Increased Biocompatibility and Bioactivity after Energetic PVD Surface Treatments
Abstract
:1. Introduction

2. Energetic Surface Treatments
2.1. Ion Implantation & Physical Vapor Deposition
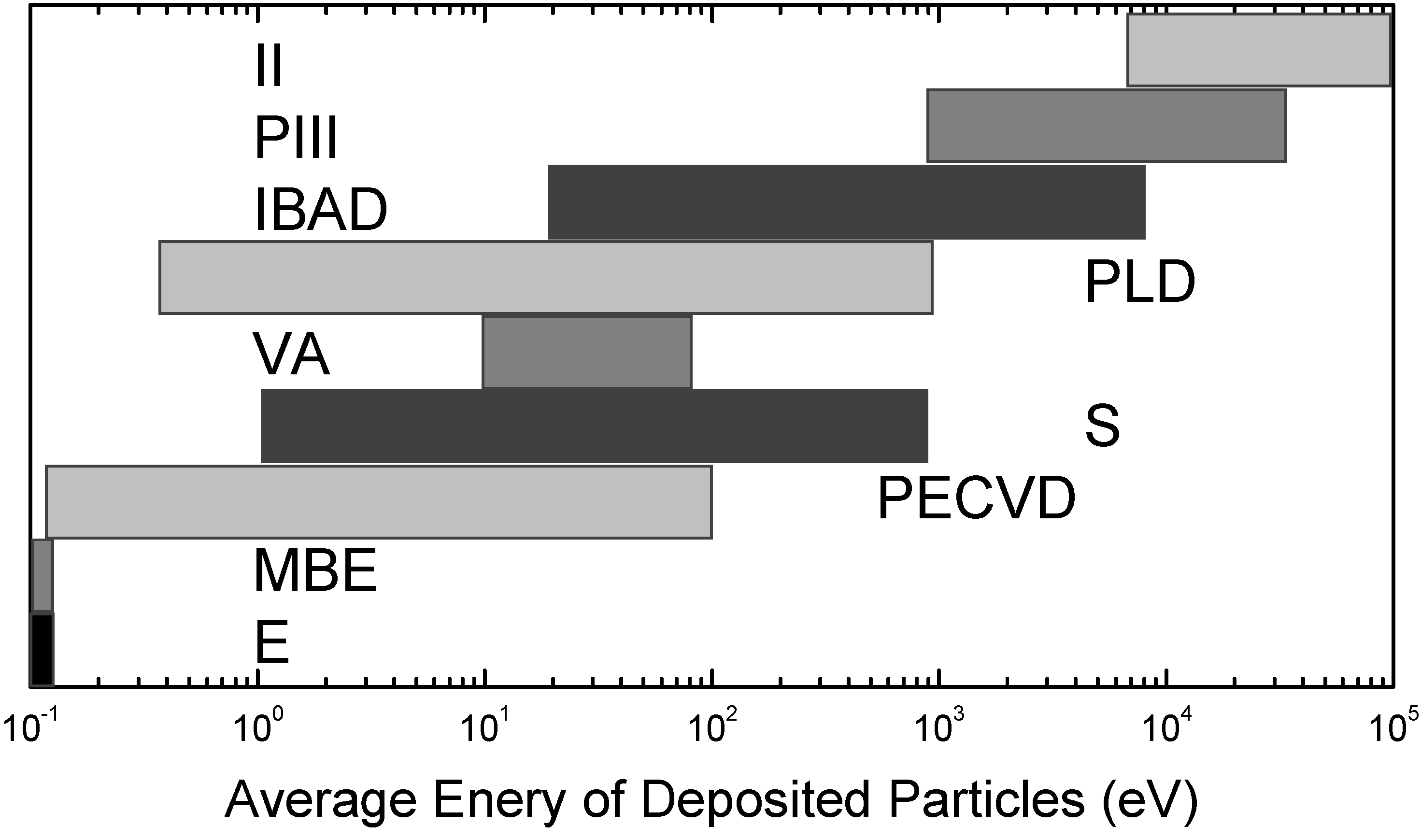
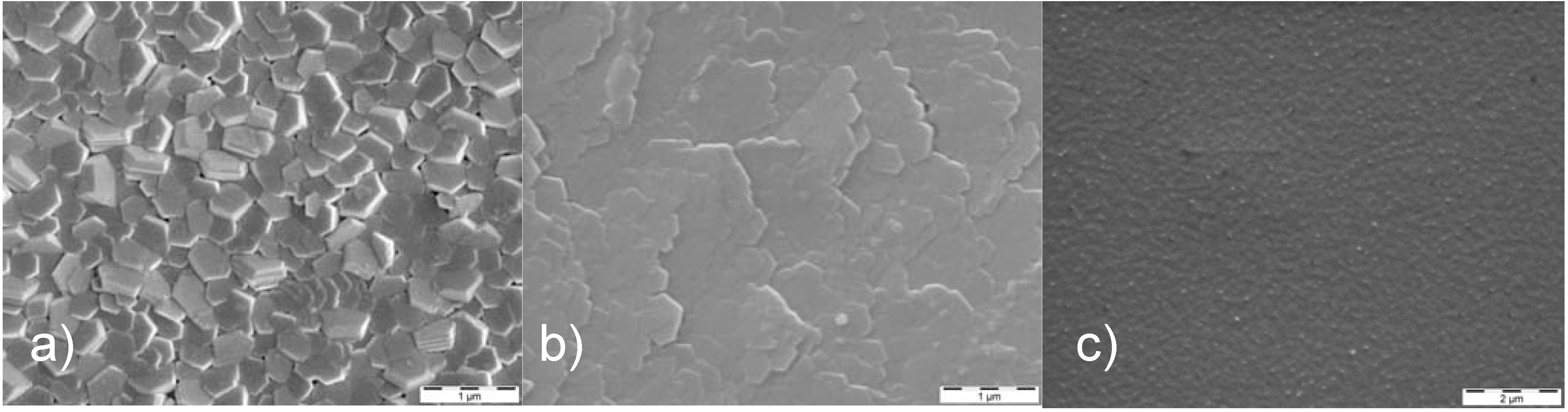
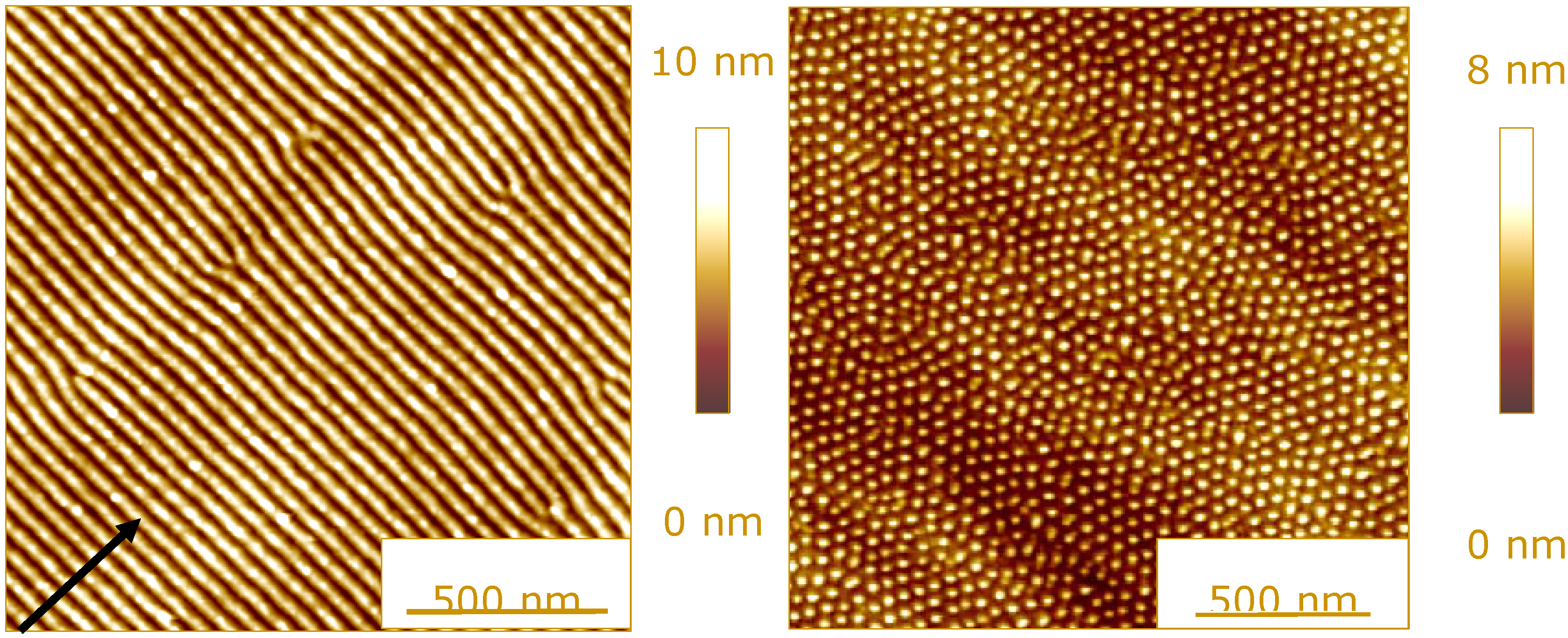
2.2. Evolution of Surface Topography under Energetic Particle Bombardment
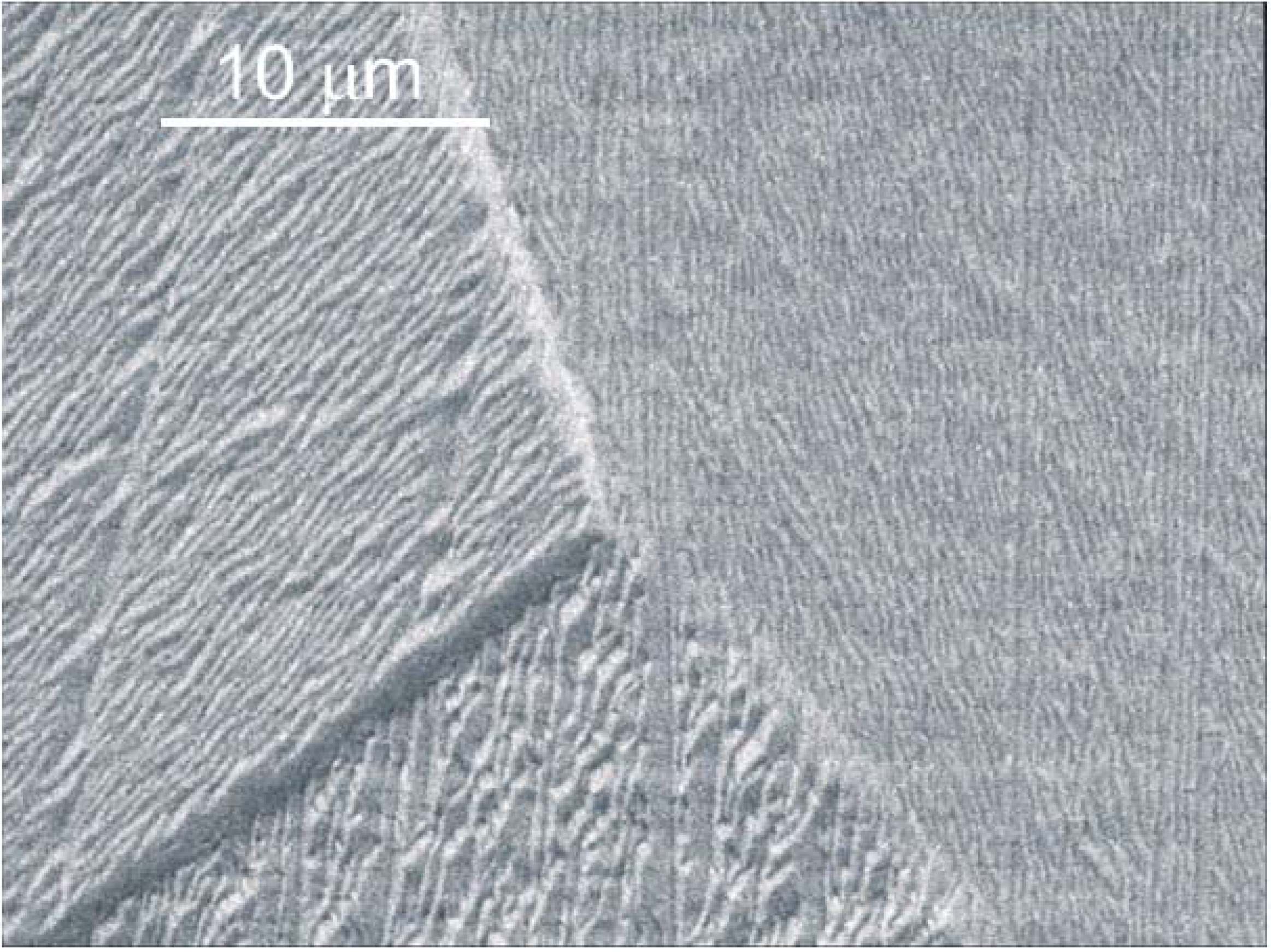
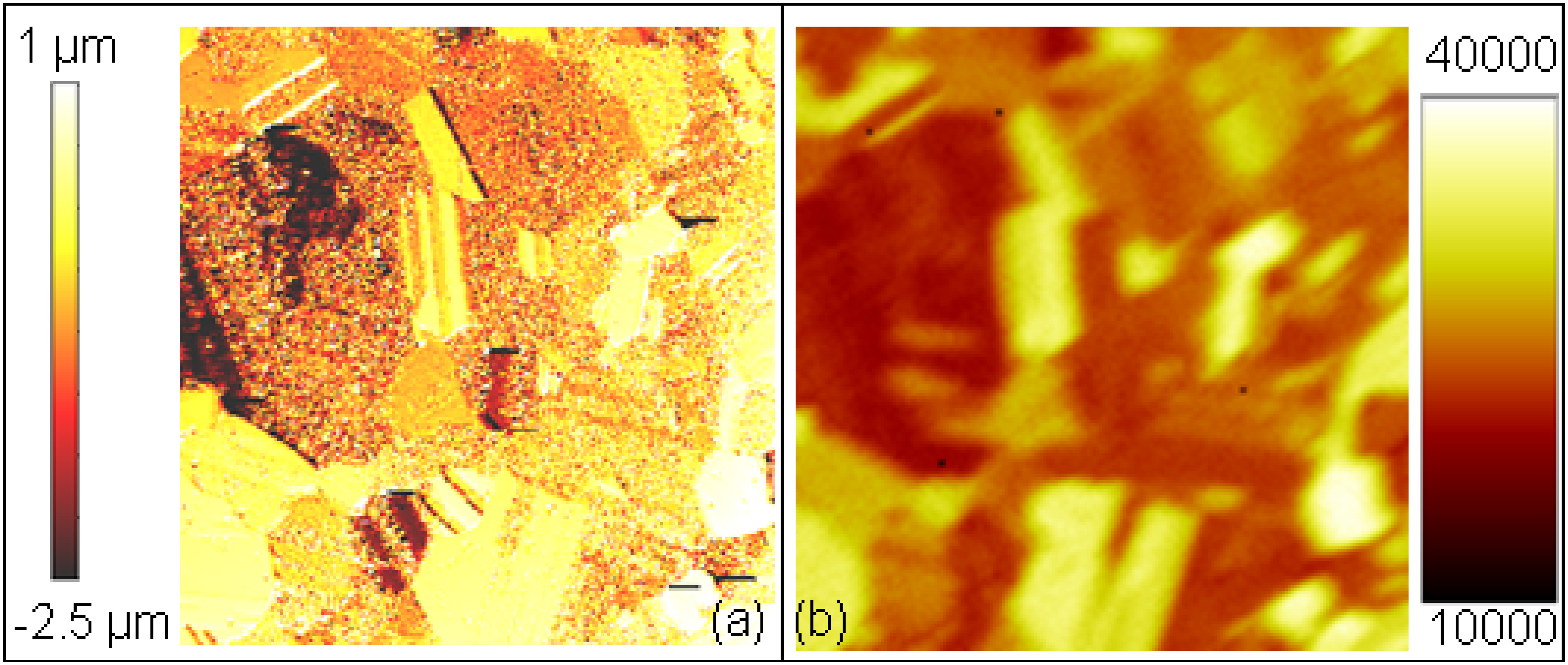
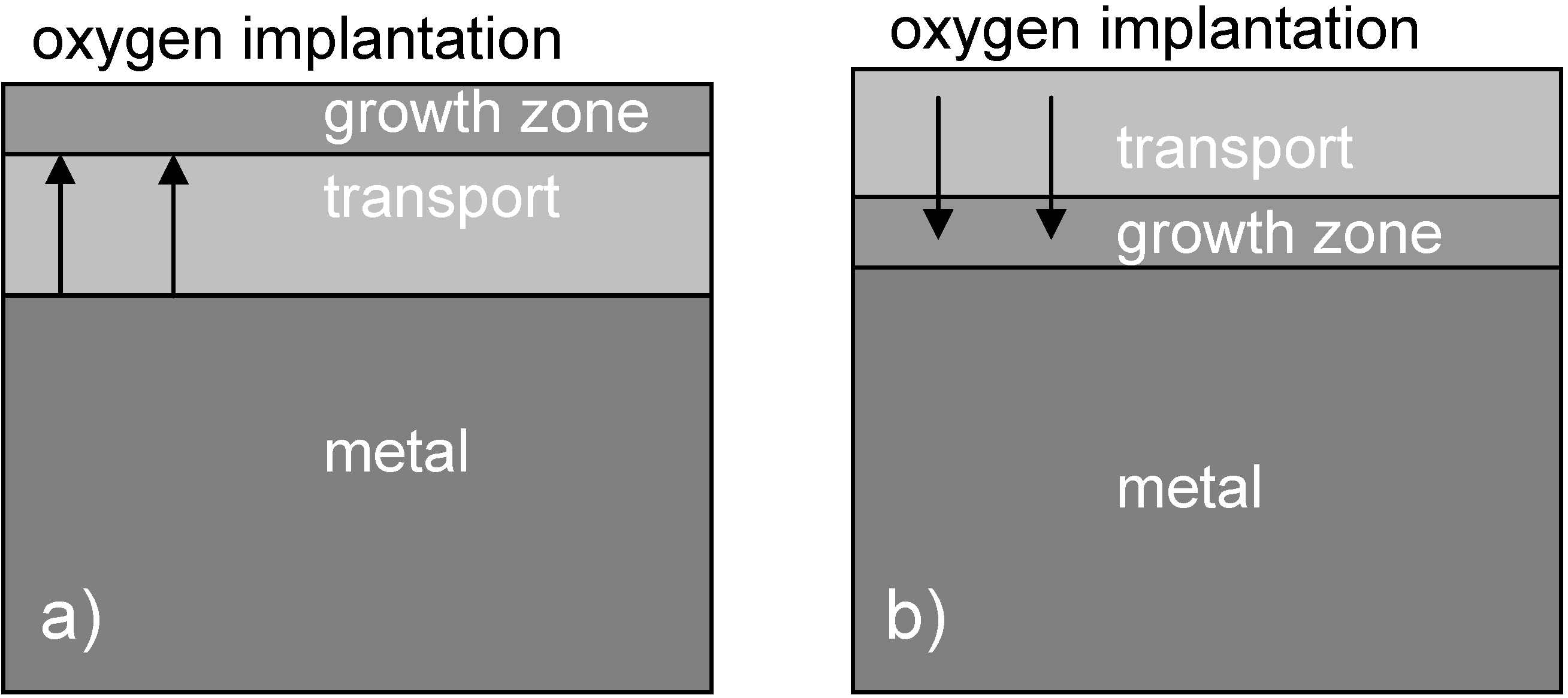
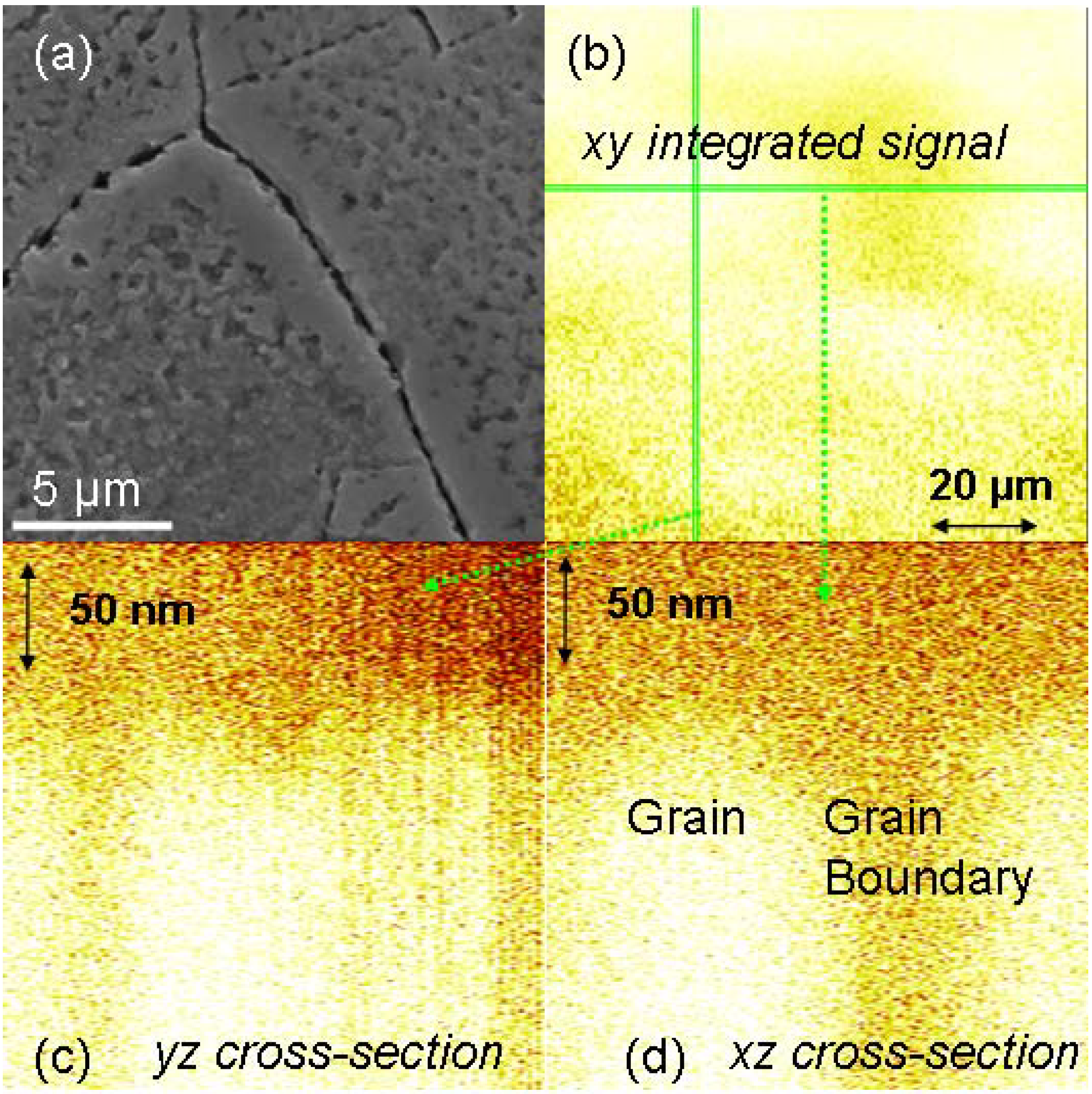
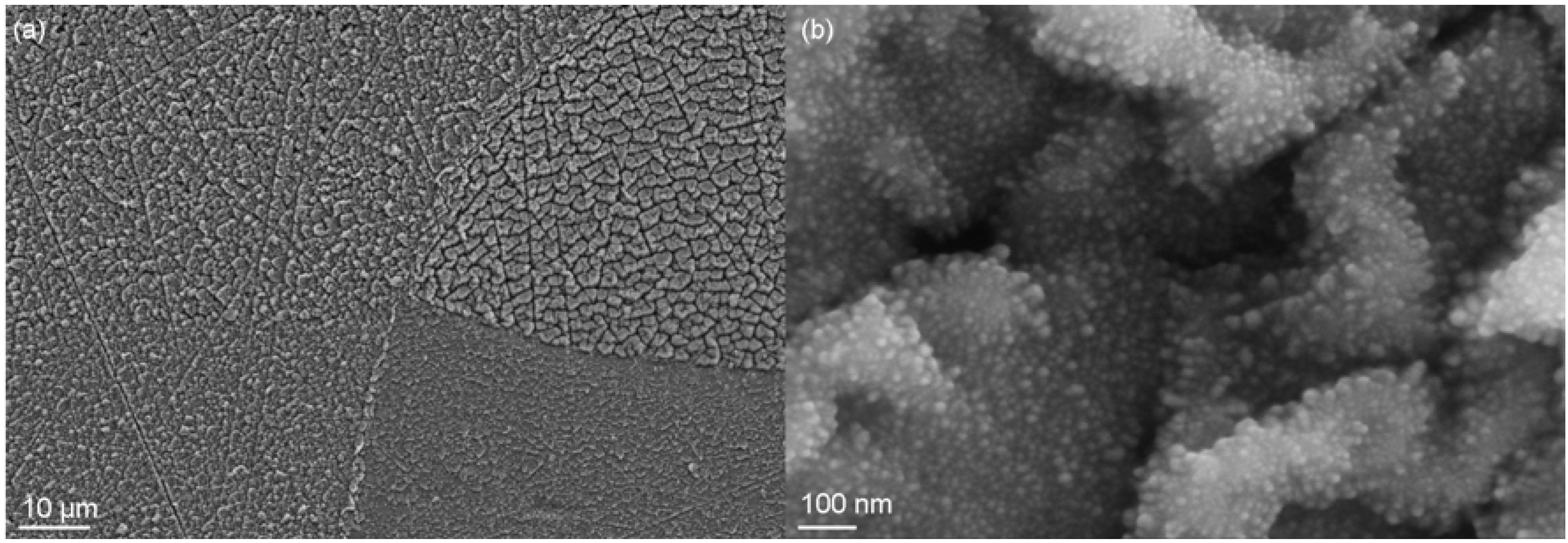

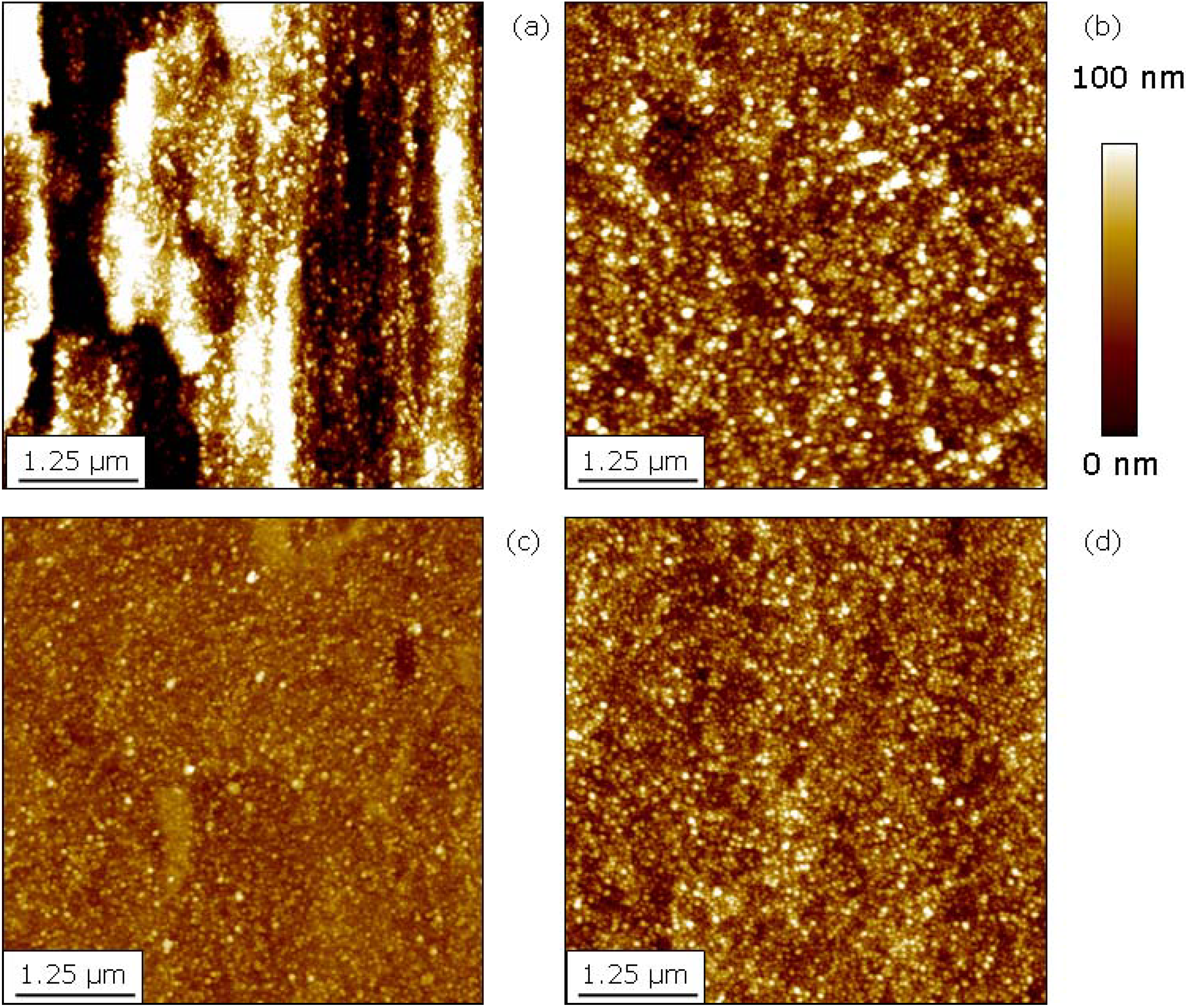
2.3. Comparison of Coating and Implantation Processes
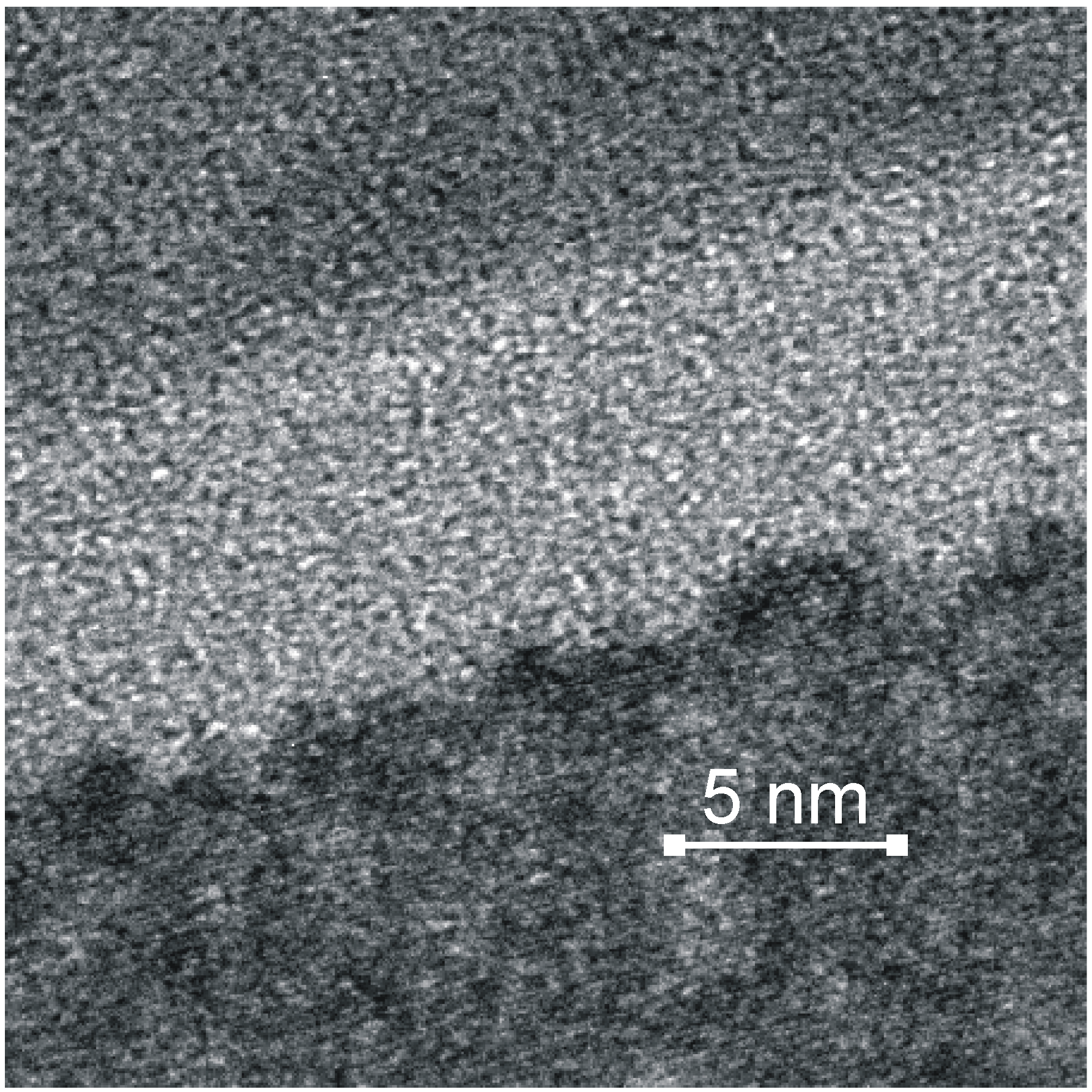

3. Biochemical Surface Interaction
3.1. Topography

3.2. Surface Hydrophilicity
3.3. Corrosion
3.4. Wear
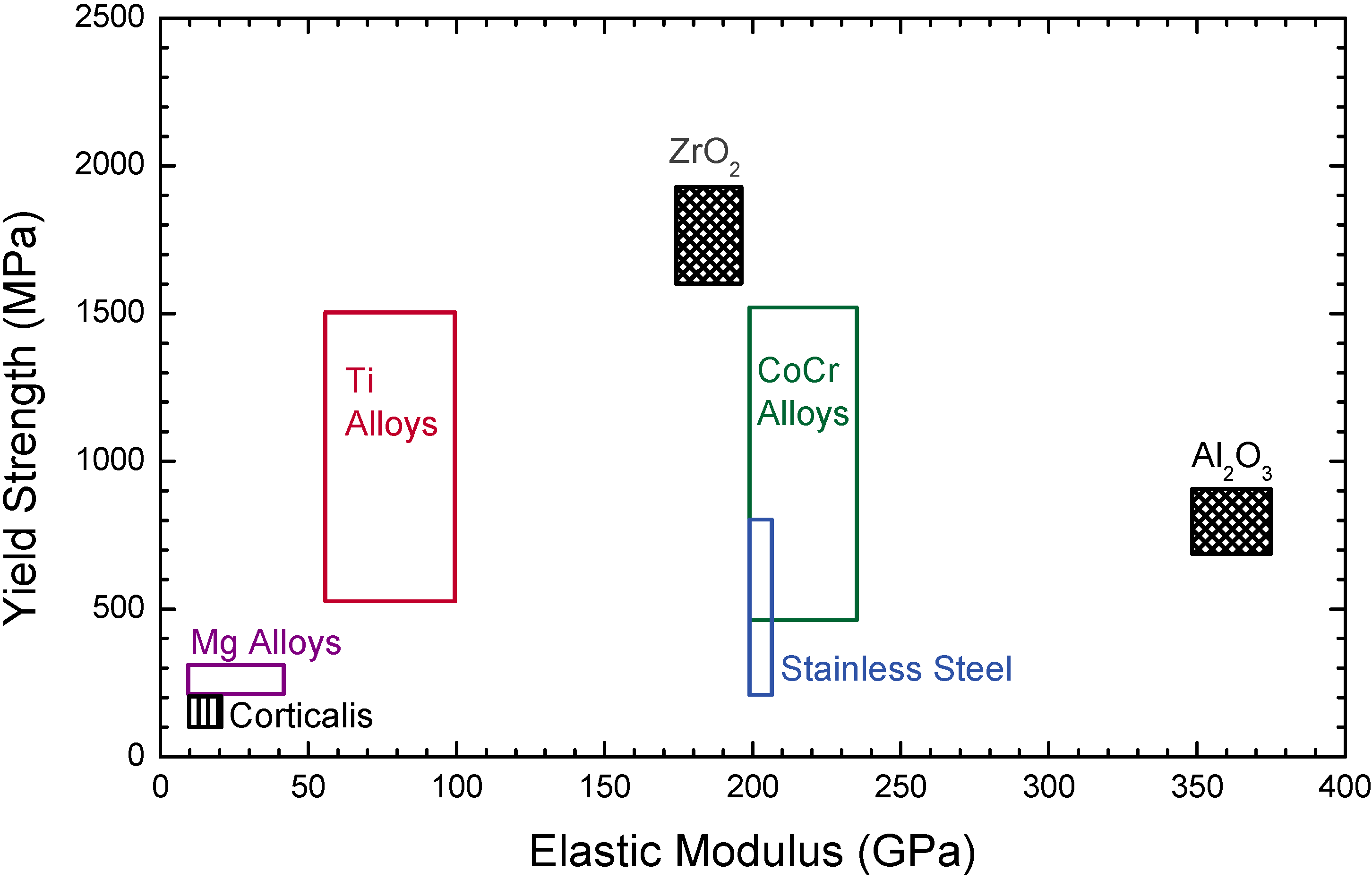
4. Metallic Implants
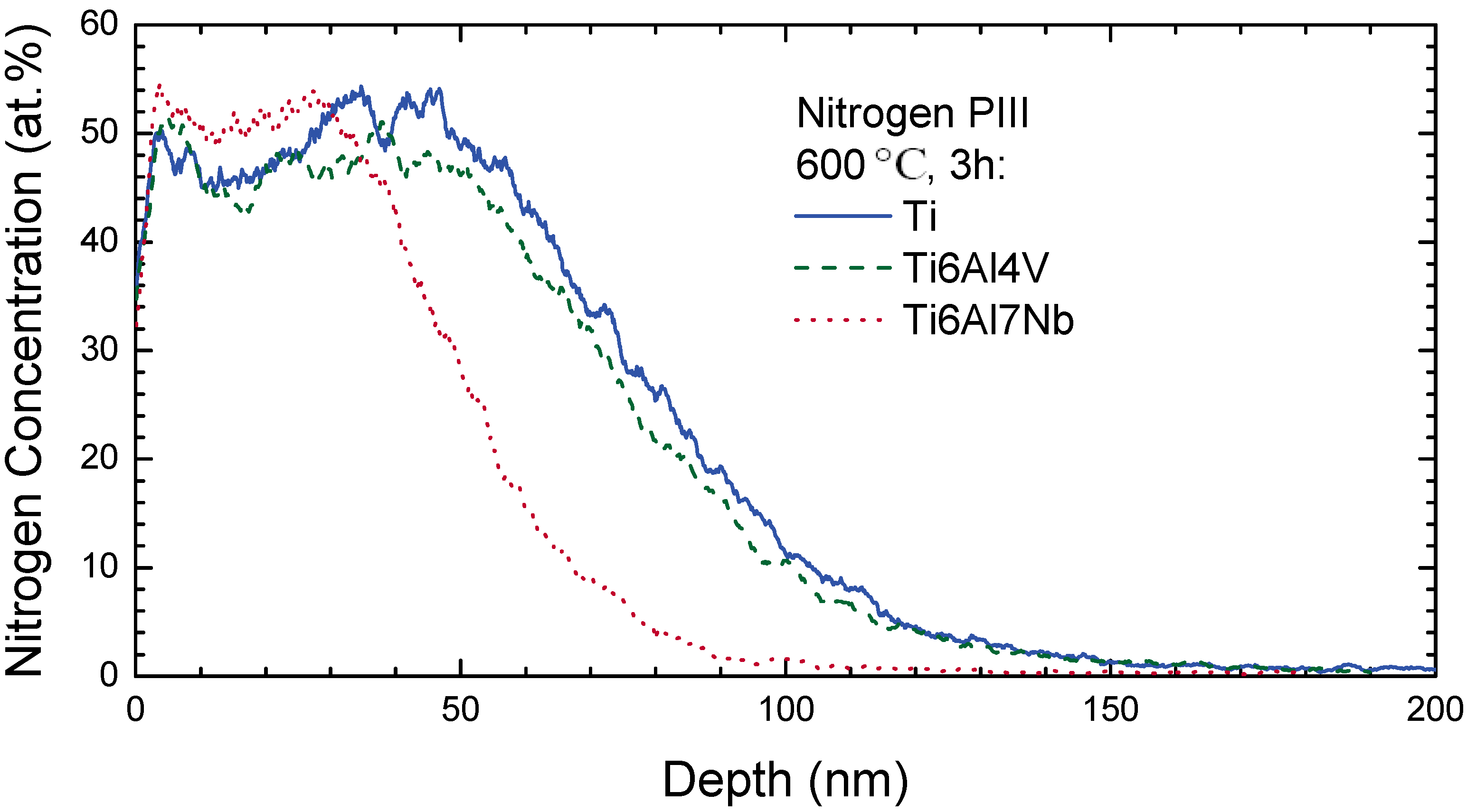
4.1. Titanium and Titanium Alloys
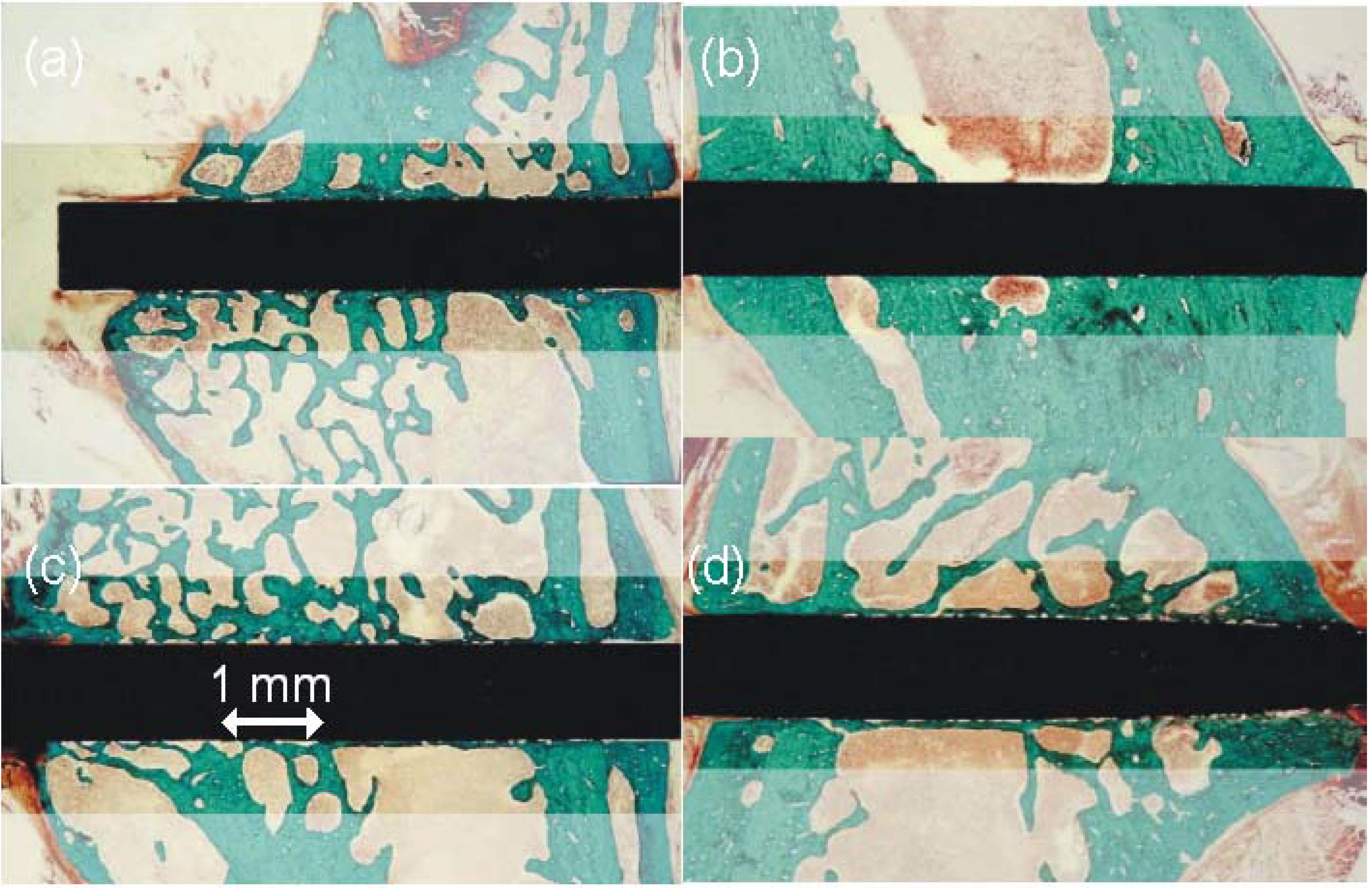
4.2. Stainless Steel
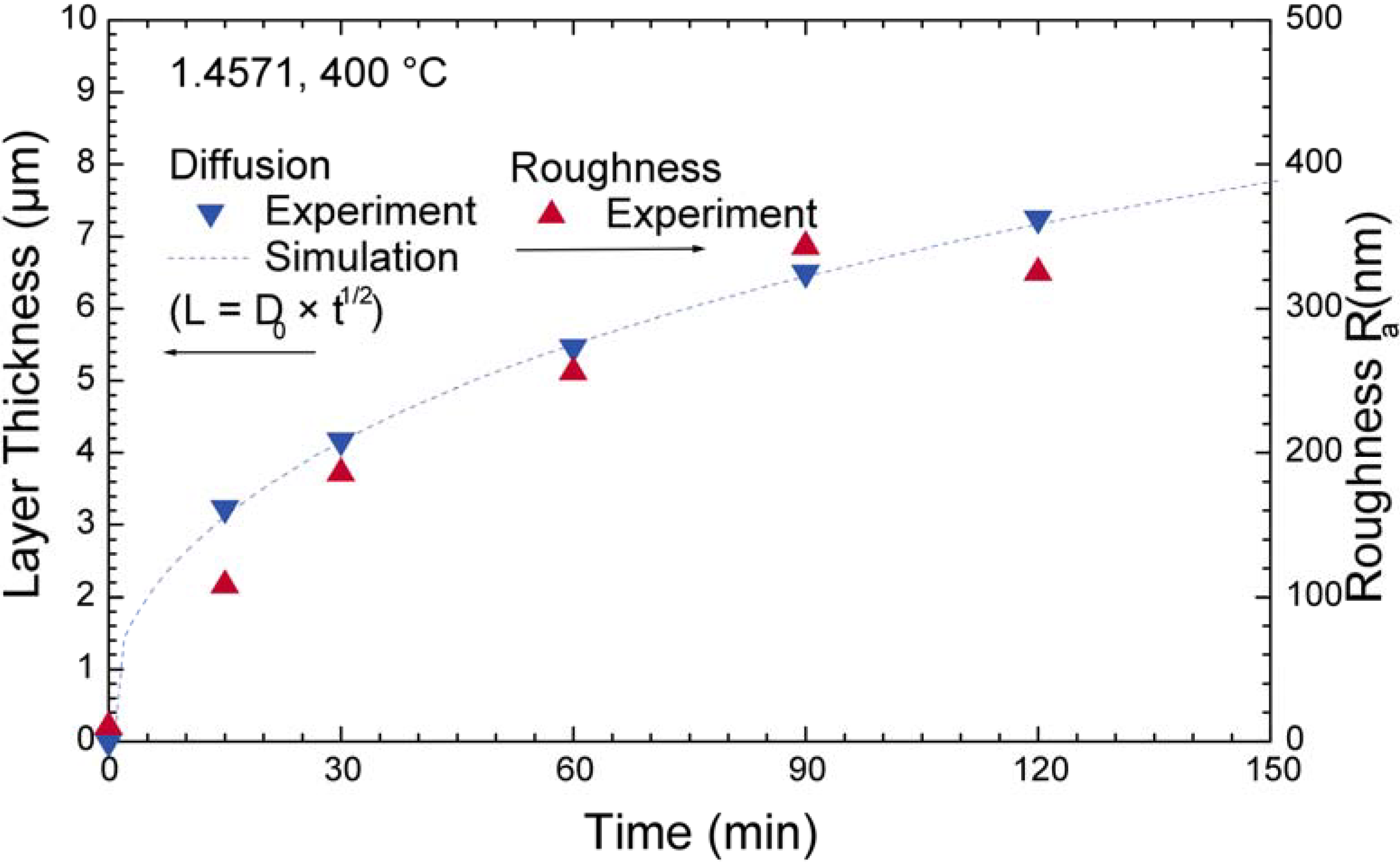
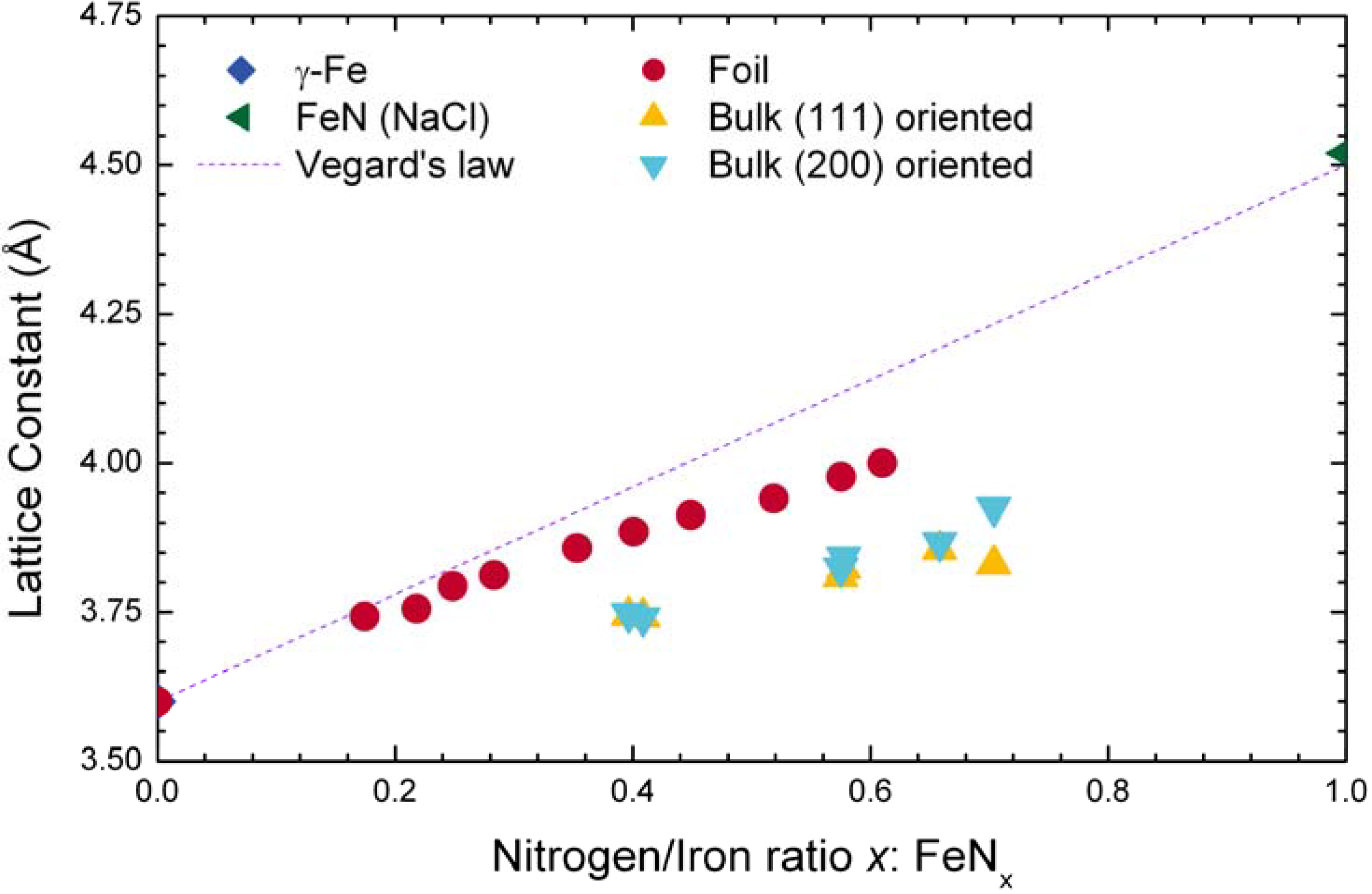

4.3. CoCr Alloys
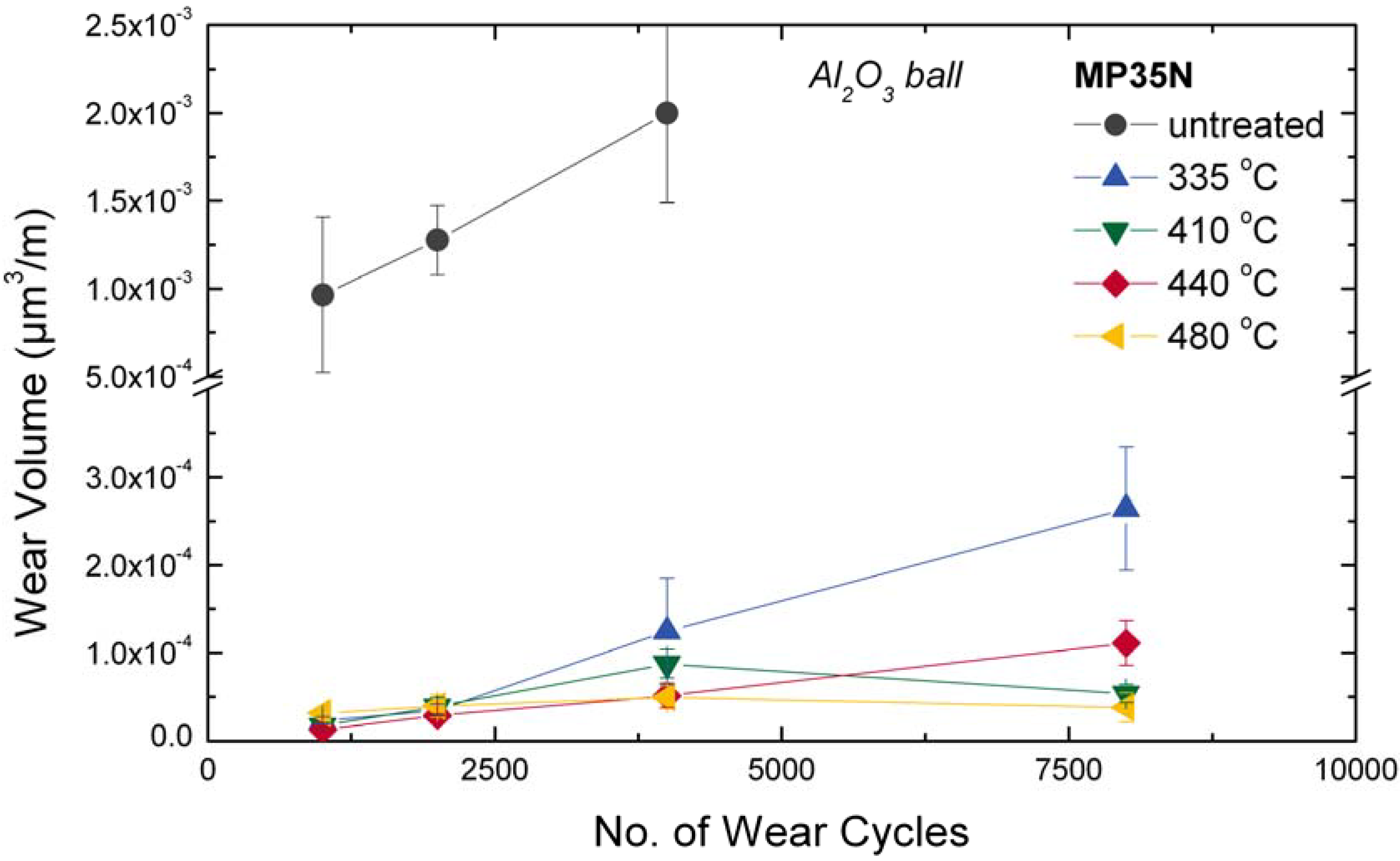
4.4. Mg Alloys
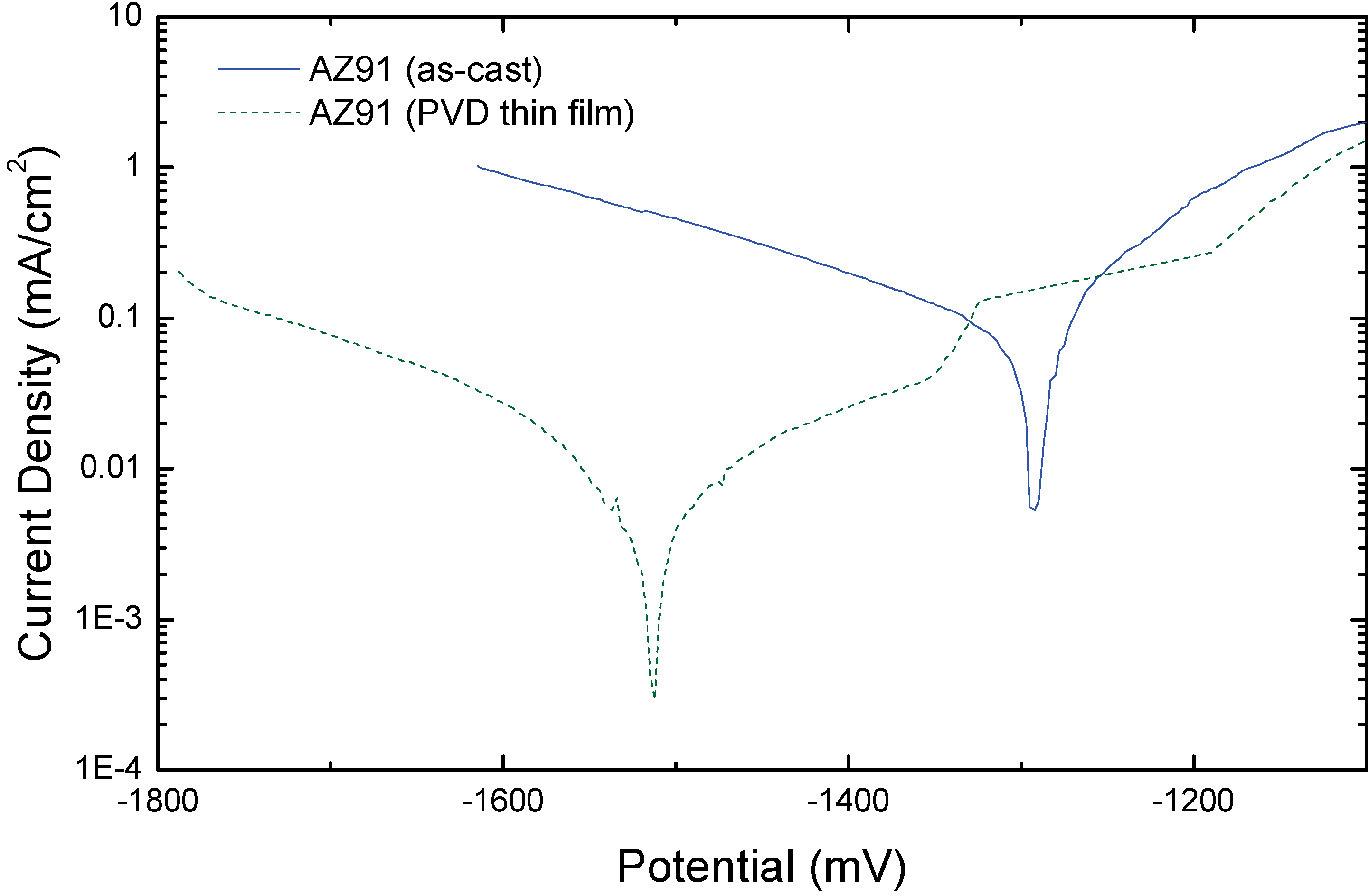
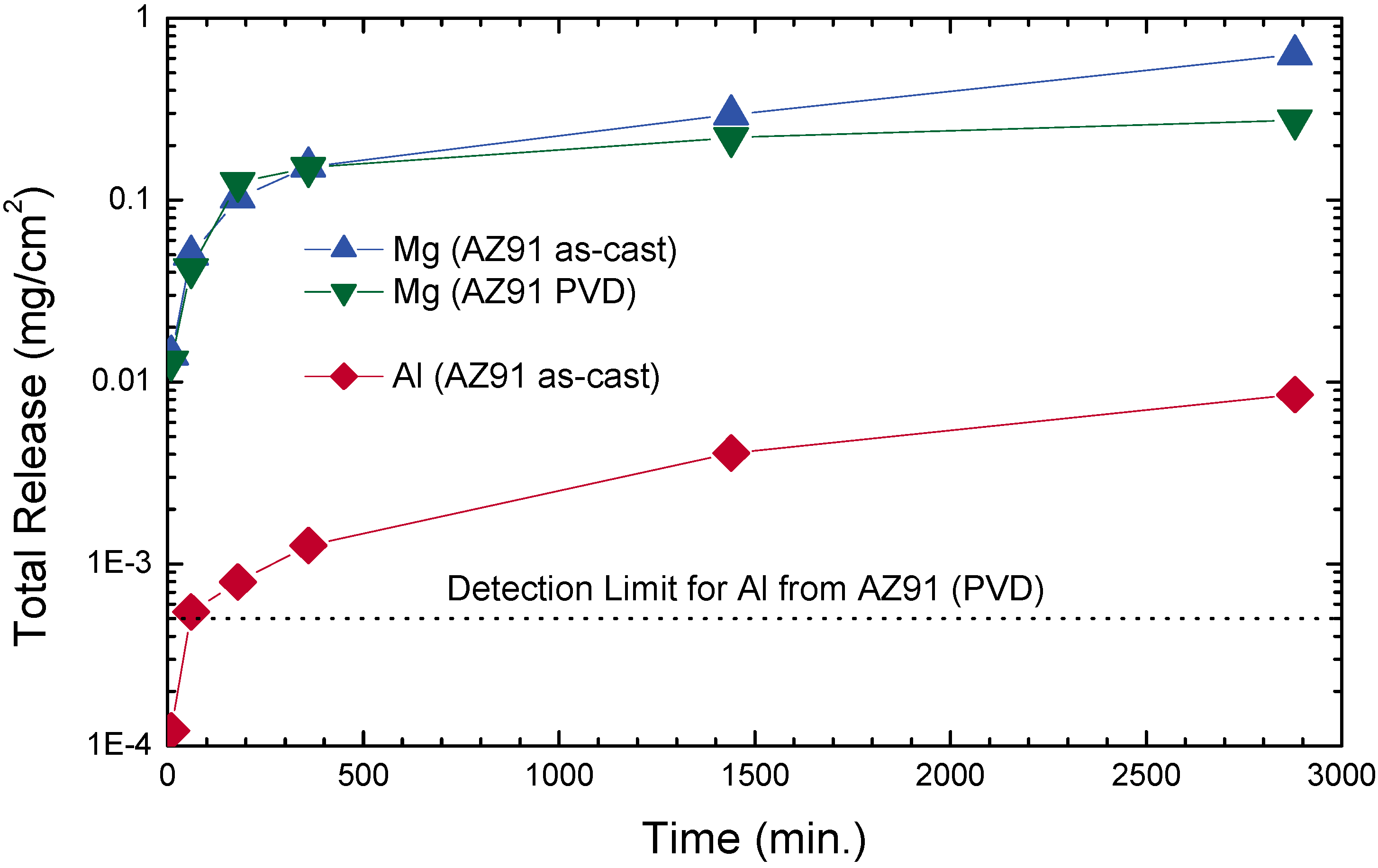
5. Summary and Conclusions
Acknowledgements
References and Notes
- Thompson, D’Arcy W. On Growth and Form; Dover: New York, NY, USA, 1992. [Google Scholar]
- Ball, P. Nature’s Patterns; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Woods, T.C.; Marks, A.R. Drug-eluting stents. Annu. Rev. Med. 2004, 55, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Keefer, L.K. Thwarting thrombus. Nature Mater. 2003, 2, 357–358. [Google Scholar] [CrossRef]
- Breme, J.; Kirkpatrick, C.J.; Thull, R., (Eds.) Metallic Biomaterial Interfaces; Wiley: Weinheim, Germany, 2008.
- Epstein, S.M. Physical and Cultural Constraint of Innovation in the Late Prehistoric Metallurgy of Cerro Huaringa, Peru. In Materials Issues in Art and Archaeology III; Vandiver, P.B., Druzik, J.R., Wheeler, G.S., Freestone, I.C., Eds.; Materials Research Society: Pittsburgh, PA, USA, 1992; pp. 747–756. [Google Scholar]
- Harris, J.E.; Iskander, Z.; Farid, S. Restorative dentistry in ancient Egypt: An archaeologic fact! J. Mich. Dent. Assoc. 1975, 57, 401–404. [Google Scholar] [PubMed]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: Orlando, FL, USA, 2004. [Google Scholar]
- Coppa, A.; Bondioli, L.; Cucina, A.; Frayer, D.W.; Jarrige, C.; Jarrige, J.-F.; Quivron, G.; Rossi, M.; Vidale, M.; Macchiarelli, R. Palaeontology: Early Neolithic tradition of dentistry. Nature 2006, 440, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Charnley, J. Arthroplasty of the hip: A new operation. Lancet 1961, 277, 1129–1133. [Google Scholar] [CrossRef]
- McKee, G.K.; Watson-Farrar, J. Replacement of arthritic hips by the McKee-Farrar prosthesis. J. Bone Joint Surg. Br. 1966, 48, 245–259. [Google Scholar] [PubMed]
- Kirkpatrick, C.J.; Fuchs, S.; Peters, K.; Brochhausen, C.; Hermanns, M.I.; Unger, R.E. Visions for regenerative medicine: Interface between scientific fact and science fiction. Artif. Organs 2006, 30, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Kasemo, B. Biological surface science. Surf. Sci. 2002, 500, 656–677. [Google Scholar] [CrossRef]
- Castner, D.G.; Ratner, B.D. Biomedical surface science: foundations to frontiers. Surf. Sci. 2002, 500, 28–60. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Shabalovskaya, S.A. Surface, corrosion and biocompatibility aspects of Nitinol as an implant material. Biomed. Mater. Eng. 2002, 12, 69–109. [Google Scholar] [PubMed]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.G.; Burdick, J.A.; Langer, R. Smart biomaterials. Science 2004, 305, 1923–1924. [Google Scholar] [CrossRef] [PubMed]
- Shastri, V.P. Future of regenerative medicine: challenges and hurdles. Artif. Organs 2006, 30, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P.; Weinkamer, R. Nature's hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Meyers, M.A.; Chen, P.-Y.; Lin, A.Y.-M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef]
- Whitesides, G.M. The ‘right’ size in nanobiotechnology. Nature Biotechnol. 2003, 21, 1161–1165. [Google Scholar] [CrossRef]
- Böhler, M.; Kanz, F.; Schwarz, B.; Steffan, I.; Walter, A.; Plenk, H.; Knahr, K. Adverse tissue reactions to wear particles from Co-alloy articulations, increased by alumina-blasting particle contamination from cementless Ti-based total hip implants. J. Bone Joint Surg. Br. 2002, 84B, 128–136. [Google Scholar] [CrossRef] [PubMed]
- The Future of the Orthopedic Devices Market to 2012; Market Research Report LABD78480; Global Markets Direct: Louisville, KY, USA, 2008.
- Frosch, K.-H.; Stürmer, K.M. Metallic Biomaterials in Skeletal Repair. Eur. J. Trauma 2006, 2, 149–159. [Google Scholar] [CrossRef]
- Kasemo, B.; Gold, J. Implant Surfaces and Interface Processes. Adv. Dent. Res. 1999, 13, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Petit, R. The use of hydroxyapatite in orthopaedic surgery: a ten-year review. Eur. J. Orthop. Surg. Traumatol. 1999, 9, 71–74. [Google Scholar] [CrossRef]
- Niinomi, M. Metallic biomaterials. J. Artif. Organs 2008, 11, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, M.; Oda, Y.; Kato, T.; Okuda, K. Influence of surface modifications to titanium on antibacterial activity in vitro. Biomaterials 2001, 22, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Takeuchi, M.; Suzuki, T.; Kubo, K.; Anpo, M.; Ogawa, T. Enhanced osteoblast function on ultraviolet light-treated zirconia. Biomaterials 2009, 30, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-J.; Lin, H.-I.; Chou, C.-M.; Hsieh, P.-Y.; Hsiao, C.-H.; Shi, Z.-Y.; He, J.-L. Inactivation of Staphylococcus aureus and Escherichia coli under various light sources on photocatalytic titanium dioxide thin film. Surf. Coat. Technol. 2009, 203, 1081–1085. [Google Scholar] [CrossRef]
- Variola, F.; Vetrone, F.; Richert, L.; Jedrzejowski, P.; Yi, J.H.; Zalzal, S.; Clair, S.; Sarkissian, A.; Perepichka, D.F.; Wuest, J.D.; Rosei, F.; Nanci, A. Improving biocompatibility of implantable metals by nanoscale modification of surfaces: an overview of strategies, fabrication methods, and challenges. Small 2009, 5, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Richert, L.; Vetrone, F.; Yi, J.-H.; Zalzal, S.F.; Wuest, J.D.; Rosei, F.; Nanci, A. Surface nanopatterning to control cell growth. Adv. Mater. 2008, 20, 1488–1492. [Google Scholar] [CrossRef]
- Zeng, R.; Dietzel, W.; Witte, F.; Hort, N.; Blawert, C. Progress and challenge for magnesium alloys as biomaterials. Adv. Eng. Mater. 2008, 10, B3–B14. [Google Scholar]
- Nastasi, M.; Mayer, J.W. Ion Implantation and Synthesis of Materials; Springer: Berlin, Germany, 2006; pp. 77–90. [Google Scholar]
- Boxman, R.L.; Martin, P.J.; Sanders, D.M. Handbook of Vacuum Arc Science and Technology; Noyes Publications: Park Ridge, IL, USA, 1995. [Google Scholar]
- Anders, A.; Anders, S.; Brown, I.G.; Dickinson, M.R.; MacGill, R.A. Metal plasma immersion ion implantation using vacuum arc plasma sources. J. Vac. Sci. Technol. B 1994, 12, 815–820. [Google Scholar] [CrossRef]
- Schneider, J.M.; Rohde, S.; Sproul, W.D.; Matthews, A. Recent developments in plasma assisted physical vapour deposition. J. Phys. D 2000, 33, R173–R186. [Google Scholar] [CrossRef]
- Thornton, J.A. The microstructure of sputter-deposited coatings. J. Vac. Sci. Technol. A 1974, 4, 3059–3065. [Google Scholar] [CrossRef]
- Ostwald, W. Über die vermeintliche Isomerie des roten und gelben Quecksilberoxyds und die Oberflächenspannung fester Körper. Z. Phys. Chem. 1900, 34, 495–503. [Google Scholar]
- Moske, M. Thin films for biomechanical coatings. Nova Acta Leopoldina 2001, 84, 137–152. [Google Scholar]
- Bohne, Y.; Manova, D.; Blawert, C.; Störmer, M.; Dietzel, W.; Mändl, S. Influence of ion energy on morphology and corrosion properties of Mg alloys formed by energetic PVD processes. Nucl. Instrum. Meth. B 2007, 257, 392–396. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar]
- Curran, J.A.; Clyne, T.W. Thermo-physical properties of plasma electrolytic oxide coatings on aluminium. Surf. Coat. Technol. 2005, 199, 168–176. [Google Scholar]
- C. Gabbi, A.; Cacchioli, F.; Ravanetti, B.; Spaggiari, P.; Borghetti, F.M.; Martini, F. Leonardi, Osteogenesis and bone integration: The effect of new titanium surface treatments. Ann. Fac. Medic. Vet. di Parma 2005, XXV, 307–318. [Google Scholar]
- Han, Y.; Sun, J.; Huang, X. Formation mechanism of HA-based coatings by micro-arc oxidation. Electrochem. Comm. 2008, 10, 510–513. [Google Scholar] [CrossRef]
- Shockley, W. Forming semiconductive devices by ionic bombardment. US Patent 2787564, 1957. [Google Scholar]
- Ohl, RS. Properties of ionic bombarded silicon. Bell System Tech. J. 1952, 31, 104–121. [Google Scholar] [CrossRef]
- Nastasi, M.; Mayer, J.W.; Hirvonen, J.K. Ion-Solid Interactions; Cambridge University Press: Cambridge, MA, USA, 1996; pp. 332–359. [Google Scholar]
- Cuomo, J.J.; Rossnagel, S.M.; Kaufman, H.R. Handbook of Ion Beam Processing Technology; Noyes Publications: Park Ridge, NJ, USA, 1989. [Google Scholar]
- Behrisch, R. Sputtering by Particle Bombardment; Springer: Berlin, Germany, 1983; Vol. II. [Google Scholar]
- Ziegler, J.F. Ion Implantation Science and Technology; Ion Implantation Technology Co.: Edgewater, FL, USA, 1996. [Google Scholar]
- Dearnaley, G. The modification of materials by ion implantation. Physics Technol. 1983, 14, 225–232. [Google Scholar] [CrossRef]
- Neumann, H.; Tartz, M.; Scholze, F.; Chassé, T.; Kersten, H.; Leiter, H. Broad beam ion sources for electrostatic space propulsion and surface modification processes: From roots to present applications. Contrib. Plasma Phys. 2007, 47, 487–497. [Google Scholar] [CrossRef]
- Conrad, J.R.; Radtke, J.L.; Dodd, R.A.; Worzala, F.J. Plasma source ion implantation technique for surface modification of materials. J. Appl. Phys. 1987, 62, 4591–4596. [Google Scholar] [CrossRef]
- Tendys, J.; Donnelly, I.J.; Kenny, M.J.; Pollock, J.T.A. Plasma immersion ion implantation using plasmas generated by radio frequency techniques. Appl. Phys. Lett. 1988, 53, 2143–2145. [Google Scholar] [CrossRef]
- Anders, A. (Ed.) Handbook of Plasma Immersion Ion Implantation and Deposition; Wiley-VCH: Berlin, Germany, 2000.
- Liedtke, D. Wärmebehandlung von Eisenwerkstoffen: Nitrieren und Nitrocarburieren; Expert-Verlag: Renningen, Germany, 2005. [Google Scholar]
- Brown, I.G.; Godechot, X.; Yu, K.M. Novel metal ion surface modification technique. Appl. Phys. Lett. 1991, 58, 1392–1394. [Google Scholar] [CrossRef]
- Edison, T.A. Process of duplicating phonograms. US Patent 484582, 1892. [Google Scholar]
- Sano, M.; Teramoto, T.; Yukimura, K.; Maruyama, T. TiN coating and ion implantation of materials with three-dimensional topology in metal DC plasma-based ion implantation. Surf. Coat. Technol. 2001, 136, 168–171. [Google Scholar] [CrossRef]
- Anders, A. Approaches to rid cathodic arc plasmas of macro- and nanoparticles: A review. Surf. Coat. Technol. 1999, 120/121, 319–330. [Google Scholar] [CrossRef]
- Brandolf, H. Vapor deposition apparatus and method. US Patent 4511593, 1985. [Google Scholar]
- Grove, W.R. On the electro-chemical polarity of gases. Phil. Trans. R. Soc. Lond. 1852, 142, 87–101. [Google Scholar] [CrossRef]
- Sigmund, P. Theory of sputtering. I. Sputtering yield of amorphous and polycrystalline targets. Phys. Rev. 1969, 184, 383–416. [Google Scholar] [CrossRef]
- Yamamura, Y.; Tawara, H. Energy dependence of ion-induced sputtering yields from monatomic solids at normal incidence. Atomic Data Nucl. Data Tables 1996, 62, 149–253. [Google Scholar] [CrossRef]
- Eckstein, W.; Preuss, R. New fit formulae for the sputtering yield. J. Nucl. Mater. 2003, 320, 209–213. [Google Scholar] [CrossRef]
- Günterschulze, A. Kathodenzerstäubung. Z. Phys. 1926, 36, 563–580. [Google Scholar] [CrossRef]
- Jacob, W. Surface reactions during growth and erosion of hydrocarbon films. Thin Solid Films 1998, 326, 1–42. [Google Scholar] [CrossRef]
- Ziberi, B.; Leibniz-Institut für Oberflächenmodifizierung. Private communication, 2009.
- Navez, M.; Sella, C.; Chaperot, D. Investigation of the attack on glass by ion bombardment. C. R. Acad. Sci. Paris 1962, 254, 240–244. [Google Scholar]
- Ziberi, B.; Frost, F.; Höche, T.; Rauschenbach, B. Ripple pattern formation on silicon surfaces by low-energy ion-beam erosion: Experiment and theory. Phys. Rev. B 2005, 72, 235310. [Google Scholar] [CrossRef]
- Facsko, S.; Dekorsy, T.; Koerdt, C.; Trappe, C.; Kurz, H.; Vogt, A.; Hartnagel, H.L. Formation of ordered nanoscale semiconductor dots by ion sputtering. Science 1999, 285, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Frost, F.; Schindler, A.; Bigl, F. Roughness evolution of ion sputtered rotating InP surfaces: Pattern formation and scaling laws. Phys. Rev. Lett. 2000, 85, 4116–4119. [Google Scholar] [CrossRef] [PubMed]
- Frost, F.; Fechner, R.; Ziberi, B.; Völlner, J.; Flamm, D.; Schindler, A. Large area smoothing of surfaces by ion bombardment: fundamentals and applications. J. Phys.: Condens. Matter 2009, 21, 224026. [Google Scholar] [CrossRef]
- Wilson, I.H. The topography of sputtered semiconductors. Radiat. Eff. 1973, 18, 95–103. [Google Scholar] [CrossRef]
- Ziberi, B.; Cornejo, M.; Frost, F.; Rauschenbach, B. Highly ordered nanopatterns on Ge and Si surfaces by ion beam sputtering. J. Phys.: Condens. Matter 2009, 21, 224003. [Google Scholar] [CrossRef]
- Bradley, R.M.; Harper, J.M.E. Theory of ripple topography induced by ion bombardment. J. Vac. Sci. Technol. A 1988, 6, 2390–2395. [Google Scholar] [CrossRef]
- Güntherschulze, A.; Tollmien, W. Neue Untersuchungen über die Kathodenzerstäubung der Glimmentladung: V. Die Oberflächenbeschaffenheit der Kathode. Z. Phys. A 1942, 119, 685–695. [Google Scholar] [CrossRef]
- Michely, T.; Comsa, G. Temperature dependence of the sputtering morphology of Pt(111). Surf. Sci. 1991, 256, 217–226. [Google Scholar] [CrossRef]
- Manova, D.; Schreck, M.; Mändl, S.; Stritzker, B.; Rauschenbach, B. Orientation dependent sputter yield of aluminium. Surf. Coat. Technol. 2002, 151/152, 72–75. [Google Scholar] [CrossRef]
- Goldschmidt, H.J. Interstitial Alloys; Butterworth: London, UK, 1967; pp. 88–444. [Google Scholar]
- Saunders, S.R.J.; Monteiro, M.; Rizzo, F. The oxidation behaviour of metals and alloys at high temperatures in atmospheres containing water vapour: A review. Prog. Mater. Sci. 2008, 53, 775–837. [Google Scholar] [CrossRef]
- Hultman, L. Thermal stability of nitride thin films. Vacuum 2000, 57, 1–30. [Google Scholar] [CrossRef]
- Wood, R.J.K. Tribo-corrosion of coatings: A review. J. Phys D: Appl. Phys. 2007, 40, 5502–5521. [Google Scholar] [CrossRef]
- Wagner, C. Formation of composite scales consisting of oxides of different metals. J. Electrochem. Soc. 1956, 103, 627–633. [Google Scholar] [CrossRef]
- Smialek, J.L.; Meier, G.M. High-temperature oxidation. In Superalloys II; Sims, C.T., Stoloff, N.S., Hagel, W.C., Eds.; Wiley: New York, NY, USA, 1987; pp. 293–326. [Google Scholar]
- Atkinson, A. Grain boundary diffusion - structural effects and mechanisms. J. Phys. Colloques 1985, 46, C4:379–C4:391. [Google Scholar]
- Kaur, I.; Mishin, Y.; Gust, W. Fundamentals of Grain and Interphase Boundary Diffiusion; Wiley: Chichester, UK, 1995. [Google Scholar]
- Fisher, J.C. Calculation of diffusion penetration curves for surface and grain boundary diffusion. J. Appl. Phys. 1951, 22, 74–77. [Google Scholar] [CrossRef]
- Brown, A.M.; Ashby, M.F. Correlations for diffusion constants. Acta Metall. 1980, 28, 1085–1101. [Google Scholar] [CrossRef]
- Atkinson, A.; Monty, C. Grain boundary diffusion in ceramics. In Surfaces and Interfaces of Ceramic Materials; Dufour, L.-C., Monty, C., Petot-Ervas, G., Eds.; Kluwer Academic Publishers: New York, NY, USA, 1988; pp. 273–284. [Google Scholar]
- Manova, D.; Attenberger, W.; Mändl, S.; Stritzker, B.; Rauschenbach, B. Aluminum diffusion and nitrogen sputter yield for nitrogen plasma immersion ion implantation into aluminum. J. Vac. Sci. Technol. A 2003, 21, 643–648. [Google Scholar] [CrossRef]
- Telbizova, T.; Parascandola, S.; Prokert, F.; Barradas, N.P.; Richter, E.; Möller, W. Ion nitriding of Al: growth kinetics and characterisation of the nitride layer. Surf. Coat. Technol. 2001, 142/144, 1028–1033. [Google Scholar] [CrossRef]
- Fouquet, V.; Pichon, L.; Straboni, A.; Drouet, M. Nitridation of Ti6Al4V by PBII: Study of the nitrogen diffusion and of the nitride growth mechanism. Surf. Coat. Technol. 2004, 186, 34–39. [Google Scholar] [CrossRef]
- Hammerl, C.; Renner, B.; Rauschenbach, B.; Assmann, W. Phase formation in titanium after high-fluence oxygen ion implantation. Nucl. Instrum. Methods B 1999, 148, 851–857. [Google Scholar] [CrossRef]
- Mändl, S.; Thorwarth, G.; Schreck, M.; Stritzker, B.; Rauschenbach, B. Raman study of titanium oxide layers produced with plasma immersion ion implantation. Surf. Coat. Technol. 2000, 125, 84–88. [Google Scholar] [CrossRef]
- Lutz, T.; Gerlach, J.W.; Mändl, S. Diffusion, phase formation and segregation effects in Ti6Al4V after oxygen PIII. Surf. Coat. Technol. 2007, 201, 6690–6694. [Google Scholar] [CrossRef]
- Mändl, S.; Fleischer, A.; Manova, D.; Rauschenbach, B. Wear behaviour of NiTi shape memory alloy after oxygen-PIII treatment. Surf. Coat. Technol. 2006, 200, 6225–6229. [Google Scholar] [CrossRef]
- Davis, J.R. ASM Specialty Handbook: Heat-resistant Materials; ASM International: Materials Park, OH, USA, 1997; pp. 237–246. [Google Scholar]
- Heinke, W.; Leyland, A.; Matthews, A.; Berg, G.; Friedrich, C.; Broszeit, E. Evaluation of PVD nitride coatings, using impact, scratch and Rockwell-C adhesion tests. Thin Solid Films 1995, 270, 431–438. [Google Scholar] [CrossRef]
- Lane, M.; Dauskardt, R.H.; Vainchtein, A.; Gao, H. Plasticity contributions to interface adhesion in thin-film interconnect structures. J. Mater. Res. 2000, 15, 2758–2769. [Google Scholar] [CrossRef]
- Diesselberg, M.; Stock, H.-R.; Mayr, P. Corrosion protection of magnetron sputtered TiN coatings deposited on high strength aluminium alloys. Surf. Coat. Technol. 2004, 177/178, 399–403. [Google Scholar] [CrossRef]
- Chandra, L.; Allen, M.; Butter, R.; Rushton, N.; Lettington, A.H.; Clyne, T.W. The effect of exposure to biological fluids on the spallation resistance of diamond-like carbon coatings on metallic substrates. J. Mater. Sci.: Mater. Med. 1995, 6, 581–589. [Google Scholar] [CrossRef]
- Attenberger, W.; Thorwarth, G.; Manova, D.; Mändl, S.; Stritzker, B.; Rauschenbach, B. Interface properties of TiO2 on Si formed by simultaneous implantation and deposition of titanium and oxygen ions. Surf. Coat. Technol. 2001, 142/144, 412–417. [Google Scholar] [CrossRef]
- Manova, D.; Hirsch, D.; Mändl, S.; Neumann, H. Microstructure of Ti thin films formed by energetic PVD processes. Nucl. Instrum. Meth. B 2009, 267, 1680–1683. [Google Scholar] [CrossRef]
- Anderson, D.G.; Putnam, D.; Lavik, E.B.; Mahmood, T.A.; Langer, R. Biomaterial microarrays: Rapid, microscale screening of polymer-cell interaction. Biomaterials 2005, 26, 4892–4897. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A. Biomaterials in tissue engineering. Bio-Technology 1995, 13, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.S.; Wilkinson, C.D. Reactions of cells to topography. J. Biomater. Sci. Polym. Ed. 1998, 9, 1313–1329. [Google Scholar] [CrossRef] [PubMed]
- Wojciak-Stothard, B.; Curtis, A.; Monaghan, W.; Macdonald, K.; Wilkinson, C. Guidance and activation of murine macrophages by nanometric scale topography. Exp. Cell Res. 1996, 226, 426–435. [Google Scholar]
- Elwing, H.; Nilsson, B.; Svensson, K.E.; Askendahl, A.; Nilsson, U.R.; Lundström, I. Conformational changes of a model protein (complement factor 3) adsorbed on hydrophilic and hydrophobic solid surfaces. J. Coll. Interf. Sci. 1988, 125, 139–145. [Google Scholar] [CrossRef]
- Elagli, K.; Hildebrand, H.J.; Breme, J. Electrochemical and in vitro biological behaviour of titanium and its alloys. Intern. Biomater. Symp. Trans. 1994, 26, 262–263. [Google Scholar]
- El Feninat, F.; Laroche, G.; Fiset, M.; Mantovani, D. Shape memory materials for biomedical applications. Adv. Eng. Mat. 2002, 4, 91–104. [Google Scholar] [CrossRef]
- Büscher, R.; Täger, G.; Dudzinski, W.; Gleising, B.; Wimmer, M.A.; Fischer, A. Subsurface microstructure of metal-on-metal hip joints and its relationship to wear particle generation. J. Biomed. Mater. Res. B 2004, 72, 206–214. [Google Scholar]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.T.; Tosetto, M.; Miller, I.; O’Connor, D.; Penney, S.C.; Lynch, I.; Keenan, A.K.; Pennington, S.R.; Dawson, K.A.; Gallagher, W.M. Surface-induced changes in protein adsorption and implications for cellular phenotypic responses to surface interaction. Biomaterials 2006, 27, 3096–3108. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A. Materials as morphogenetic guides in tissue engineering. Curr. Opin. Biotechnol. 2003, 14, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Flemming, R.G.; Murphy, C.J.; Abrams, G.A.; Goodman, S.L.; Nealey, P.F. Effects of synthetic micro- and nano-structured surfaces on cell behaviour. Biomaterials 1999, 20, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.C.; Webster, T.J. The effect of nanotopography on calcium and phosphorus deposition on metallic materials in vitro. Biomaterials 2006, 27, 3064–3074. [Google Scholar] [CrossRef] [PubMed]
- Asadchikov, V.E.; Duparré, A.; Jakobs, S.; Karabekov, A.Y.; Kozhevnikov, I.V.; Krivonosov, Y.S. Comparative study of the roughness of optical surfaces and thin films by use of x-ray scattering and atomic force microscopy. Appl Opt. 1999, 38, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Duparré, A.; Ferre-Borrull, J.; Gliech, S.; Notni, G.; Steinert, J.; Bennett, J.M. Surface characterization techniques for determining the root-mean-square roughness and power spectral densities of optical components. Appl. Opt. 2002, 41, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Raz, P.; Lohmann, C.H.; Turner, J.; Wang, L.; Poythress, N.; Blanchard, C.; Boyan, B.D.; Schwartz, Z. 1α,25(OH)2D3 Regulation of integrin expression is substrate dependent. J. Biomed. Mater. Res. A 2004, 71, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Lohmann, C.H.; Sisk, M.; Cochran, D.L.; Sylvia, V.L.; Simpson, J.; Dean, D.D.; Boyan, B.D. Local factor production by MG63 osteoblast-like cells in response to surface roughness and 1,25-(OH)2D3 is mediated via protein kinase C- and protein kinase A-dependent pathways. Biomaterials 2001, 22, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.; Schwartz, Z.; Swain, L.D.; Ramirez, V.; Poser, J.; Boyan, B.D. Stimulation of plasma membrane and matrix vesicle enzyme activity by transforming growth factor-beta in osteosarcoma cell cultures. J. Cell Physiol. 1990, 145, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.A. Transforming growth factor-beta: a general review. Eur. Cytokine Netw. 1996, 7, 363–374. [Google Scholar] [PubMed]
- Martin, J.Y.; Dean, D.D.; Cochran, D.L.; Simpson, J.; Boyan, B.D.; Schwartz, Z. Proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) cultured on previously used titanium surfaces. Clin. Oral Implants Res. 1996, 7, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Batzer, R.; Kieswetter, K.; Liu, Y.; Cochran, D.L.; Szmuckler-Moncler, S.; Dean, D.D.; Schwartz, Z. Titanium surface roughness alters responsiveness of MG63 cells to 1α,25(OH)2D3. J. Biomed. Mater. Res. A 1998, 39, 77–85. [Google Scholar] [CrossRef]
- Hofmann, A.; Ritz, U.; Verrier, S.; Eglin, D.; Alini, M.; Fuchs, S.; Kirkpatrick, C.J.; Rommens, P.M. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials 2008, 29, 4217–4226. [Google Scholar] [CrossRef] [PubMed]
- Lossdörfer, S.; Schwartz, Z.; Wang, L.; Lohmann, C.H.; Turner, J.D.; Wieland, M.; Cochran, D.L.; Boyan, B.D. Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J. Biomed. Mater. Res. A 2004, 70, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, I.; Abrahams, G.A.; Bertics, P.J.; Murphy, C.J.; Nealey, P.F. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J. Cell. Sci. 2003, 116, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Monsees, T.K.; Barth, K.; Tippelt, S.; Heidel, K.; Gorbunov, A.; Pompe, W.; Funk, R.H.W. Effects of different titanium alloys and nanosize surface patterning on adhesion, differentiation, and orientation of osteoblast-like cells. Cells Tissues Organs 2005, 180, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-B.; Lin, J.-H. Modulation of morphology and functions of human hepatoblastoma cells by nano-grooved substrata. Acta Biomaterialia 2009, 5, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Elter, P.; Sickel, F.; Ewald, A. Nanoscaled periodic surface structures of medical stainless steel and their effect on osteoblast cells. Acta Biomaterialia 2009, 5, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, E.; Lienz, P.; Biehl, V.; Velten, D.; Breme, J.; Hildebrand, H.F. Cell orientation and cytoskeleton organisation on ground titanium surfaces. Biomol. Eng. 2002, 19, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.E.; Skazik, C.; Harwardt, M.; Bartneck, M.; Denecke, B.; Klee, D.; Salber, J.; Zwadlo-Klarwasser, G. Topographical control of human macrophages by a regularly microstructured polyvinylidene fluoride surface. Biomaterials 2008, 29, 4056–4064. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, S.; Azari, F.; Li, H.; Bateni, M.R.; Szpunar, J.A.; Vali, H.; Tabrizian, M. The significance of crystallographic texture of titanium alloy substrates on pre-osteoblast responses. Biomaterials 2006, 27, 3532–3539. [Google Scholar] [PubMed]
- Habibovic, P.; Yuan, H.; van der Valk, C.M.; Meijer, G.; van Blitterswijk, C.A.; de Groot, K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials 2005, 26, 3565–3575. [Google Scholar] [CrossRef] [PubMed]
- Erli, H.-J.; Rüger, M.; Ragoß, C.; Jahnen-Dechent, W.; Hollander, D.A.; Paar, O.; von Walter, M. The effect of surface modification of a porous TiO2/perlite composite on the ingrowth of bone tissue in vivo. Biomaterials 2006, 27, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Fujibayashi, S.; Neo, M.; Suzuki, J.; Matsushita, T.; Kokubo, T.; Nakamura, T. Osteoinductive porous titanium implants: Effect of sodium removal by dilute HCl treatment. Biomaterials 2006, 27, 2682–2691. [Google Scholar] [CrossRef] [PubMed]
- Van Wachem, P.B.; Beugeling, T.; Feijen, J.; Bantjes, A.; Detmers, J.P.; van Aken, W.G. Interaction of cultured human endothelial cells with polymeric surfaces of different wettabilities. Biomaterials 1985, 6, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Groth, T.; Zlatanov, I.; Altankov, G. Adhesion of human peripheral lymphocytes on biomaterials preadsorbed with fibronectin and vitronectin. J. Biomater. Sci. Polym. Edn. 1994, 6, 729–739. [Google Scholar] [CrossRef]
- Lim, J.Y.; Liu, X.; Vogler, E.A.; Donahue, H.J. Systematic variation in osteoblast adhesion and phenotype with substratum surface characteristics. J. Biomed. Mater. Res. A 2004, 68, 504–512. [Google Scholar] [CrossRef]
- Grinnell, F. Fibronectin adsorption on material surfaces. Ann. NY Acad. Sci. 1987, 516, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Juliano, D.J.; Saavedra, S.S.; Truskey, G.A. Effect of the conformation and orientation of adsorbed fibronectin on endothelial cell spreading and the strength of adhesion. J. Biomed. Mater. Res. 1993, 27, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, P.N.; Pate, J.W. Bio-electric phenomena as etiological agents in intravascular thrombosis. Surgery 1953, 34, 491–500. [Google Scholar] [PubMed]
- Baurschmidt, P.; Schaldach, M. The electrochemical aspects of the thrombogenicity of a material. J. Bioeng. 1977, 1, 261–278. [Google Scholar]
- Cloud, A.N.; Kumar, S.; Kavdia, M.; Abu-Safe, H.H.; Gordon, M.H. Protein adsorption on low temperature alpha alumina films for surgical instruments. Surf. Coat. Technol. 2008, 203, 913–917. [Google Scholar] [CrossRef]
- Böhler, M.; Knahr, K.; Plenk, H.; Walter, A.; Salzer, M. Long-term results of uncemented alumina acetabular implants. J. Bone Joint Surg. Br. 1994, 76B, 53–59. [Google Scholar] [PubMed]
- Fischer, H.; Niedhart, C.; Kaltenborn, N.; Prange, A.; Marx, R.; Niethard, F.U.; Telle, R. Bioactivation of inert alumina ceramics by hydroxylation. Biomaterials 2005, 26, 6151–6157. [Google Scholar] [CrossRef] [PubMed]
- Groth, T.; Altankov, G.; Kostadinova, A.; Krasteva, N.; Albrecht, W.; Paul, D. Altered vitronectin receptor (αv integrin) function in fibroblasts adhering on hydrophobic glass. J. Biomed. Mater. Res. A 1999, 44, 341–351. [Google Scholar] [CrossRef]
- Pešákova, V.; Kubies, D.; Hulejová, H.; Himmlová, L. The influence of implant surface properties on cell adhesion and proliferation. J. Mater. Sci.: Mater. Med. 2007, 18, 465–473. [Google Scholar] [CrossRef]
- Davies, A.P.; Willert, H.G.; Campbell, P.A.; Learmonth, I.D.; Case, C.P. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J. Bone Joint Surg. Am. 2005, 87, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Willert, H.-G.; Buchhorn, G.H.; Fayyazi, A.; Flury, R.; Windler, M.; Köster, G.; Lohmann, C.H. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints: A clinical and histomorphological study. J. Bone Joint Surg. Am. 2005, 87, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Wolner, C.; Nauer, G.E.; Trummer, J.; Putz, V.; Tschegg, S. Possible reasons for the unexpected bad biocompatibility of metal-on-metal hip implants. Mater. Sci. Eng. C 2006, 26, 34–40. [Google Scholar] [CrossRef]
- Brady, M.P.; Gleeson, B.; Wright, I.G. Alloy design strategies for promoting protective oxide-scale formation. JOM 2000, 52, 16–21. [Google Scholar] [CrossRef]
- Hennig, F.F.; Raithel, H.J.; Schaller, K.H.; Döhler, J.R. Nickel-, chrome- and cobalt-concentrations in human tissue and body fluids of hip prosthesis patients. J. Trace Elem. Electrolytes Health Dis. 1992, 6, 239–243. [Google Scholar] [PubMed]
- Jacobs, J.J.; Skipor, A.K.; Patterson, L.M.; Hallab, N.J.; Paprosky, W.G.; Black, J.; Galante, J.O. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J. Bone Joint Surg. Am. 1998, 80, 1447–1458. [Google Scholar] [PubMed]
- Agins, H.J.; Alcock, N.W.; Bansal, M.; Salvati, E.A.; Wilson, P.D., Jr.; Pellicci, P.M.; Bullough, P.G. Metallic wear in failed titanium-alloy total hip replacements. A histological and quantitative analysis. J. Bone Joint. Surg. Am. 1988, 70, 347–356. [Google Scholar] [PubMed]
- Browne, M.; Gregson, P.J. Surface modification of titanium alloy implants. Biomaterials 1994, 15, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Gawkrodger, D.J. Nickel dermatitis: How much nickel is safe? Contact Dermatitis 1996, 35, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Gotoh, E. Comparsion of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, S.; Milošev, I.; Gomez-Barrena, E.; Trebše, R.; Salo, J.; Konttinen, Y.T. Special modes of corrosion under physiological and simulated physiological conditions. Acta Biomaterialia 2008, 4, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Wapner, K.L. Implications of metallic corrosion in total knee arthroplasty. Clin. Orthop. Relat. Res. 1991, 271, 12–20. [Google Scholar] [PubMed]
- Kirkpatrick, C.J.; Barth, S.; Gerdes, T.; Krump-Konvalinkova, V.; Peters, K. Pathomechanismen der gestörten Wundheilung durch metallische Korrosionsprodukte. Mund-, Kiefer- GesichtsChir. 2002, 6, 183–190. [Google Scholar] [CrossRef]
- Fleury, C.; Petit, A.; Mwale, F.; Antoniou, J.; Zukor, D.J.; Tabrizian, M.; Huk, O.L. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: Morphology, cytotoxicity, and oxidative stress. Biomaterials 2006, 27, 3351–3360. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.L.; Wataha, J.C.; Hanks, C.T. Effects of metal ions on osteoblast-like cell metabolism and differentiation. J. Biomed. Mater. Res. 1997, 34, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kalbacova, M.; Roessler, S.; Hempel, U.; Tsaryk, R.; Peters, K.; Scharnweber, D.; Kirkpatrick, J.C.; Dieter, P. The effect of electrochemically simulated titanium cathodic corrosion products on ROS production and metabolic activity of osteoblasts and monocytes/macrophages. Biomaterials 2007, 28, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Unger, R.E.; Barth, S.; Gerdes, T.; Kirkpatrick, C.J. Induction of apoptosis in human microvascular endothelial cells by divalent cobalt ions. Evidence for integrin-mediated signalling via the cytoskeleton. J. Mater. Sci.: Mater. Med. 2001, 12, 955–958. [Google Scholar] [CrossRef]
- Peters, K.; Schmidt, H.; Unger, R.E.; Kamp, G.; Pröls, F.; Berger, B.J.; Kirkpatrick, C.J. Paradoxical effects of hypoxia-mimicking divalent cobalt ions in human endothelial cells in vitro. Mol. Cell. Biochem. 2005, 270, 157–166. [Google Scholar] [CrossRef] [PubMed]
- D’Antò, V.; Eckhardt, A.; Hiller, K.-A.; Spagnuolo, G.; Valletta, R.; Ambrosio, L.; Schmalz, G.; Schweikl, H. The influence of Ni(II) on surface antigen expression in murine macrophages. Biomaterials 2009, 30, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Zreiqat, H.; Valenzuela, S.M.; Nissan, B.B.; Roest, R.; Knabe, C.; Radlanski, R.J.; Renz, H.; Evans, P.J. The effect of surface chemistry modification of titanium alloy on signalling pathways in human osteoblasts. Biomaterials 2005, 26, 7579–7586. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.C.; Pereira, M.L.; Sousa, J.P. Evaluation of nickel toxicity on liver, spleen, and kidney of mice after administration of high-dose metal ion. J. Biomed. Mater. Res. A 1998, 40, 40–47. [Google Scholar] [CrossRef]
- Schliephake, H.; Lehmann, H.; Kunz, U.; Schmelzeisen, R. Ultrastructural findings in soft tissues adjacent to titanium miniplates used in jaw fracture treatment. Int. J. Oral Maxillofac. Surg. 1993, 22, 20–25. [Google Scholar] [CrossRef] [PubMed]
- MacQuarrie, R.A.; Fang, C.Y.; Coles, C.; Anderson, G.I. Wear-particle-induced osteoclast osteolysis: the role of particulates and mechanical strain. J. Biomed. Mater. Res. B 2004, 69, 104–112. [Google Scholar] [CrossRef]
- Pioletti, D.P.; Kottelat, A. The influence of wear particles in the expression of osteoclastogenesis factors by osteoblasts. Biomaterials 2004, 25, 5803–5808. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Sasaki, K.; Sasaki, A.; Takakubo, Y.; Hasegawa, H.; Ogino, T.; Konttinen, Y.T.; Salo, J.; Takagi, M. Enhanced osteolytic potential of monocytes/macrophages derived from bone marrow after particle stimulation. J. Biomed. Mater. Res. B 2008, 84, 191–204. [Google Scholar] [CrossRef]
- Clohisy, J.C.; Hirayama, T.; Frazier, E.; Han, S.K.; Abu-Amer, Y. NF-κB signalling blockade abolishes implant particle-induced osteoclastogenesis. J. Orthop. Res. 2004, 22, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amer, Y.; Darwech, I.; Clohisy, J.C. Aseptic loosening of total joint replacements: Mechanisms underlying osteolysis and potential therapies. Arthritis Res. Ther. 2007, 9, S6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yu, X.; Collin-Osdoby, P.; Osdoby, P. RANKL stimulates inducible nitricoxide synthase expression and nitric oxide production in developing osteoclasts. An autocrine negative feedback mechanism triggered by RANKLinduced interferon-beta via NF-κB that restrains osteoclastogenesis and bone resorption. J. Biol. Chem. 2006, 281, 15809–15820. [Google Scholar] [CrossRef] [PubMed]
- Doorn, P.F.; Campbell, P.A.; Worrall, J.; Benya, P.D.; McKellop, H.A. Metal wear particle characterization from metal on metal total hip replacements: TEM study of periprosthetic tissues and isolated particles. J. Biomed. Mater. Res. 1998, 42, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.M.; Hamer, A.J.; Stockley, I.; Eastell, R. Polyethylene wear rate and osteolysis: Critical threshold versus continuous dose–response relationship. J. Orthop. Res. 2005, 23, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Wintermantel, E.; Ha, S.-W. Biokompatible Werkstoff und Bauweisen; Springer: Berlin, Germany, 2002; pp. 121–125. [Google Scholar]
- Mckelvey, A.L.; Ritchie, R.O. Fatigue-crack propagation in Nitinol, a shape-memory and superelastic endovascular stent material. J. Biomed. Mater. Res. A 1999, 47, 301–308. [Google Scholar] [CrossRef]
- Bugbee, W.D.; Culpepper, W.J.; Engh, C.A., Jr.; Engh, C.A., Sr. Long-term clinical conse-quences of stress-shielding after total hip arthroplasty without cement. J. Bone Joint Surg. Am. 1997, 79, 1007–1012. [Google Scholar] [PubMed]
- Hanawa, T. In vivo metallic biomaterials and surface modification. Mater. Sci. Eng. A 1999, 267, 260–266. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Sioshansi, P.; Olivier, R.W.; Matthews, F.D. Wear improvement of surgical titanium alloys by ion implantation. J. Vac. Sci. Technol. A 1985, 3, 2670–2674. [Google Scholar] [CrossRef]
- Fujii, N.; Kusakari, H.; Maeda, T.A. A histological study on tissue responses to titanium implantation in rat maxilla: The process of epithelial regeneration and bone reaction. J. Periodontol. 1998, 69, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Cook, S.; Salkeld, S.L.; Gaisser, D.M.; Wagner, W.R. The effect of surface macrotexture on the mechanical and histologic characteristics of hydroxylapatite-coated dental implants. J. Oral Implantol. 1993, 19, 288–294. [Google Scholar] [PubMed]
- Dion, I.; Roques, X.; More, N.; Labrousse, L.; Caix, J.; Lefebvre, F.; Rouais, F.; Gautreau, J.; Baquey, C. Ex vivo leucocyte adhesion and protein adsorption on TiN. Biomaterials 1993, 14, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Van Raay, J.J.A.M.; Rozing, P.M.; van Blitterswijk, C.A.; van Haastert, R.M.; Koerten, H.K. Biocompatibility of wear-resistant coatings in orthopaedic surgery in vitro testing with human fibroblast cell cultures. J. Mater. Sci.: Mater. Med. 1995, 6, 80–84. [Google Scholar] [CrossRef]
- Williams, J.M.; Buchanan, R.A. Ion implantation of surgical Ti6Al4V alloy. Mater. Sci. Eng. 1985, 69, 237–246. [Google Scholar] [CrossRef]
- Chen, A.; Scheuer, J.T.; Ritter, C.; Alexander, R.B.; Conrad, J.R. Comparison between conventional and plasma source ion-implanted femoral knee components. J. Appl. Phys. 1991, 70, 6757–6760. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zu, X.T.; Qiu, S.Y.; Wang, L.; Ma, W.G.; Wei, C.F. Surface characterization and corrosion resistance of Ti–Al–Zr alloy by niobium ion implantation. Nucl. Instrum. Meth. B 2006, 244, 397–402. [Google Scholar] [CrossRef]
- Krupa, D.; Baszkiewicz, J.; Kozubowski, J.A.; Barcz, A.; Sobczak, J.W.; Biliński, A.; Lewandowska-Szumieł, M.; Rajchel, B. Effect of phosphorus-ion implantation on the corrosion resistance and biocompatibility of titanium. Biomaterials 2002, 23, 3329–3340. [Google Scholar] [CrossRef] [PubMed]
- Nayab, S.N.; Jones, F.H.; Olsen, I. Effects of calcium ion implantation on human bone cell interaction with titanium. Biomaterials 2005, 26, 4717–4727. [Google Scholar] [CrossRef] [PubMed]
- Krupa, D.; Baszkiewicz, J.; Kozubowski, J.A.; Barcz, A.; Sobczak, J.W.; Biliński, A.; Lewandowska-Szumieł, M.; Rajchel, B. Effect of calcium-ion implantation on the corrosion resistance and iocompatibility of titanium. Biomaterials 2001, 22, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Maitz, M.F.; Poon, R.W.Y.; Liu, X.Y.; Pham, M.-T.; Chu, P.K. Bioactivity of titanium following sodium plasma immersion ion implantation and deposition. Biomaterials 2005, 26, 5465–5473. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T.; Kon, M.; Doi, H.; Ukai, H.; Murakami, K.; Hamanaka, H.; Asaoka, K. Amount of hydroxyl radical on calcium-ion-implanted titanium and point of zero charge of constituent oxide of the surface-modified layer. J. Mater. Sci: Mater. Med. 1998, 9, 89–92. [Google Scholar] [CrossRef]
- Javorsky, C.S.; Strohaecker, T.R.; Borges da Costa, J.A.T.; Vasconcellos, M.A.Z. Ion beam analysis of plasma nitrided Ti6Al4V-ELI. Nucl. Instrum. Meth. B 2001, 175/177, 726–731. [Google Scholar] [CrossRef]
- Manova, D.; Gerlach, J.W.; Neumann, H.; Assmann, W.; Mändl, S. Phase formation in Ti after high fluence/high temperature nitrogen implantation. Nucl. Instrum. Meth. B 2006, 242, 282–284. [Google Scholar] [CrossRef]
- Mändl, S. PIII treatment of Ti alloys and NiTi for medical applications. Surf. Coat. Technol. 2007, 201, 6833–6838. [Google Scholar] [CrossRef]
- Lanning, B.R.; Wei, R. High intensity plasma ion nitriding of orthopedic materials. Part II. Microstructural analysis. Surf. Coat. Technol. 2004, 186, 314–319. [Google Scholar] [CrossRef]
- Fouquet, V.; Pichon, L.; Straboni, A.; Drouet, M. Nitridation of Ti6Al4V by PBII: Study of the nitrogen diffusion and of the nitride growth mechanism. Surf. Coat. Technol. 2004, 186, 34–39. [Google Scholar] [CrossRef]
- Alves Jr., C.; Guerra Neto, C.LB.; Morais, G.H.S.; da Silva, C.F.; Hajek, V. Nitriding of titanium disks and industrial dental implants using hollow cathode discharge. Surf. Coat. Technol. 2006, 200, 3657–3663. [Google Scholar] [CrossRef]
- Thorwarth, G.; Mändl, S.; Rauschenbach, B. Rutile formation and oxygen diffusion in oxygen-PIII treated titanium. Surf. Coat. Technol. 2001, 136, 236–240. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Schenk-Meuser, K.; Linez, P.; Biehl, V.; Duschner, H.; Breme, J.; Hildebrand, H. Interactions between cells and titanium surfaces. Biomol. Eng. 2002, 19, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Chen, Y.-R.; Luo, J.-M.; Yi, J.; Lu, R.; Xiao, J.; Xue, Z.-N.; Liu, X.-H. In vitro investigation of blood compatibility of Ti with oxide layers of rutile structure. J. Biomater. Appl. 1994, 8, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Ducherow, M.; Fleischer, A.; Mändl, S. Change in wear behaviour of Ti and Ti6Al4V after plasma immersion ion implantation. Plasma Proc. Polymers 2007, 4, S602–S606. [Google Scholar] [CrossRef]
- Wei, R.; Booker, T.; Rincon, C.; Arps, J. High-intensity plasma ion nitriding of orthopedic materials. Part I. Tribological study. Surf. Coat. Technol. 2004, 186, 305–313. [Google Scholar] [CrossRef]
- Alonso, F.; Rinner, M.; Loinaz, A.; Oñate, J.I.; Ensinger, W.; Rauschenbach, B. Characterization of Ti-6Al-4V modified by nitrogen plasma immersion ion implantation. Surf. Coat. Technol. 1997, 93, 305–308. [Google Scholar] [CrossRef]
- Shibata, H.; Tokaji, K.; Ogawa, T.; Hori, C. The effect of gas nitriding on fatigue behaviour in titanium alloys. Int. J. Fatigue 1994, 16, 370–376. [Google Scholar] [CrossRef]
- Nolan, D.; Huang, S.W.; Leskovsek, V.; Braun, S. Sliding wear of titanium nitride thin films deposited on Ti-6Al-4V alloy by PVD and plasma nitriding processes. Surf. Coat. Technol. 2006, 200, 5698–5705. [Google Scholar] [CrossRef]
- Ababneh, M. Oberflächenoptimierung hochfester Titanlegierungen für modulare Hüftendo-prothesen; Hieronymus: München, Germany, 2002. [Google Scholar]
- Bader, R.; Steinhauser, E.; Guder, S.; Gregory, J.K.; Gradinger, R.; Mittelmeier, W. Modell zur In-vitro-Prüfung von Hüftendoprothesen-Stielen am Interface Implantatoberfläche-Knochenzemen. In Eigenschaften und Prüftechniken Mechanisch Beanspruchter Implantate; DVM-Arbeitskreis “Biowerkstoffe“, DVM: Berlin, Germany, 2002; pp. 165–174. [Google Scholar]
- Nan, H.; Yuanru, C.; Guangjun, C.; Chenggang, L.; Zhongguang, W.; Guo, Y.; Huehe, S.; Xianghuai, L.; Zhihong, Z. Research on the fatigue behavior of titanium based biomaterial coated with titanium nitride film by ion beam enhanced deposition. Surf. Coat. Technol. 1996, 88, 127–131. [Google Scholar] [CrossRef]
- Vadiraj, A.; Kamaraj, M. Characterization of fretting fatigue damage of PVD TiN coated biomedical titanium alloys. Surf. Coat. Technol. 2006, 200, 4538–4542. [Google Scholar] [CrossRef]
- Thull, R.; Trautner, K.; Karle, E.J. Testing of biomaterials. Biomed. Tech. 1992, 37, 162–169. [Google Scholar] [CrossRef]
- Mändl, S.; Krause, D.; Thorwarth, G.; Sader, R.; Zeilhofer, F.; Horch, H.H.; Rauschenbach, B. PIII treatment of medical implants. Surf. Coat. Technol. 2001, 142/144, 1046–1050. [Google Scholar] [CrossRef]
- Mändl, S.; Sader, R.; Krause, D.; Thorwarth, G.; Zeilhofer, H.-F.; Horch, H.H.; Rauschenbach, B. Investigation on plasma immersion ion implantation treated medical implants. Biomol. Eng. 2002, 19, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Keller, T. Osseointegration einer mit Plasma-Immersions-Ionen-Implantation behandelten Autokompressionsklammer aus Nitinol: eine tierexperimentelle Studie; Dissertation, Ludwig-Maximilians-Universität: München, Germany, 2004. [Google Scholar]
- Piscanec, S.; Ciacchi, L.C.; Vesselli, E.; Comelli, G.; Sbaizero, O.; Meriani, S.; De Vita, A. Bioactivity of TiN-coated titanium implants. Acta Mater. 2004, 52, 1237–1245. [Google Scholar] [CrossRef]
- Putters, J.L.M.; Kaulesar Sukul, D.M.K.S.; De Zeeuw, G.R.; Bijma, A.; Besselink, P.A. Comparative cell culture effects of shape memory metal (Nitinol), nickel and titanium: A biocompatibility estimation. Eur. Surg. Res. 1992, 24, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, R. Corrosion testing of stents: A novel fixture to hold entire device in deployed form and finish. J. Biomed. Mater. Res. 1999, 48, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Dodd, R.A.; Crone, W.C. Corrosion and wear-corrosion behaviour of NiTi modified by plasma source ion implantation. Biomaterials 2003, 24, 3931–3939. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, S.; Lindner, J.K.N.; Mändl, S. Determination of diffusing species from marker experiments in the system Ni-Ti-O. Nucl. Instrum. Meth. B 2007, 257, 714–717. [Google Scholar] [CrossRef]
- Yeung, K.W.K.; Poon, R.W.Y.; Chu, P.K.; Chung, C.Y.; Liu, X.Y.; Lu, W.W.; Chan, D.; Chan, S.C.W.; Luk, K.D.K.; Cheung, K.M.C. Surface mechanical properties, corrosion resistance, and cytocompatibility of nitrogen plasma-implanted nickel–titanium alloys: A comparative study with commonly used medical grade materials. J. Biomed. Mater. Res. A 2005, 82, 403–414. [Google Scholar]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Chatterjee-Fischer, R. Wärmebehandlung von Eisenwerkstoffen: Nitrieren und Nitrocarburieren; Expert Verlag: Renningen-Malmsheim, Germany, 1995. [Google Scholar]
- Gavriljuk, V.G.; Berns, H. High Nitrogen Steels; Springer: Berlin, Germany, 1999. [Google Scholar]
- Blawert, C.; Mordike, B.L.; Jirásková, Y.; Schneeweiss, O. Structure and composition of expanded austenite produced by nitrogen plasma immersion ion implantation of stainless steels X6CrNiTi1810 and X2CrNiMoN2253. Surf. Coat. Technol. 1999, 116/119, 189–198. [Google Scholar] [CrossRef]
- Menthe, E.; Rie, K.-T. Further investigation of the structure and properties of austenitic stainless steel after plasma nitriding. Surf. Coat. Technol. 1999, 116/119, 199–204. [Google Scholar] [CrossRef]
- Ichii, K.; Fujimara, K.; Takase, T. Structure of the ion-nitrided layer of 18-8 stainless steel. Technol. Rep. Kansai Univ. 1986, 27, 135–144. [Google Scholar]
- Marchev, K.; Landis, M.; Vallerio, R.; Cooper, C.V.; Giessen, B.C. The m phase layer on ion nitrided austenitic stainless steel (III): An epitaxial relationship between the m phase and the γ parent phase and a review of structural identifications of this phase. Surf. Coat. Technol. 1999, 116/119, 184–188. [Google Scholar] [CrossRef]
- Williamson, D.L.; Davis, J.A.; Wilbur, P.J. Effect of austenitic stainless steel composition on low-energy, high-flux, nitrogen ion beam processing. Surf. Coat. Technol. 1998, 103/104, 178–184. [Google Scholar] [CrossRef]
- Williamson, D.L.; Davis, J.A.; Wilbur, P.J.; Vajo, J.J.; Wei, R.; Matossian, J.N. Relative roles of ion energy, ion flux, and sample temperature in low-energy nitrogen ion implantation of Fe-Cr-Ni stainless steel. Nucl. Instrum. Meth. B 1997, 127/128, 930–934. [Google Scholar] [CrossRef]
- Collins, G.A.; Hutchings, R.; Short, K.T.; Tendys, J. Ion-assisted surface modification by plasma immersion ion implantation. Surf. Coat. Technol. 1998, 103/104, 212–217. [Google Scholar] [CrossRef]
- Williamson, D.L.; Öztürk, O.; Wei, R.; Wilbur, J.P. Metastable phase formation and enhanced diffusion in f.c.c. alloys under high dose, high flux nitrogen implantation at high and low ion energies. Surf. Coat. Technol. 1994, 65, 15–23. [Google Scholar] [CrossRef]
- Möller, W.; Parascandola, S.; Kruse, O.; Günzel, R.; Richter, E. Plasma-immersion ion implantation for diffusive treatment. Surf. Coat. Technol. 1999, 116-119, 1–10. [Google Scholar] [CrossRef]
- Parascandola, S.; Möller, W.; Williamson, D.L. The nitrogen transport in austenitic stainless steel at moderate temperatures. Appl. Phys. Lett. 2000, 76, 2194–2196. [Google Scholar] [CrossRef]
- Mändl, S.; Rauschenbach, B. Concentration dependent nitrogen diffusion coefficient in expanded austenite formed by ion implantation. J. Appl. Phys. 2002, 91, 9737–9742. [Google Scholar] [CrossRef]
- Christiansen, T.; Somers, M.A.J. Controlled dissolution of colossal quantities of nitrogen in stainless steel. Metall. Mater. Trans. A 2006, 37, 675–682. [Google Scholar] [CrossRef]
- Mändl, S.; Rauschenbach, B. Anisotropic strain in nitrided austenitic stainless steel. J. Appl. Phys. 2000, 88, 3323–3329. [Google Scholar] [CrossRef]
- Fewell, M.P.; Priest, J.M. High-order diffractometry of expanded austenite using synchrotron radiation. Surf. Coat. Technol. 2008, 202, 1802–1815. [Google Scholar] [CrossRef]
- Christiansen, T.; Somers, M.A.J. Reconstruction of stress and composition profiles from X-ray diffraction experiments - how to avoid ghost stresses? Mater. Sci. Forum 2004, 443/444, 91–94. [Google Scholar] [CrossRef]
- Mändl, S. Nitriding of stainless steel: PIII or low energy nitriding? Plasma Proc. Polymers 2007, 4, 239–245. [Google Scholar] [CrossRef]
- Rissanen, L.; Neubauer, M.; Lieb, K.P.; Schaaf, P. The new cubic iron-nitride phase FeN prepared by reactive magnetron sputtering. J. Alloys Comp. 1998, 274, 74–82. [Google Scholar] [CrossRef]
- Manova, D.; Mändl, S.; Neumann, H.; Rauschenbach, B. Wear behaviour of martensitic stainless steels after PIII surface treatment. Surf. Coat. Technol. 2005, 200, 137–140. [Google Scholar] [CrossRef]
- Leitão, E.; Barbosa, M.A.; De Groot, K. In vitro testing of surface-modified biomaterials. J. Mater. Sci.: Mater. Med. 1998, 9, 543–548. [Google Scholar] [CrossRef]
- Arslan, E.; Iğdil, M.C.; Yazici, H.; Tamerler, C.; Bermek, H.; Trabzon, L. Mechanical properties and biocompatibility of plasma-nitrided laser-cut 316L cardiovascular stents. J. Mater. Sci.: Mater. Med. 2008, 19, 2079–2086. [Google Scholar] [CrossRef]
- Leitão, E.; Silva, R.A.; Barbosa, M.A. Electrochemical and surface modifications on N+-ion-implanted 316L stainless steel. J. Mater. Sci.: Mater. Med. 1997, 8, 365–368. [Google Scholar] [CrossRef]
- Fernandez, E.G.; Carrie, D.; Betriu, A.; Rumoroso, J.R.; Serra, A.; Puigfel, M. Bionert stent angiographic study. Intervent. Card. 2008, 3, 55–58. [Google Scholar] [CrossRef]
- Bell, T. Current status of supersaturated surface engineered S-phase materials. Key Eng. Mater. 2008, 373/374, 289–295. [Google Scholar] [CrossRef]
- Milošev, I.; Strehblow, H.H. The composition of the surface passive film formed on CoCrMo alloy in simulated physiological solution. Electrochim. Acta 2003, 48, 2767–2774. [Google Scholar] [CrossRef]
- Doorn, P.F.; Campbell, P.A.; Worrall, J.; Benya, P.D.; McKellop, H.A. Metal wear particle characterization from metal on metal hip replacements: Transmission electron microscopy study of periprosthetic tissues and isolated particles. J. Biomed. Mater. Res. A 1998, 42, 103–111. [Google Scholar] [CrossRef]
- Escobedo, J.C.; Ortiz, J.C.; Almanza, J.M.; Cortés, D.A. Hydroxyapatite coating on a cobalt base alloy by investment casting. Scripta Mater. 2006, 54, 1611–1615. [Google Scholar] [CrossRef]
- Tomita, A.; Kusuda, M.; Otsuki, S.; Oka, Y.; Nishimura, Y.; Murakami, A.; Yatsuzuka, M. Enhancement of adhesive strength of DLC film prepared by PBIID on Co–Cr alloy for biomaterial. Thin Solid Films 2006, 506/507, 59–62. [Google Scholar] [CrossRef]
- Maruyama, M.; Capello, W.N.; D’Antonio, J.A.; Jaffe, W.L.; Bierbaum, B.E. Effect of low-friction ion-treated femoral heads on polyethylene wear rates. Clin. Orthop. 2000, 370, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, D.; Ogawa, M.; Hara, Y.; Nishimura, Y.; Odusanya, O.; Azuma, K.; Matsuda, S.; Yatsuzuka, M.; Murakami, A. Effect of nitrogen plasma-based ion implantation on joint prosthetic material. Surf. Coat. Technol. 2002, 156, 301–305. [Google Scholar] [CrossRef]
- Lutz, J.; Mändl, S. Wear mechanism, wear rate and contact pressure in PIII nitrided CoCr alloys. Plasma Proc. Polymers 2009, 6. [Google Scholar] [CrossRef]
- Eichentopf, I.-M.; Lehmann, A.; Lutz, J.; Gerlach, J.W.; Mändl, S. Mechanical surface properties of cocr alloys after nitrogen PIII. Plasma Proc. Polymers 2007, 4, S44–S48. [Google Scholar] [CrossRef]
- Lutz, J.; Lehmann, A.; Mändl, S. Nitrogen diffusion in medical CoCrNiW alloys after plasma immersion ion implantation. Surf. Coat. Technol. 2008, 202, 3747–3753. [Google Scholar] [CrossRef]
- Lutz, J.; Mändl, S. Effect of ion energy on layer growth processes during nitriding of CoCr alloys. Nucl. Instrum. Meth. B 2009, 267, 1522–1525. [Google Scholar] [CrossRef]
- Öztürk, O.; Türkan, U.; Eroğlu, A.E. Metal ion release from nitrogen ion implanted CoCrMo orthopedic implant material. Surf. Coat. Technol. 2006, 200, 5687–5697. [Google Scholar] [CrossRef]
- Diaz, C.; Lutz, J.; Mändl, S.; García, J.A.; Martínez, R.; Rodríguez, R.J. Improved tribo-corrosion of biomedical alloys by ion implantation techniques. Nucl. Instrum. Meth. B 2009, 267, 1630–1633. [Google Scholar] [CrossRef]
- Saris, N.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An update on physiological, clinical and analytical aspects. Clinica Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. takes for Calcium, Phosphorus, Magn. Vitamin D, and Fluoride; National Academy Press: Washington, DC, USA, 1997; pp. 190–249. [Google Scholar]
- Lambotte, A. L’utilisation du magnesium comme materiel perdu dans l’osteosynthèse. Bull. Mém. Soc. Nat. Chir. 1932, 28, 1325–1334. [Google Scholar]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Kainer, K.U. Magnesium-Eigenschaften, Anwendungen, Potentiale; VCH: Weinheim, Germany, 2000. [Google Scholar]
- Witte, F.; Reifenrath, J.; Mueller, P.P.; Crostack, H.A.; Nellesen, J.; Bach, F.W.; Bormann, D.; Rudert, M. Cartilage repair on magnesium scaffolds used as a subchondral bone replacement. Mat.- wiss. u. Werkstofftech. 2006, 37, 504–508. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Bohne, Y.; Seeger, D.M.; Blawert, C.; Dietzel, W.; Mändl, S.; Rauschenbach, B. Influence of ion energy on properties of mg alloy thin films formed by ion beam sputter deposition. Surf. Coat. Technol. 2006, 200, 6527–6532. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raman, R.K.S. In vitro degradation and mechanical integrity of calcium-containing magnesium alloys in modified-simulated body fluid. Biomaterials 2008, 29, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-C.; Kim, J.-G.; Lee, J.-Y.; Seok, H.-K. Influence of Ca on the corrosion properties of magnesium for biomaterials. Mater. Lett. 2008, 62, 4146–4148. [Google Scholar] [CrossRef]
- Li, Z.J.; Gu, X.N.; Lou, S.Q.; Zheng, Y.F. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Estrin, Y.; Fu, H.; Song, G.; Zúberová, Z. The effect of pre-processing andgrain structure on the bio-corrosion and fatigue resistance of magnesium alloy AZ31. Adv. Eng. Mater. 2007, 9, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.L.; Xu, L.P.; Yang, K. Formation by ion plating of Ti-coating on pure Mg for biomedical applications. Scr. Mater. 2005, 53, 523. [Google Scholar] [CrossRef]
- Liu, C.L.; Xin, Y.C.; Tian, X.B.; Zhao, J.; Chu, P.K. Corrosion resistance of titanium ion implanted AZ91 magnesium alloy. J. Vac. Sci. Technol. A 2007, 25, 334–339. [Google Scholar] [CrossRef]
- Bohne, Y.; Manova, D.; Blawert, C.; Störmer, M.; Dietzel, W.; Mändl, S. Deposition and properties of novel microcrystalline Mg alloy coatings. Surf. Eng. 2007, 23, 339–343. [Google Scholar] [CrossRef]
- Blawert, C.; Lutz, J.; Prager-Duschke, A.; Scharnagl, N.; Störmer, M.; Manova, D.; Mändl, S. Different underlying corrosion mechanism for mg bulk alloys and mg thin films. Plasma Proc. Polymers 2009, 6. [Google Scholar] [CrossRef]
- Wang, H.; Eliaz, N.; Xiang, Z.; Hsu, H.-P.; Spector, M.; Hobbs, L.W. Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy. Biomaterials 2006, 27, 4192–4203. [Google Scholar] [CrossRef] [PubMed]
- Vetrone, F.; Variola, F.; de Oliveira, P.T.; Zalzal, S.F.; Yi, J.-H.; Sam, J.; Bombonato-Prado, K.F.; Sarkissian, A.; Perepichka, D.F.; Wuest, J.D.; Rosei, F.; Nanci, A. Nanoscale oxidative patterning of metallic surfaces to modulate cell activity and fate. Nano Lett. 2009, 9, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.; Iwaya, Y.; Kono, H.; Sato, H. Surface modification of titanium by etching in concentrated sulfuric acid. Dent. Mater. 2006, 22, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Li, Y.C.; Lin, J.G.; Yamada, Y.; Hodgson, P.D.; Wen, C.E. In vitro bioactivity evaluation of titanium and niobium metals with different surface morphologies. Acta Biomater. 2008, 4, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mändl, S. Increased Biocompatibility and Bioactivity after Energetic PVD Surface Treatments. Materials 2009, 2, 1341-1387. https://doi.org/10.3390/ma2031341
Mändl S. Increased Biocompatibility and Bioactivity after Energetic PVD Surface Treatments. Materials. 2009; 2(3):1341-1387. https://doi.org/10.3390/ma2031341
Chicago/Turabian StyleMändl, Stephan. 2009. "Increased Biocompatibility and Bioactivity after Energetic PVD Surface Treatments" Materials 2, no. 3: 1341-1387. https://doi.org/10.3390/ma2031341



