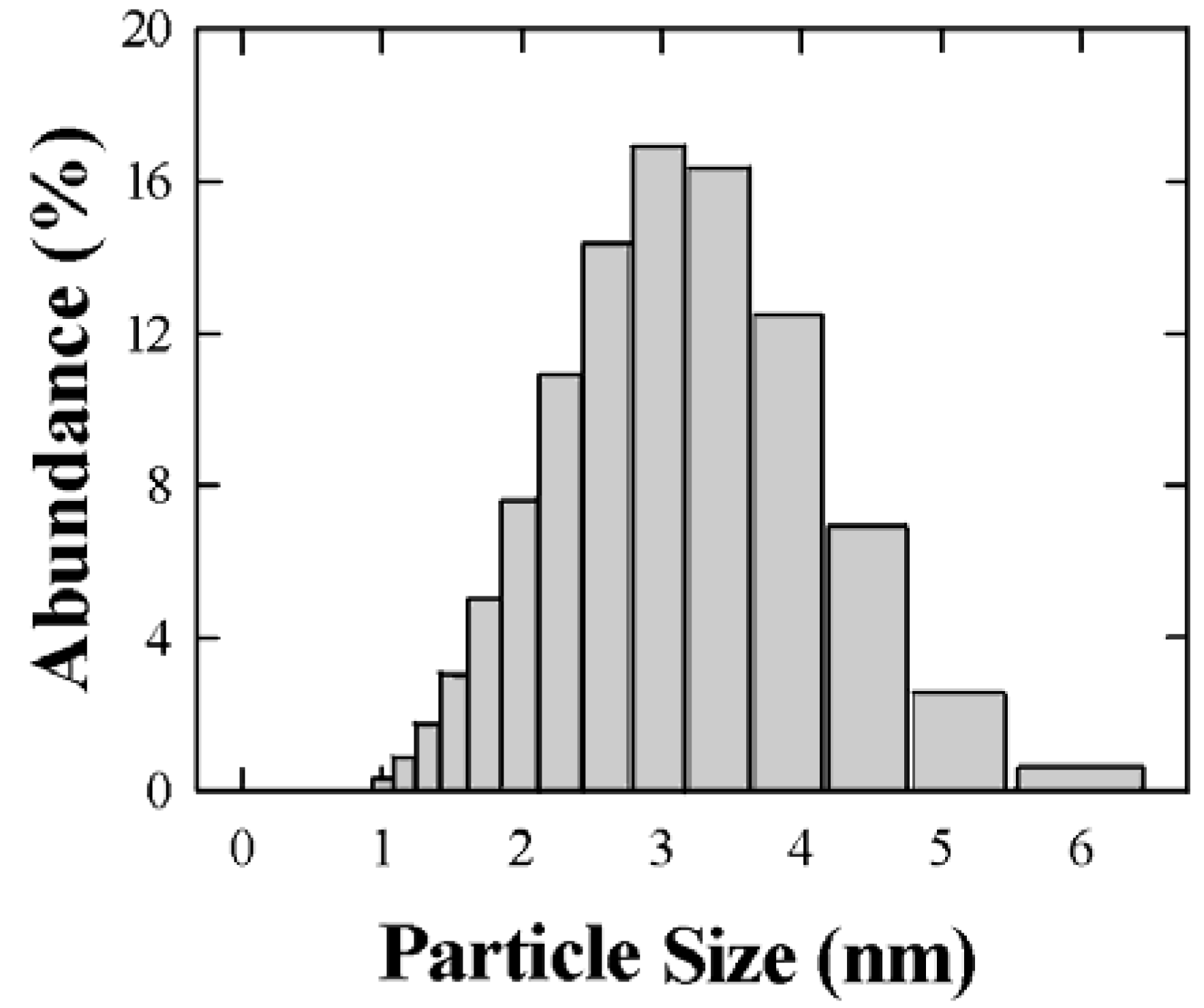
4.2. Characterization of nanoparticles
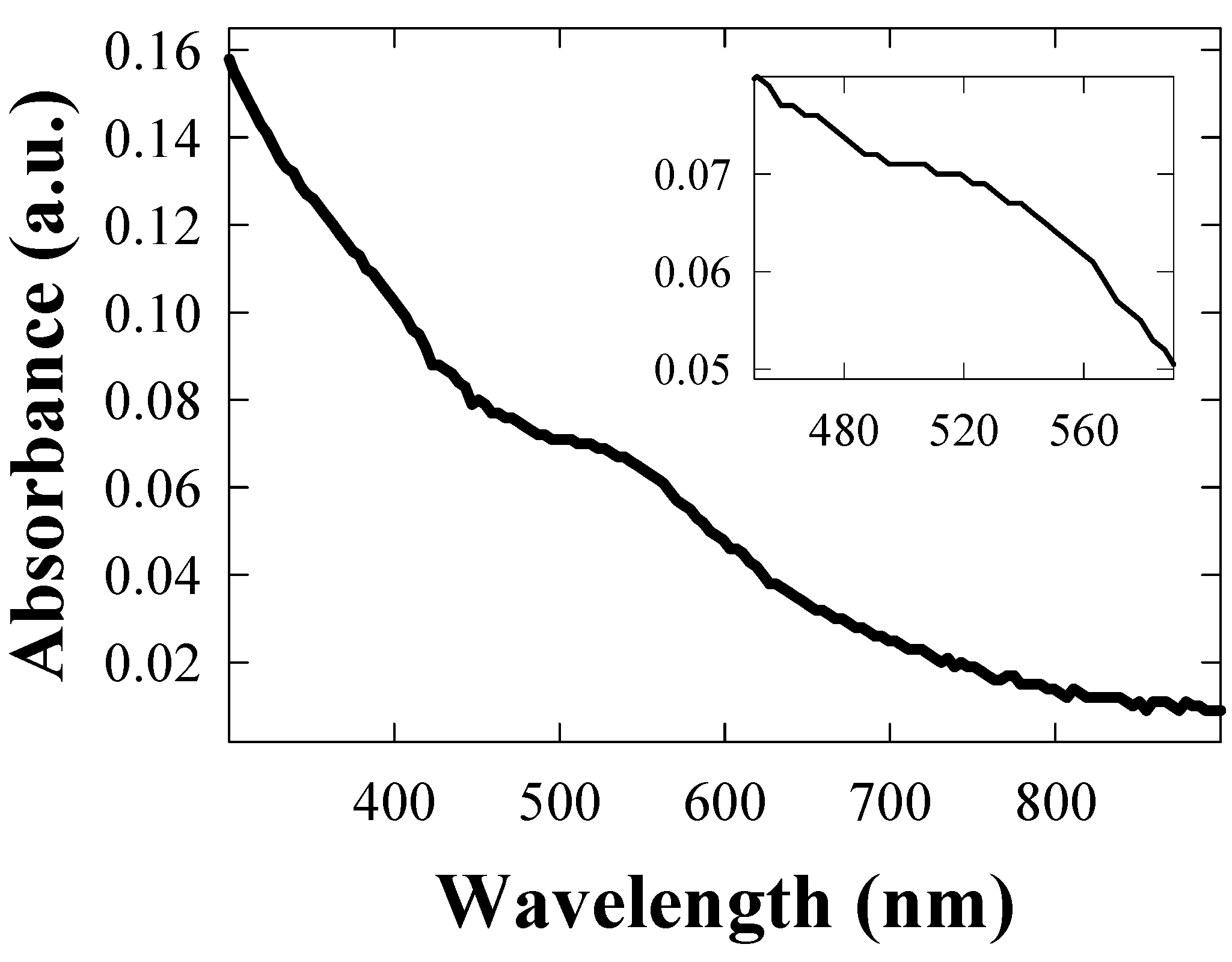
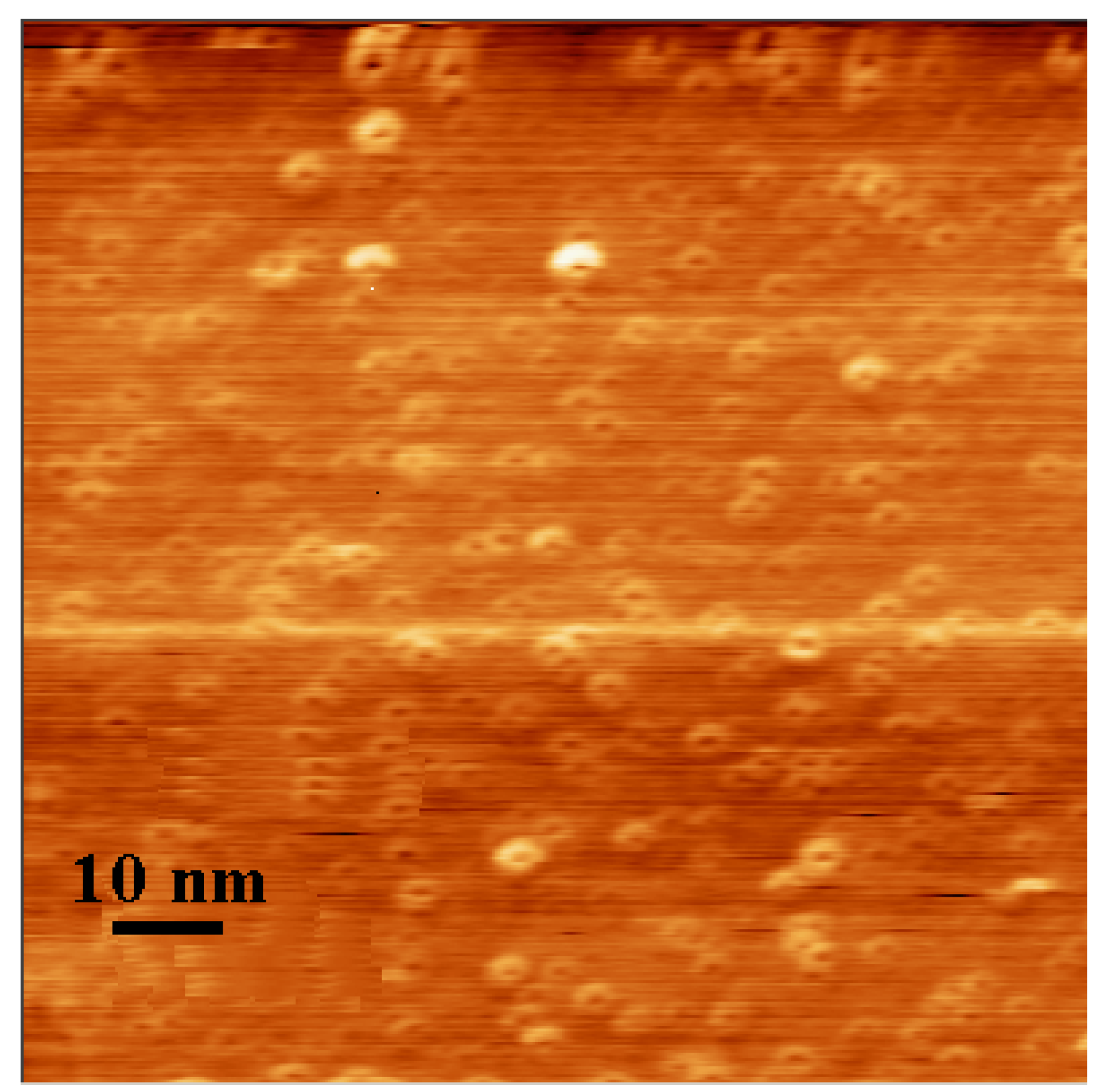
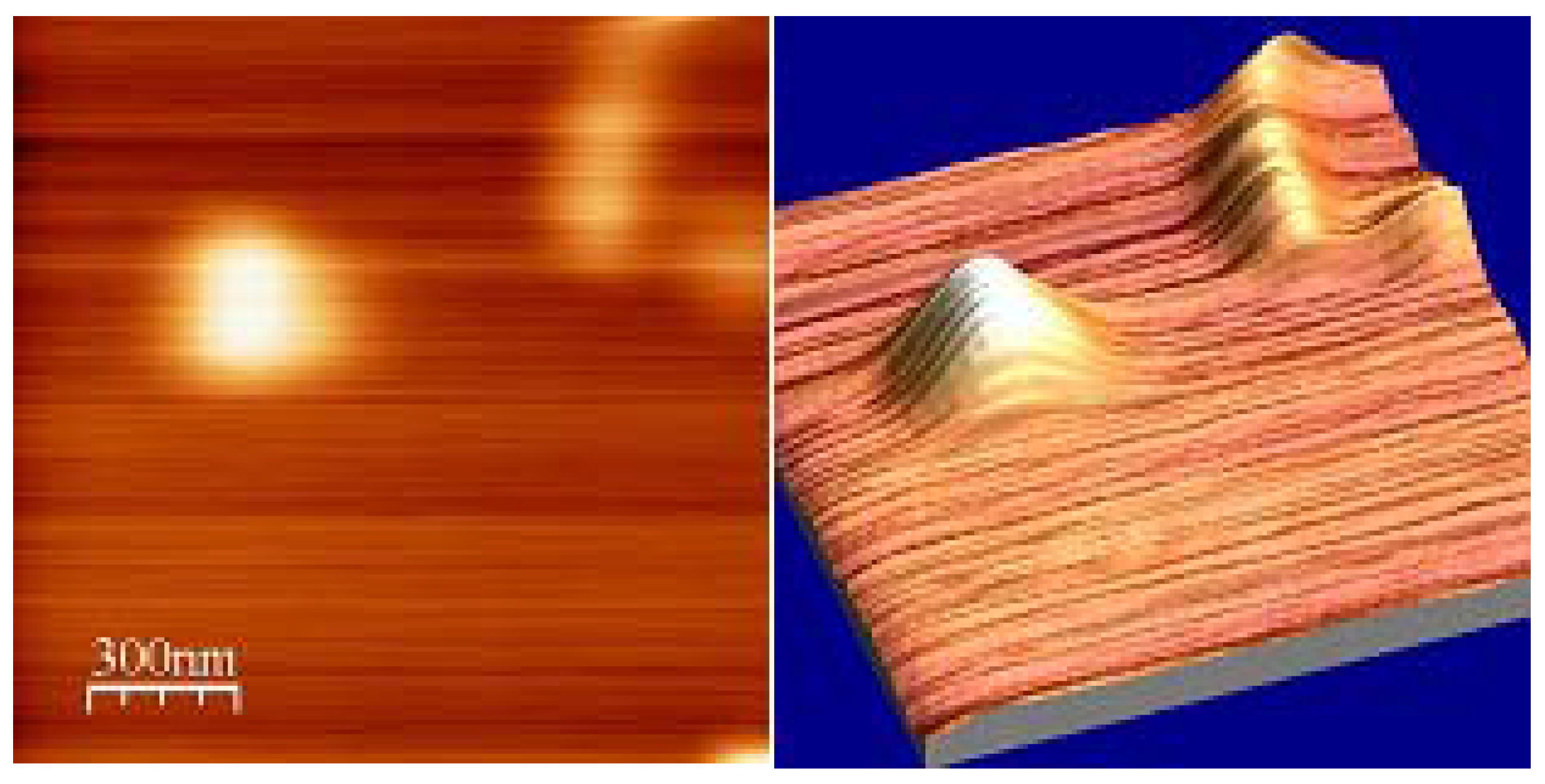
4.3. Characterization of polycarbonate film

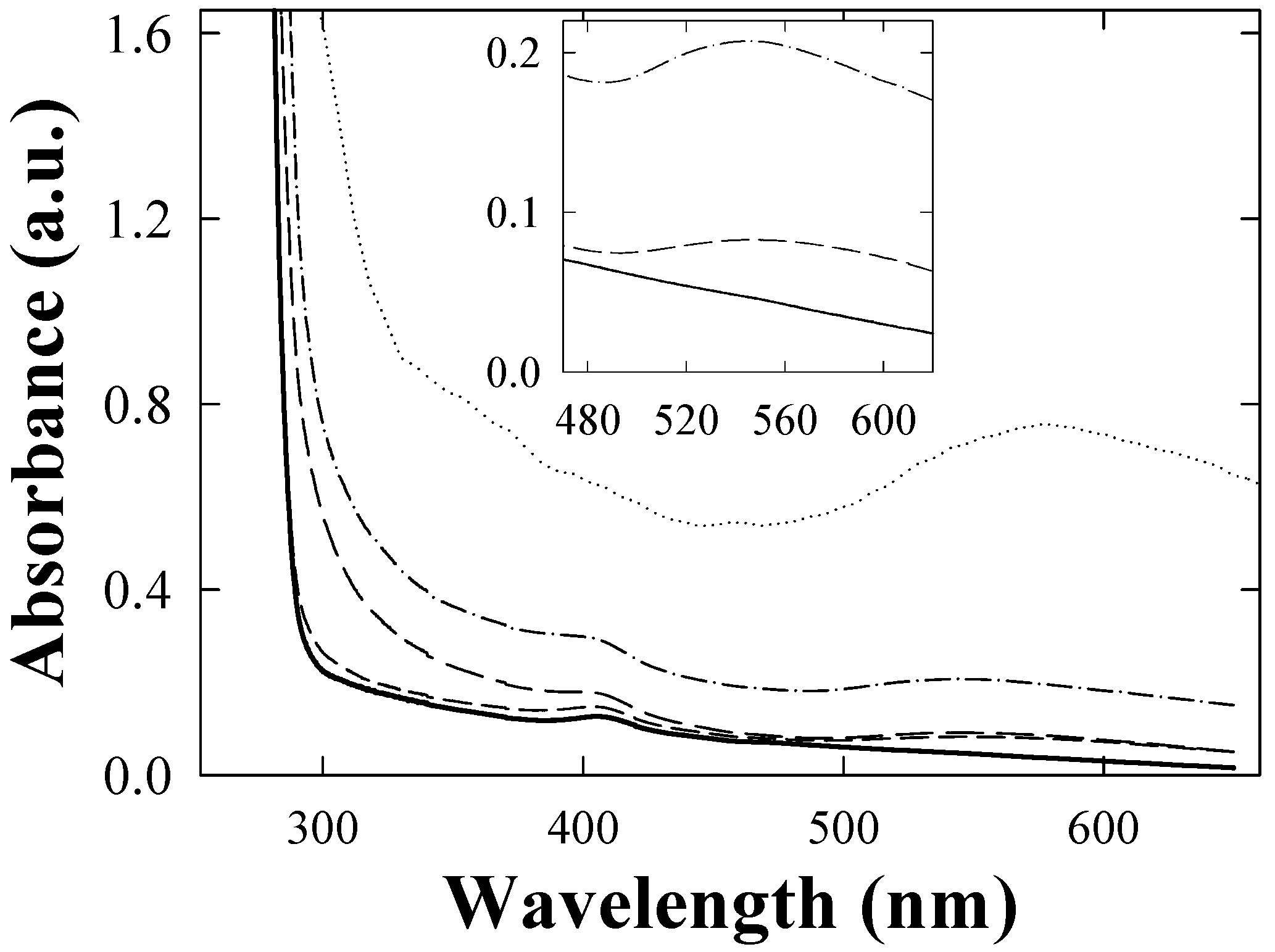
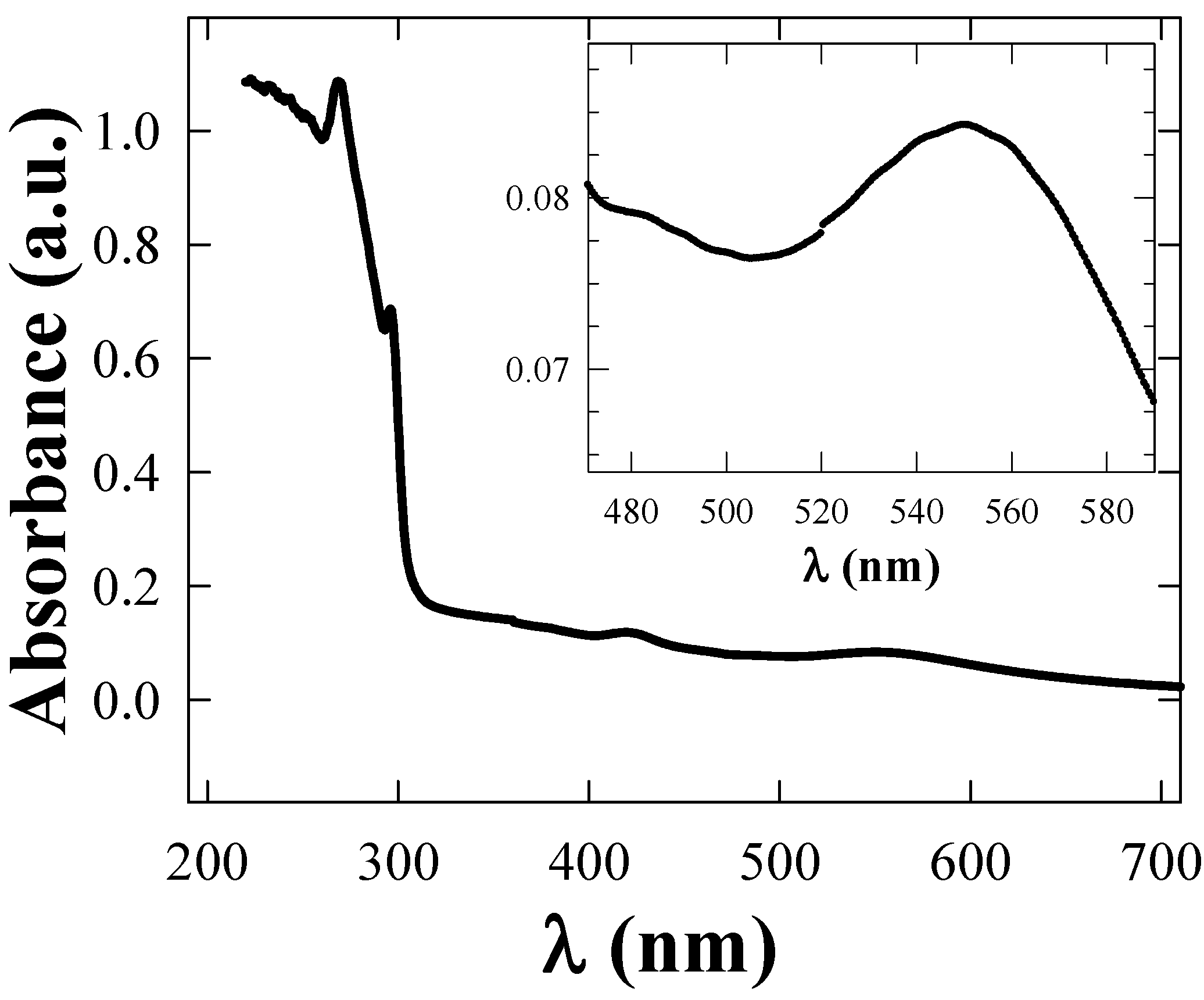
5. Discussion
6. Conclusions
Acknowledgements
References and Notes
- Bhattcharya, R.; Mukherjee, P. Biological properties of naked metal nanoparticles. Adv. Drug Deliver. Rev. 2008, 60, 1289–1306. [Google Scholar]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer: Berlin, Germany, 1995. [Google Scholar]
- Kerker, M. The Scattering of Light and other Electromagnetic Radiation; Academic Press: New York, NY, USA, 1969. [Google Scholar]
- Bohren, C.F; Uffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley: New York, NY, USA, 1983. [Google Scholar]
- Shumyntseva, V.V.; Carrara, S.; Bavastrello, V.; Riley, D.J.; Bulko, T.V.; Skryabin, K.G.; Arhakov, A.I.; Nicolini, C. Direct electron transfer between cytocrome P450scc and gold nanoparticles on screen-printed rhodium–graphite electrodes. Biosens. Bioelectron. 2005, 21, 217–222. [Google Scholar]
- Martins, R.; Baptista, P.; Silva, L.; Raniero, L.; Doria, G.; Franco, R.; Fortunato, E. Identification of unamplified genomic DNA sequences using gold nanoparticle probes and a novel thin film photodetector. J. Non-Cryst. Solids 2008, 354, 2580–2584. [Google Scholar]
- Suni, I.I. Impedance methods for electrochemical sensors using nanomaterials. Trend. Anal. Chem. 2008, 27, 604–611. [Google Scholar]
- Pingarròn, J.M.; Yanez-Sedeno, P.; Gonzalez-Cortès, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar]
- Martins, R.; Baptista, P.; Raniero, L. Amorphous/nanocrystalline silicon biosensor for the specific identification of unamplified nucleic acid sequences using gold nanoparticle probes. Appl. Phys. Lett. 2007, 90, 023903:1–023903:3. [Google Scholar]
- Schmid, G. Nanoclusters building blocks for future nanoelectronic devices. Adv. Eng. Mater. 2001, 3, 737–743. [Google Scholar]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self assembled monolayers of thiols on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar]
- Akamatsu, K.; Deki, S. TEM investigation and electron diffraction study on dispersion of gold nanoparticles into a nylon 11 thin film during heat treatment. J. Colloid Interf. Sci. 1999, 214, 353–361. [Google Scholar]
- Kay, E.; Hecq, M. Metal cluster in plasma polymerized matrices: Gold. J. Appl. Phys. 1984, 55, 370–374. [Google Scholar]
- Koziol, K.K.K.; Dolgner, K.; Tsuboi, N.; Kruse, J.; Zaporojtchenko, V.; Deki, S.; Faupel, F. Dispersion of gold in polycarbonates by induced crystallization. Macromolecules 2004, 37, 2182–2185. [Google Scholar]
- Corbierre, M.K.; Cameron, N.S.; Sutton, M.; Mochrie, S.G.J.; Lurio, L.B.; Rühm, A.; Lennox, R.B. Polymer-stabilized gold nanoparticles and their incorporation into polymer matrices. J. Am. Chem. Soc. 2001, 123, 10411–10412. [Google Scholar]
- Heilmann, A.; Werner, J.; Stenzel, O.; Homilius, F. Changes of the optical and electrical properties of plasma polymer-metal composite films during thermal annealing. Thin Solid Films 1993, 246, 77–85. [Google Scholar]
- Horcas, I.; Fernandez, R.; Gomez-Rodrigues, J.M.; Colchero, J.; Gomez-Herrero, J.A.; Baro, M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instr. 2007, 78, 013705:1–013705:8. [Google Scholar]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar]
- Hostetler, M.J.; Wingate, J.E.; Zhong, C.J.; Harris, J.E.; Vachet, R.W.; Clark, M.R.; Londono, J. D.; Green, S.J.; Stokes, J.J.; Wignall, G.D.; Glish, G.L.; Porter, M.D.; Evans, N.D.; Murray, R.W. Alkanethiolate gold cluster molecules with core diameters from 1.5 to 5.2 nm: Core and monolayer properties as a function of core size. Langmuir 1998, 14, 17–30. [Google Scholar]
- Henglein, A. Radiolytic preparation of ultrafine colloidal gold particles in aqueous solution: optical spectrum controlled growth and some chemical reactions. Langmuir 1999, 15, 6738–6744. [Google Scholar]
- Jin, Y.; Wang, P.; Yin, D.; Liu, J.; Qin, L.; Yu, N.; Xie, G.; Li, B. Gold nanoparticles prepared by sonochemical method in thiol–functionalized ionic liquid. Colloid Surface A 2007, 302, 366–370. [Google Scholar]
- Taleb, A.; Petit, C.; Pileni, M.P. Optical properties of self-assembled 2D and 3D superlattices of silver nanoparticles. J. Phys. Chem. B 1998, 102, 2214–2220. [Google Scholar]
- Link, S.; El-Sayed, M.A. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A.; Turkevich, J. Method for gold nanoparticles synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Larosa, C.; Stura, E.; Eggenhöffner, R.; Nicolini, C. Optimization of Optical Properties of Polycarbonate Film with Thiol Gold-Nanoparticles. Materials 2009, 2, 1193-1204. https://doi.org/10.3390/ma2031193
Larosa C, Stura E, Eggenhöffner R, Nicolini C. Optimization of Optical Properties of Polycarbonate Film with Thiol Gold-Nanoparticles. Materials. 2009; 2(3):1193-1204. https://doi.org/10.3390/ma2031193
Chicago/Turabian StyleLarosa, Claudio, Enrico Stura, Roberto Eggenhöffner, and Claudio Nicolini. 2009. "Optimization of Optical Properties of Polycarbonate Film with Thiol Gold-Nanoparticles" Materials 2, no. 3: 1193-1204. https://doi.org/10.3390/ma2031193