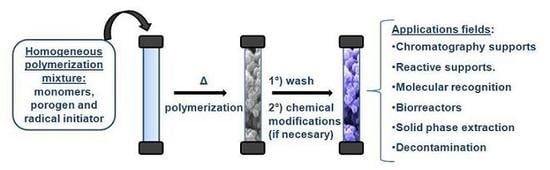
Macroporous Monolithic Polymers: Preparation and Applications
Abstract
:1. Introduction
1.1. Mass Transport in Processes That Use Polymers in Particle Form
1.2. Increase in the Mass Transfer by Convection
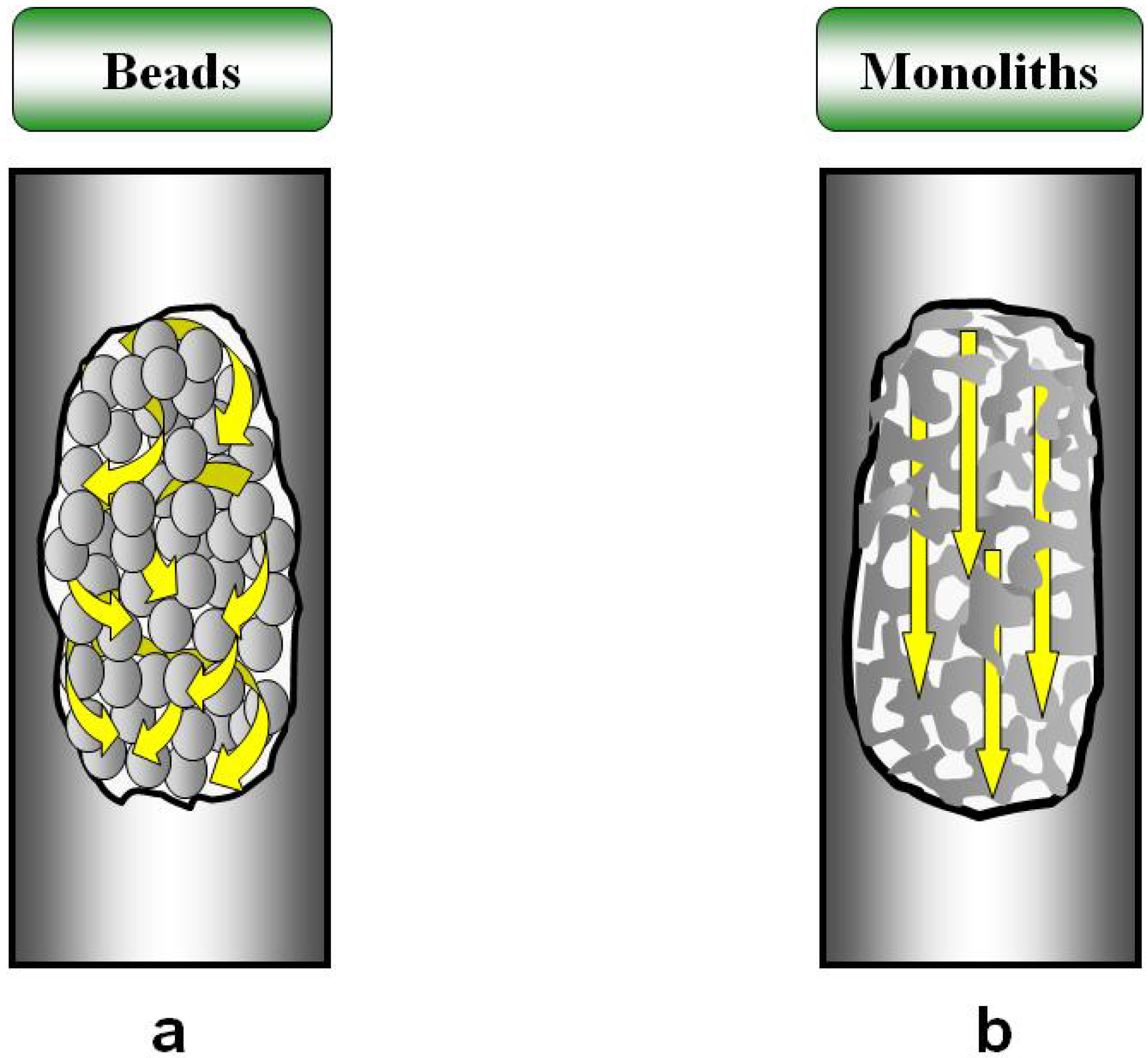
2. Continuous Macroporous Rods

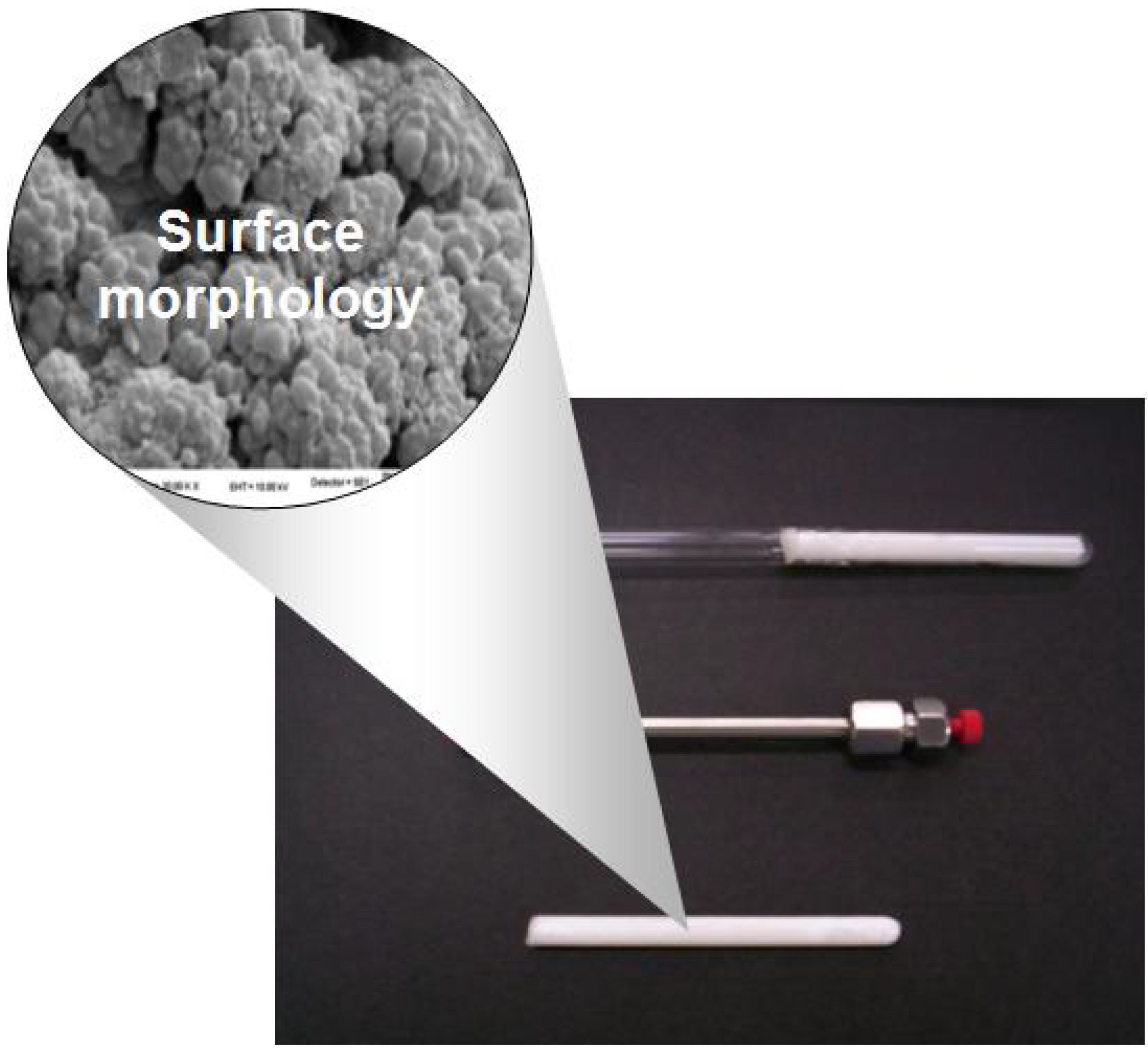
3. Classical Mechanism of Pore Formation in Macroporous Polymers
Formation of Pores in a Tube during Polymerization
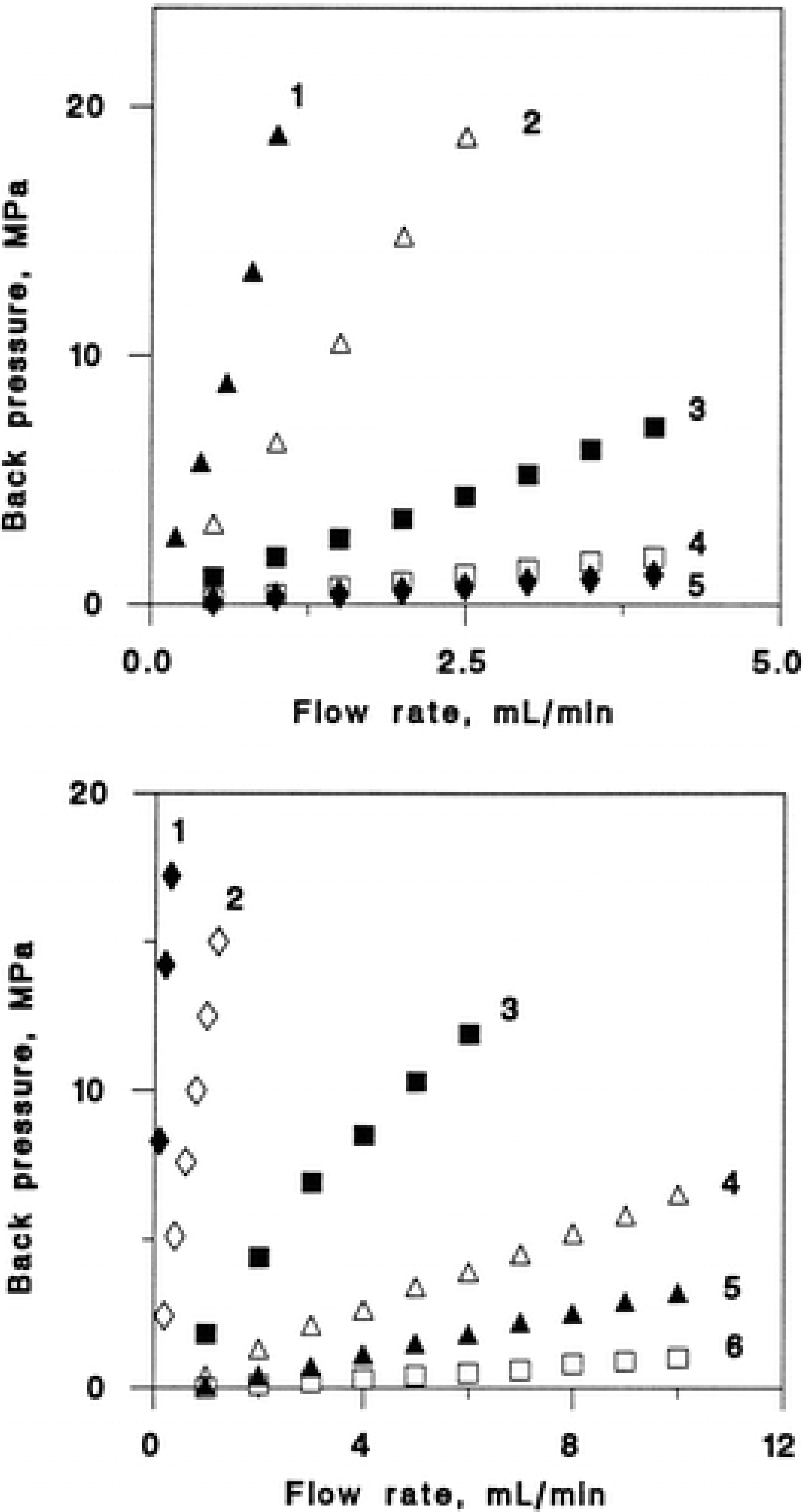
4. Hydrodynamic Properties
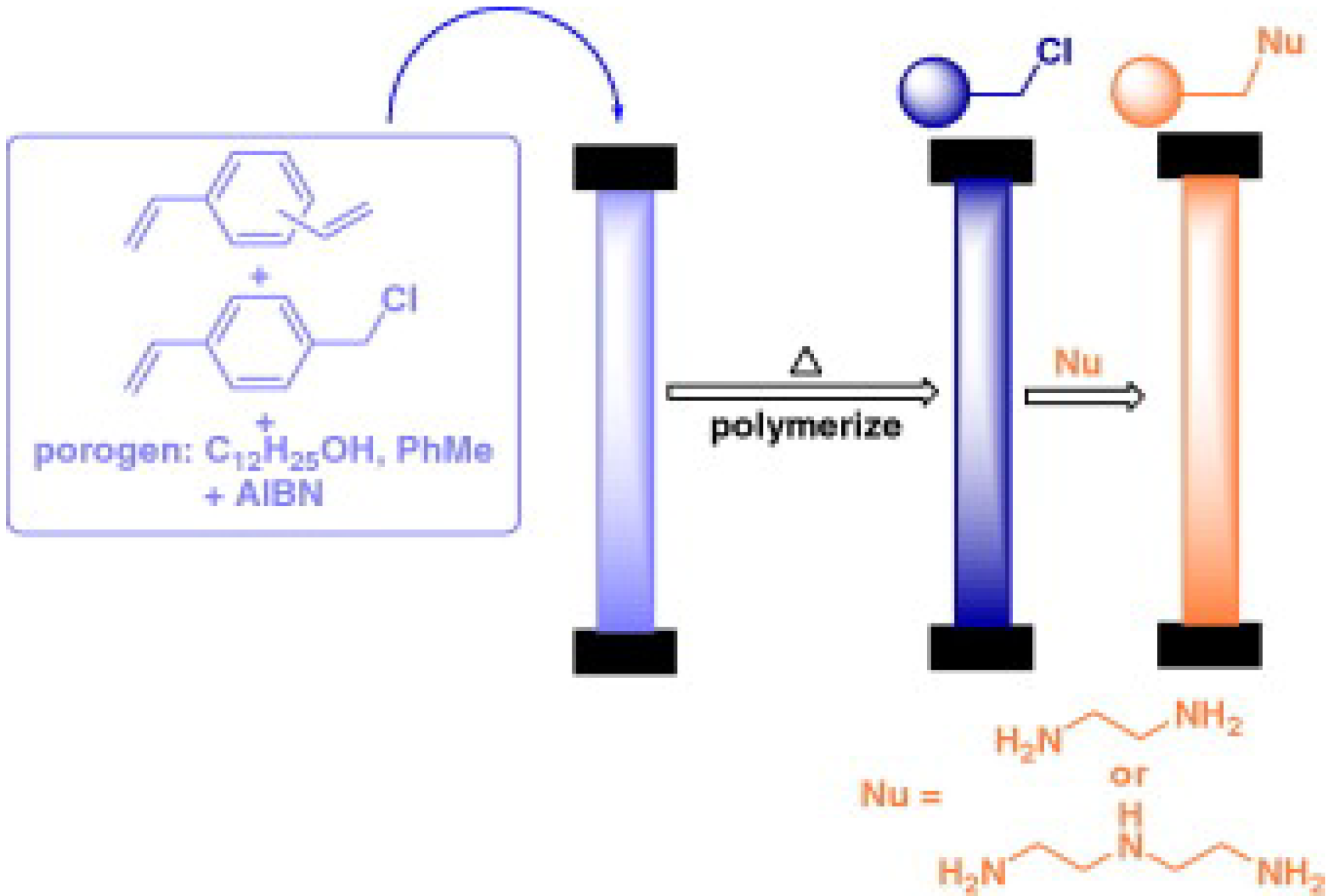
5. Chemistry of Polymer Rods
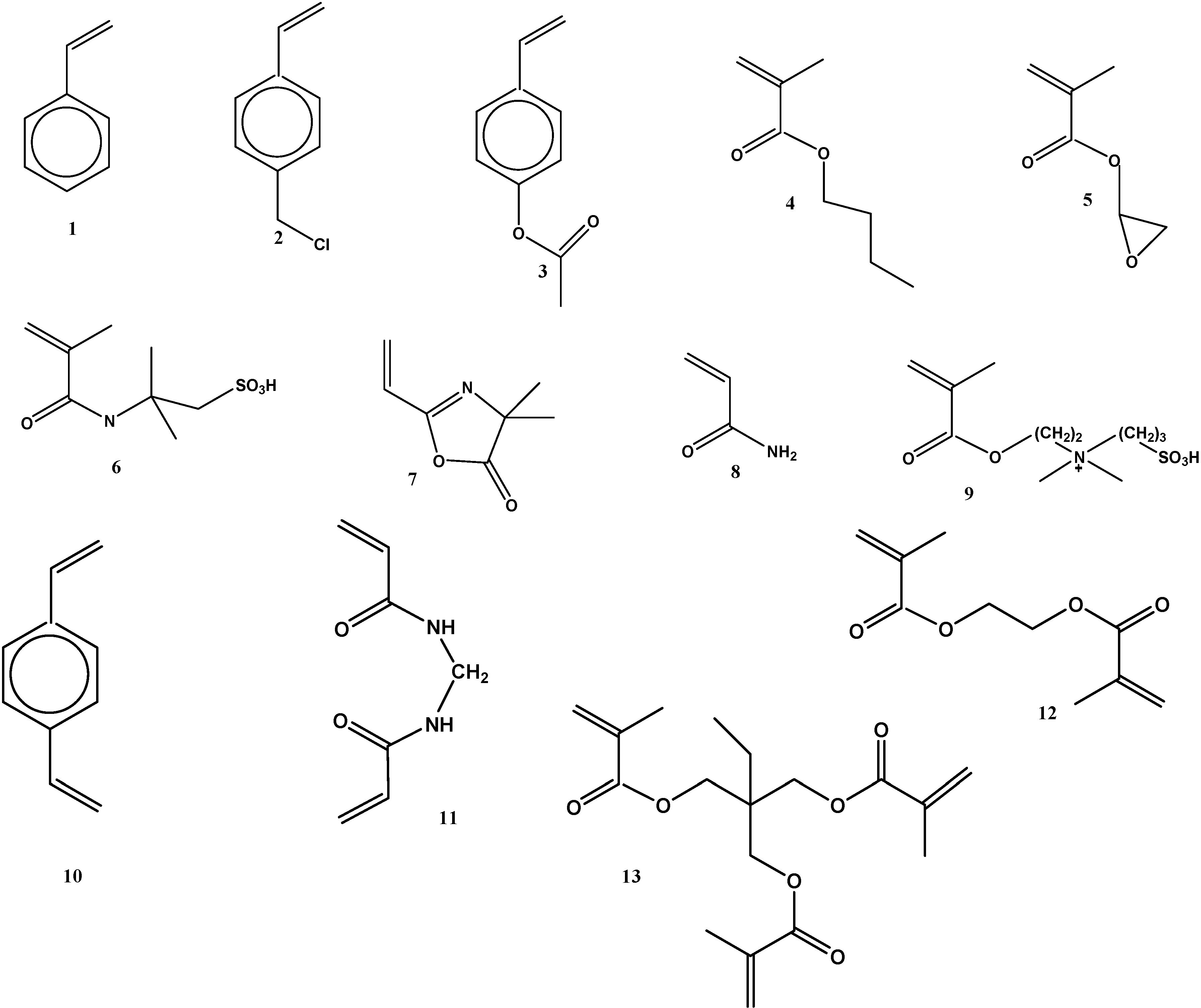
5.1. Chemical Structure of the Monomers and/or Post-Chemical Derivatization Reactions on the Product
5.2. Surface Grafting
5.3. Synthesis of Molecularly Imprinted Monoliths
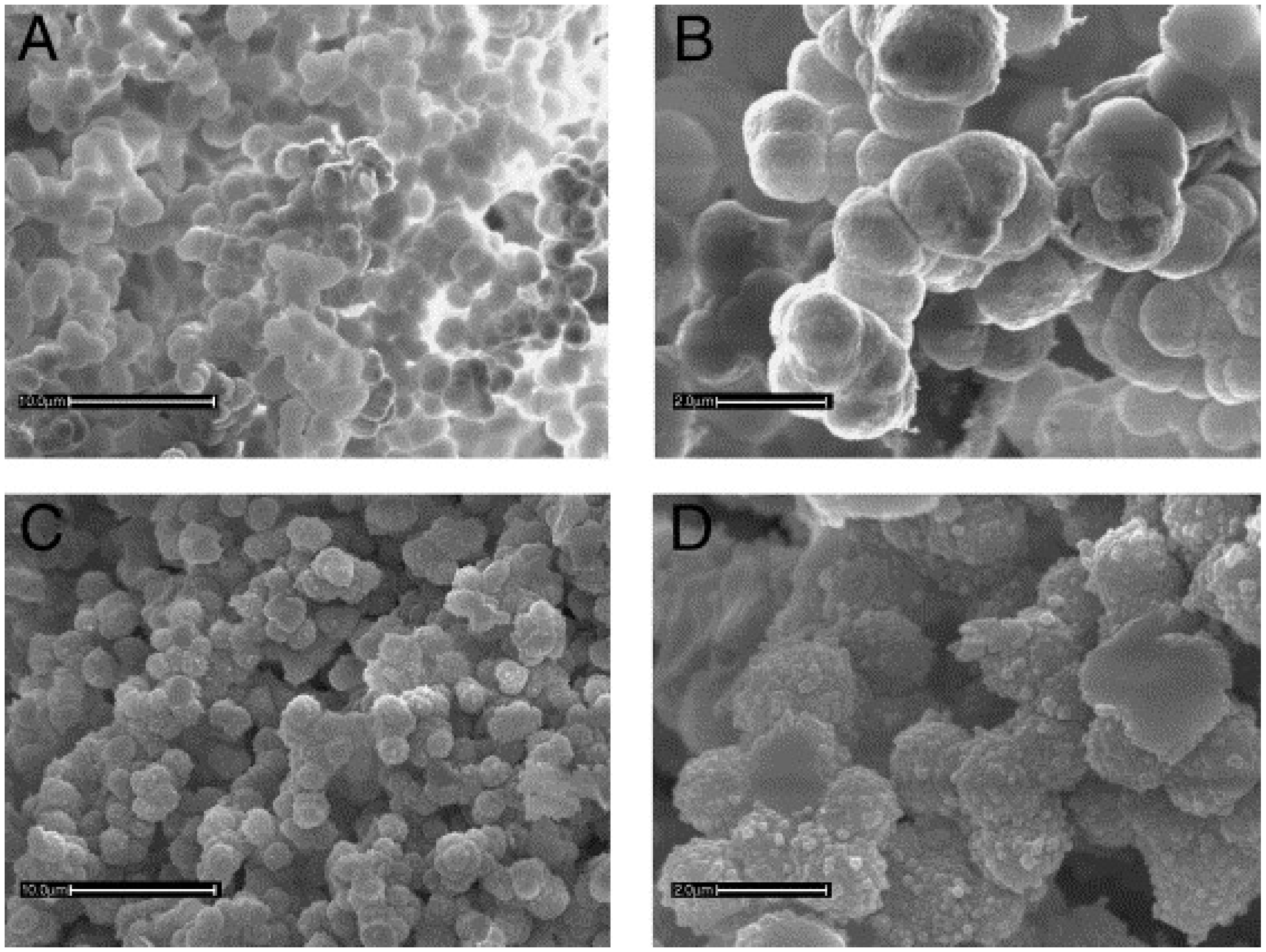
6. Important Applications
6.1. Continuous Porous Polymers as Stationary Phases in HPLC

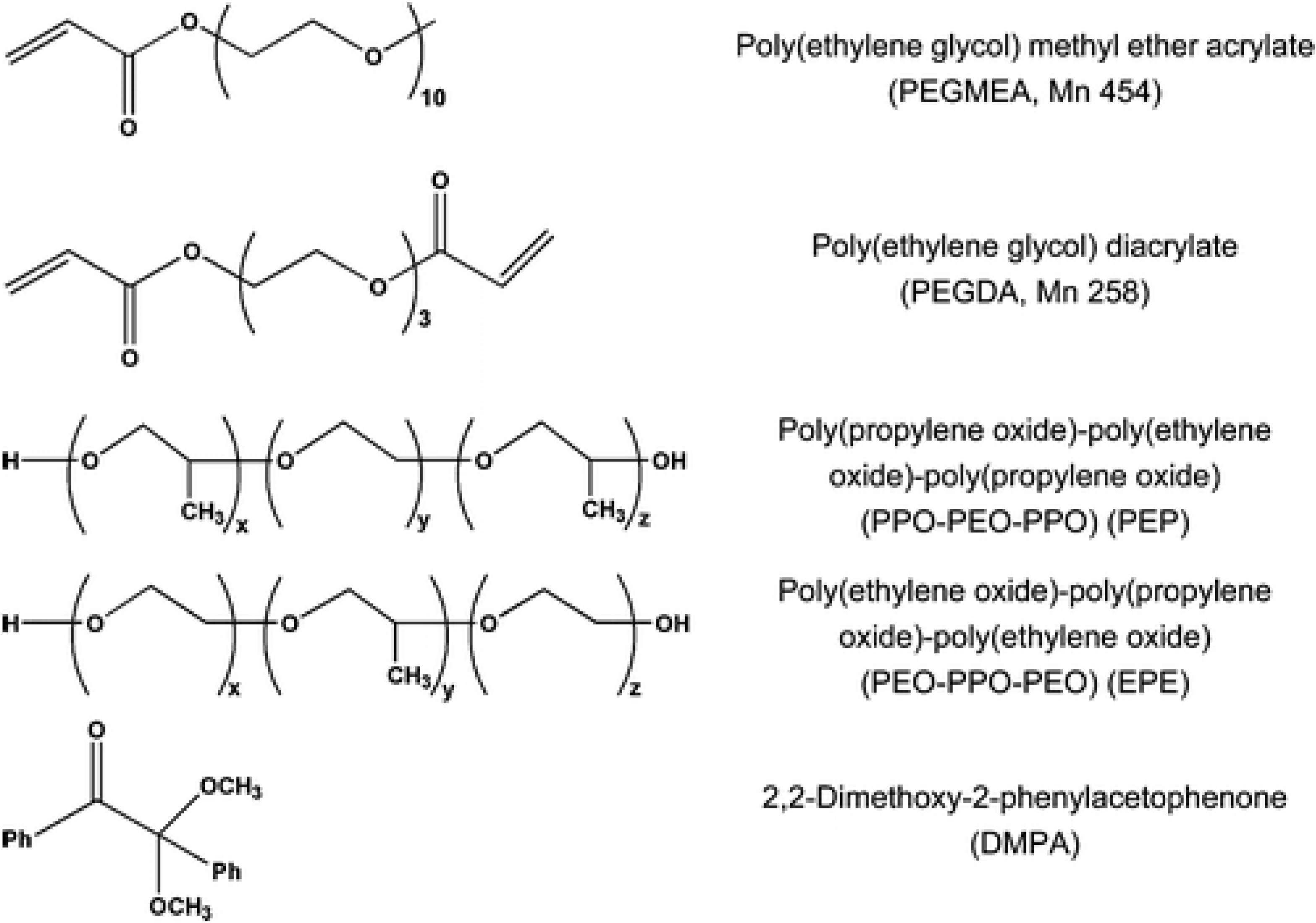
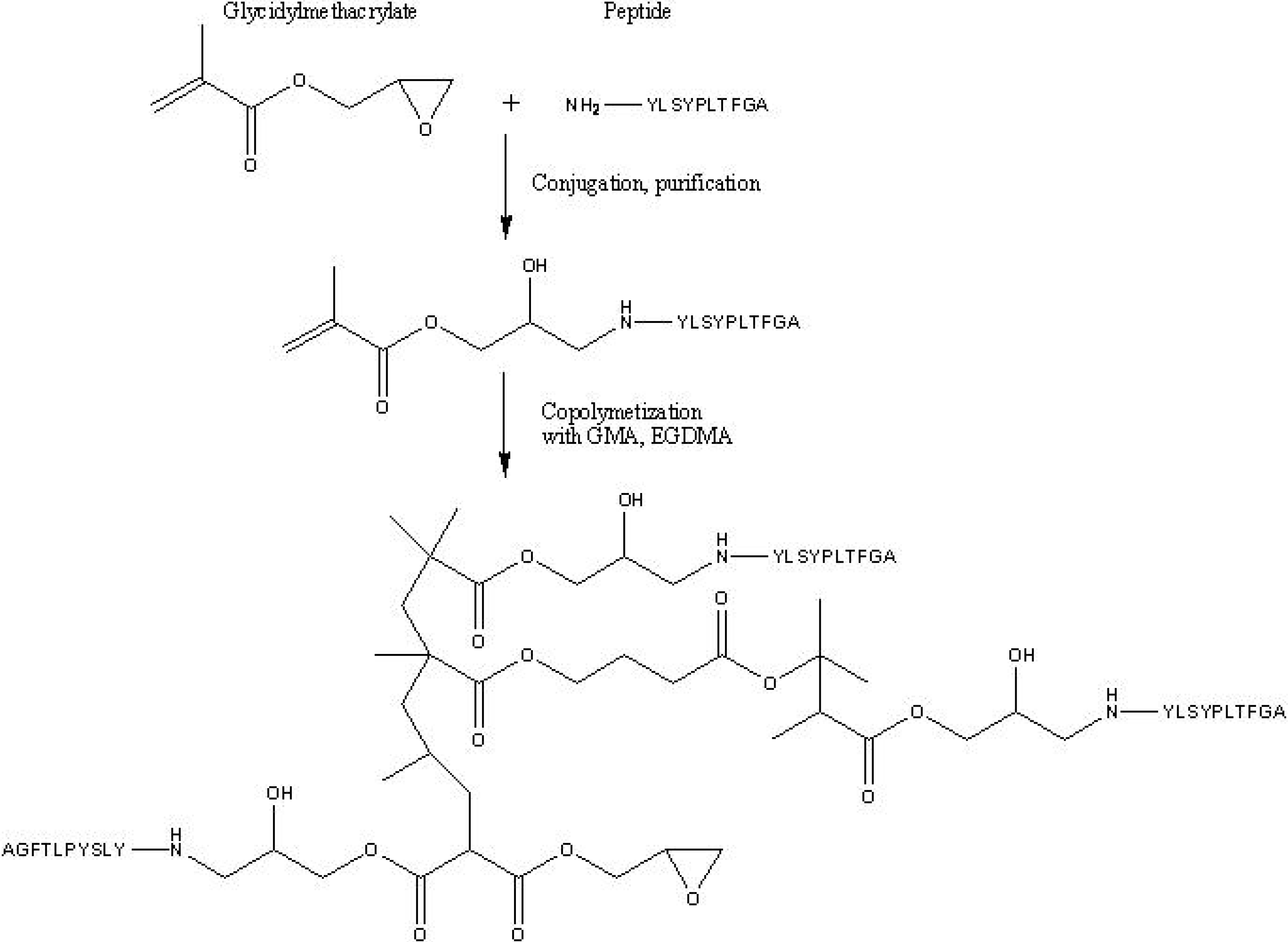
6.2. Continuous Porous Polymers as Reactive Supports
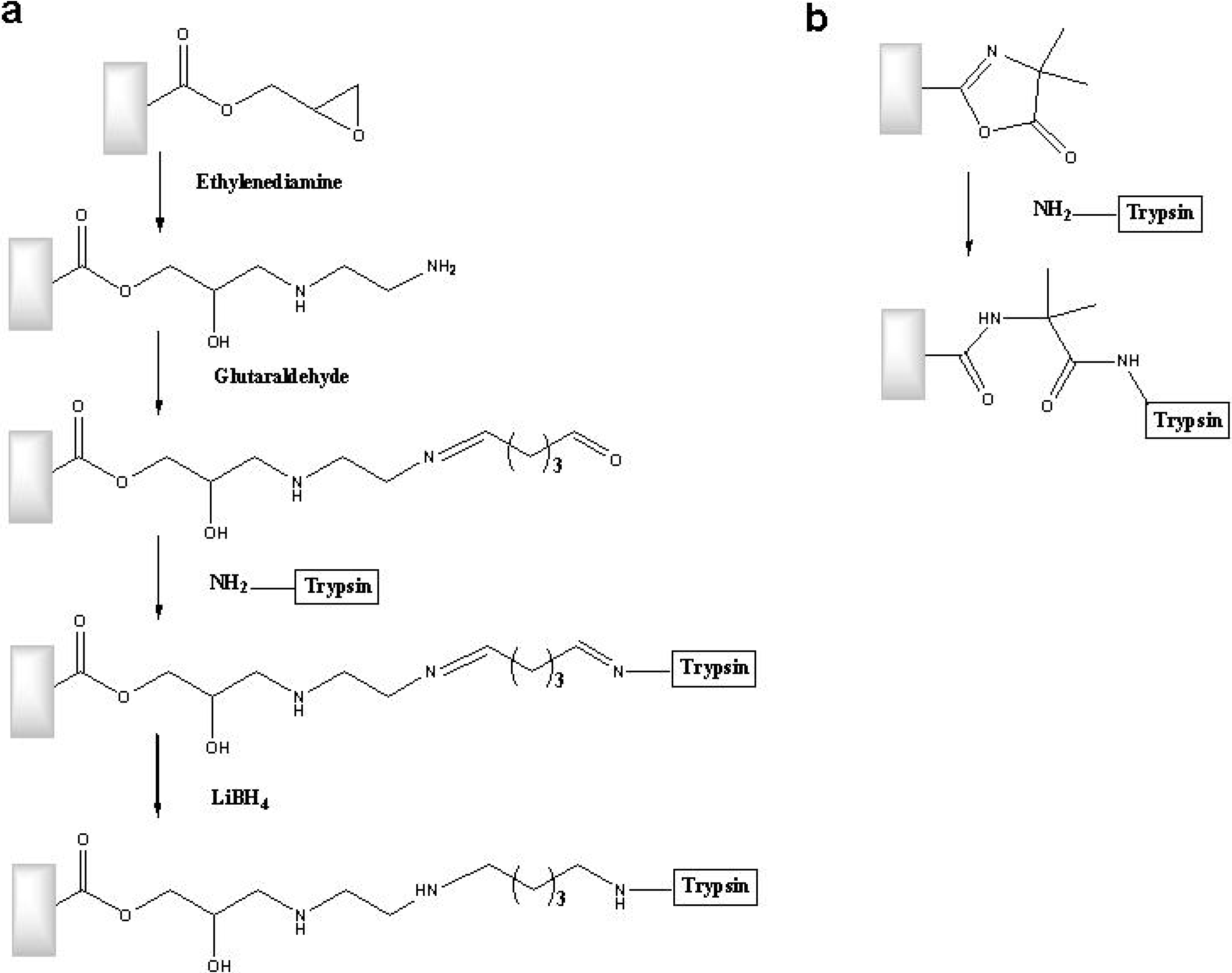
6.3. Continuous Porous Polymers as Bioreactors
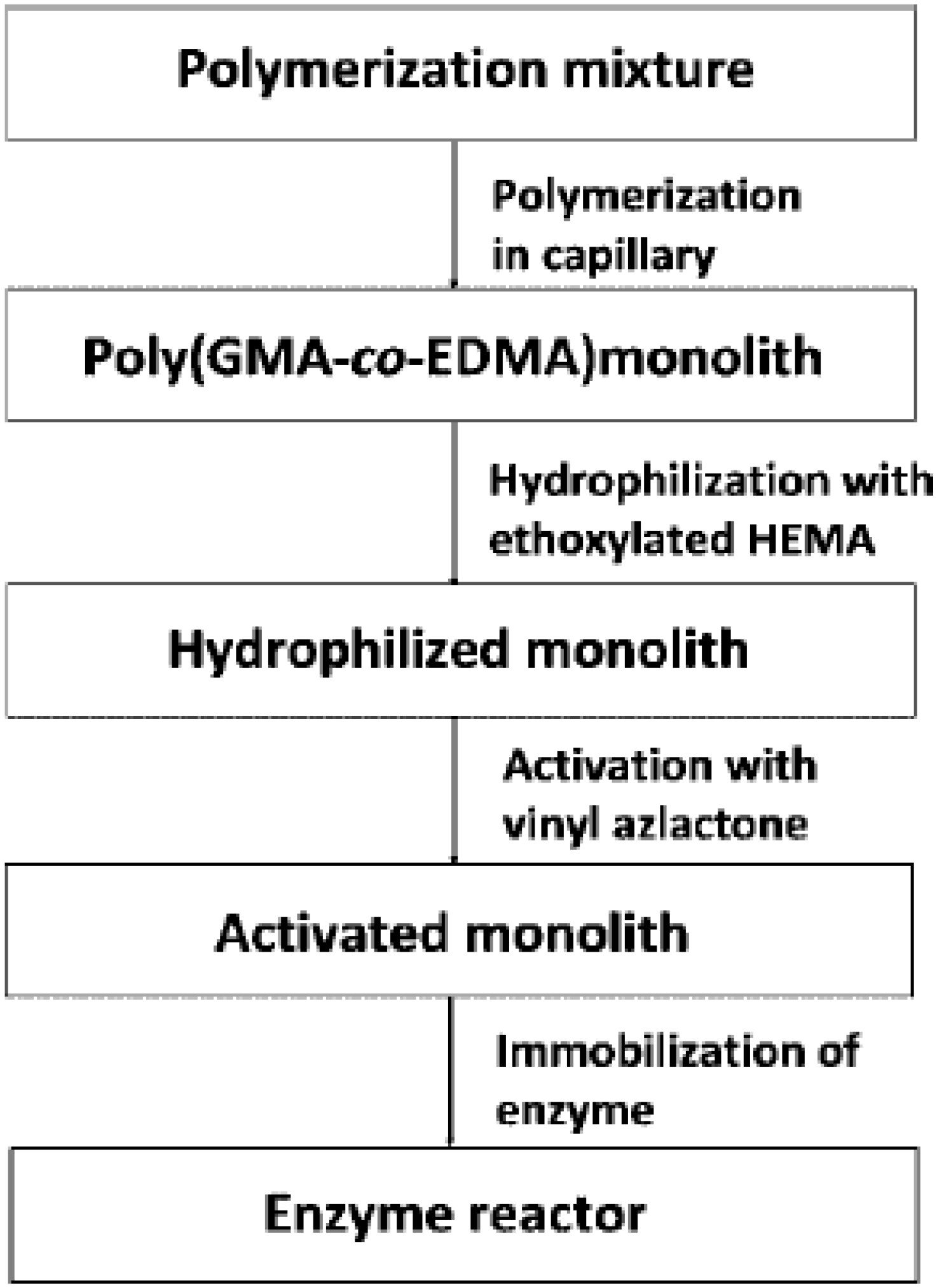
6.4. Continuous Porous Polymers in Detection in Solid-Phase
6.5. Continuous Porous Polymers in Solid-Phase Extraction
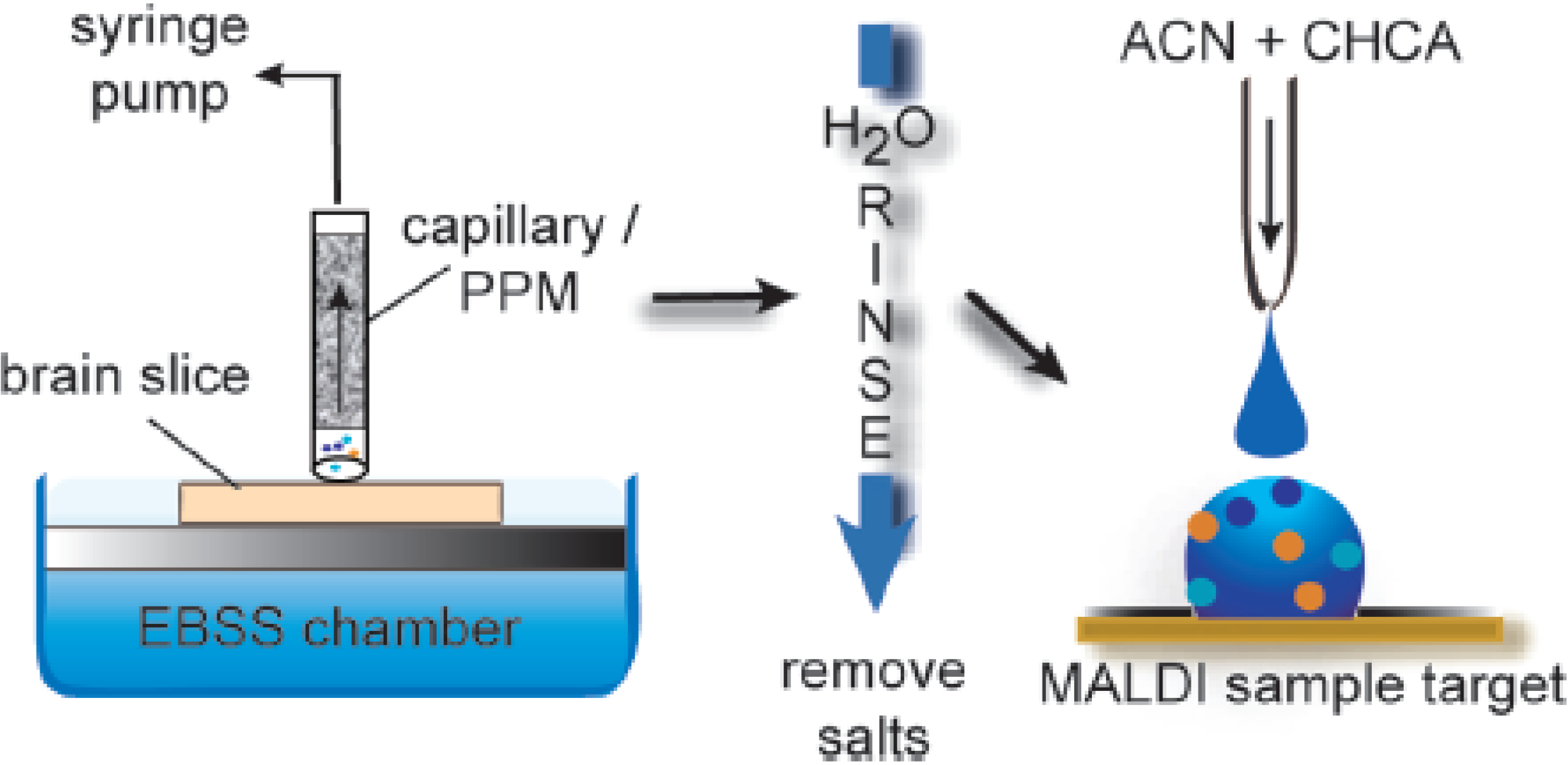
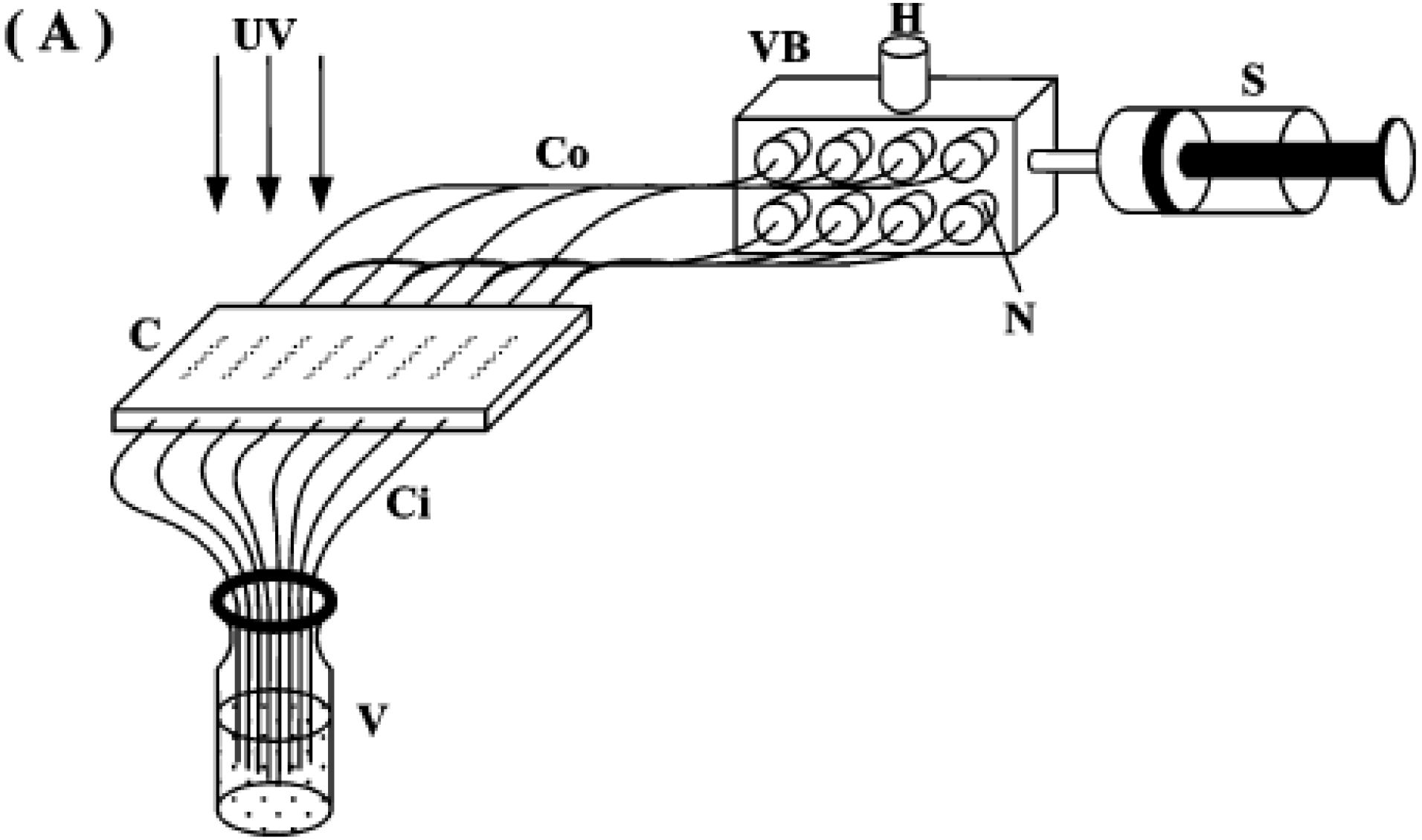
6.6. Continuous Porous Polymers in Decontamination
7. Concluding Remarks
Acknowledgments
References and Notes
- Kubín, M.; Špaček, P.; Chromeček, R. Gel permeation chromatography on porous poly(ethylene glycol methacrylate). Coll. Czechosl. Chem. Commun. 1967, 32, 3881–3887. [Google Scholar] [CrossRef]
- Qi, T.; Sonoda, A.; Makita, Y.; Kanoh, H.; Ooi, K.; Hirotsu, T. Synthesis and borate uptake of two novel chelating resins. Ind. Eng. Chem. Res. 2002, 41, 133–138. [Google Scholar] [CrossRef]
- Viklund, C.; Svec, F.; Fréchet, J.M.J.; Irgum, K. Monolithic "molded", porous materials with high flow characteristics for separations, catalysis, or solid-phase chemistry: Control of porous properties during polymerization. Chem. Mater. 1996, 8, 744–750. [Google Scholar] [CrossRef]
- Cooper, A.I.; Holmes, A.B. Synthesis of molded monolithic porous polymers using supercritical carbon dioxide as the porogenic solvent. Adv. Mat. 1999, 11, 1270–1274. [Google Scholar] [CrossRef]
- Hebb, A.; Senoo, K.; Bhat, R.; Cooper, A. Structural control in porous cross-linked poly(methacrylate) monoliths using supercritical carbon dioxide as a ‘pressure-adjustable’ porogenic solvent. Chem. Mater. 2003, 15, 2061–2069. [Google Scholar] [CrossRef]
- Xie, S.; Svec, F.; Fréchet, J.M.J. Preparation of porous hydrophilic monoliths: Effect of the polymerization conditions on the porous properties of poly (acrylamide-co-N,N´-methylenebisacrylamide) monolithic rods. J. Polym. Sci. A Polym. Chem. 1997, 35, 1013–1021. [Google Scholar] [CrossRef]
- Viklund, C.; Irgum, K.; Svec, F.; Fréchet, J.M.J. Preparation of porous poly(styrene-co-divinylbenzene) monoliths with controlled pore size distributions initiated by stable free radicals and their pore surface functionalization by grafting. Macromolecules 2001, 34, 4361–4369. [Google Scholar] [CrossRef]
- Svek, F.; Huber, C. Monolithic materials: Promises, challenges, achievements. Anal. Chem. 2006, 2101–2107. [Google Scholar]
- Svec, F.; Fréchet, J.M.J. Molded rigid monolithic porous polymers: an inexpensive, efficient, and versatile alternative to beads for the design of materials for numerous applications. Ind. Eng. Chem. Res. 1999, 38, 34–48. [Google Scholar] [CrossRef]
- Vlakh, E.G.; Tennikova, T.B. Applications of polymethacrylate-based monoliths in high-performance liquid chromatography. J. Chromatogr. A 2009, 1216, 2637–2650. [Google Scholar] [CrossRef] [PubMed]
- Nir, A.; Pismen, L. Simultaneous intraparticle forced convection, diffusion and reaction in a porous catalyst. Chem. Eng. Sci. 1977, 32, 35–41. [Google Scholar] [CrossRef]
- Afeyan, N.B.; Gordon, N.F.; Mazsaroff, I.; Varady, L.; Fulton, S.P.; Yang, Y.B.; Regnier, F.E. Flow-through particles for the high-performance liquid chromatographic separation of biomolecules: perfusion chromatography. J. Chromatogr. 1990, 519, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.E.; Lu, Z.P.; Loureiro, J.M.; Carta, G. Peak resolution in linear chromatography. Effects of intraparticle convection. J. Chromatogr. A 1993, 653, 189–198. [Google Scholar] [CrossRef]
- Miller, S. Separations in a monolith. Anal. Chem. 2004, 76, 5, 99–101. [Google Scholar] [CrossRef]
- Hjertén, S.; Liao, J.L.; Zhang, R. High-performance liquid chromatography on continuous polymer beds. J. Chromatogr. 1989, 473, 273–275. [Google Scholar] [CrossRef]
- Tennikova, T.B.; Svec, F.; Belenkii, B.G. High performance membrane chromatography. A novel method of protein separation. J. Liq. Chromatogr. 1990, 13, 63–70. [Google Scholar] [CrossRef]
- Svec, F.; Frechét, J.M.J. Continuous rods of macroporous polymer as high-performance liquid chromatography separation media. Anal. Chem. 1992, 64, 820–822. [Google Scholar] [CrossRef]
- Minakuchi, H.; Nakanishi, K.; Soga, N.; Ishizuka, N.; Tanaka, N. Octadecylsilylated porous silica rods as separation media for reversed-phase liquid chromatography. Anal. Chem. 1996, 68, 3498–3501. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kobayashi, H.; Nakanishi, H.; Minakuchi, H.; Ishizuka, N. Monolithic LC columns. Anal. Chem. 2001, 73, A420–A429. [Google Scholar] [CrossRef]
- Siouffi, A. Silica gel-based monoliths prepared by the sol-gel method: Facts and figures. J. Chromatogr. A 2003, 1000, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Podgornik, A.; Barut, M.; Strancar, A.; Josic, D.; Koloini, T. Construction of large-volume monolithic columns. Anal. Chem. 2000, 72, 5693–5699. [Google Scholar] [CrossRef] [PubMed]
- Guiochon, G. Monolithic columns in high performance liquid chromatography. J. Chromatogr. A 2007, 1168, 101–168. [Google Scholar] [CrossRef] [PubMed]
- Unger, K.; Skudas, R.; Schulte, M.M. Particle packed columns and monolithic columns in high-performence liquid chromatography-comparison and critical appraisal. J. Chromatogr. A 2008, 1184, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Švec, F. Porous monoliths: Emerging stationary phases for HPLC and related methods. LC-GC North America Suppl.: LC Column Technol. Suppl. 2004, June, 18–21. [Google Scholar]
- Švec, F. High throughput peptide mass mapping using an integrated capillary device coupled to a mass spectrometer. LC-GC Europe 2005, 17, 2–5. [Google Scholar]
- Švec, F.; Geiser, L. Monolithic stationary phases for HPLC and sample preparation. LCGC North America 2006, 24, 22–27. [Google Scholar]
- Wu, R.; Hu, L.; Wang, F.; Ye, M.; Zou, H. Recent development of monolithic stationary phases with emphasis on microscale chromatographic separation. J. Chromatogr. A 2008, 1184, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Švec, F. Less common applications of monoliths: Preconcentration and solid phase extraction. J. Chromatogr. B 2006, 841, 52–64. [Google Scholar] [CrossRef]
- Svec, F.; Kurganov, A.A. Less common applications of monoliths III. Gas chromatography. J. Chromatogr. A. 2008, 1184, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Vicklund, C.; Ponten, E.; Glad, B.; Irgum, K.; Horstedt, P.; Svec, F. “Molded” macroporous poly(glycidyl methacrylate-co-trimethylolpropane trimethacrylate) Materials with fine controlled porous properties: preparation of monoliths using photoinitiated polymerization. Chem. Mater. 1997, 9, 463–471. [Google Scholar] [CrossRef]
- Peters, E.; Svec, F.; Frechet, J.M.J. Preparation of large-diameter “molded” porous polymer monoliths and the control of pore structure homogeneity. Chem. Mater. 1997, 9, 1898–1902. [Google Scholar] [CrossRef]
- Nguyen, A.M.; Irgum, K. Epoxy-based monoliths. A novel hydrophilic separation material for liquid chromatography of biomolecules. Chem. Mater. 2006, 18, 6308–6315. [Google Scholar] [CrossRef]
- Mihelic, I.; Krajnc, M.; Koloini, T.; Podgornik, A. Kinetic modelo of a methacrylate-based monolith polymerization. Ind. Eng. Chem. Res. 2001, 40, 3495–3501. [Google Scholar] [CrossRef]
- Danquah, M.; Forde, G. Preparation of macroporous methacrylate monolithic material with convective flow properties for bioseparation: investigating the kinetiocs of pore formation and hydrodynamic performance. Chem. Eng. J. 2008, 140, 593–599. [Google Scholar] [CrossRef]
- Yang, G.; Liu, H.; Bai, L.; Jiang, M.; Zhu, T. Preparation and characterization of novel poly(vinyl ester resin) monoliths. Microp. Mesop. Mater. 2008, 112, 351–356. [Google Scholar] [CrossRef]
- Arrua, R.D.; Strumia, M.; Pastrana, G.; Serrano, D.; Alvarez, C. Synthesis of macroporous polymer rods based on an acrylamide derivative monomer. J. Polym. Sci. A Polym. Chem. 2006, 44, 6616–6623. [Google Scholar] [CrossRef]
- Arrua, R.D.; San Roman, J.; Gallardo, A.; Strumia, M.; Alvarez, C. Preparation of polymeric macroporous rod systems: Study of the influence of the reaction parameters on the porous. Mat. Chem. Phys. 2008, 112, 1055–1060. [Google Scholar] [CrossRef]
- Arrua, R.D. Synthesis of porous continuous polymers. Potential application as affinity chromatography supports. Thesis Doctoral, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Córdoba, Argentina, February 2009. [Google Scholar]
- Holdšvendová, P.; Coufal, P.; Suchánková, J.; Tesařová, E.; Bosáková, Z. Methacrylate monolithic columns for capillary liquid chromatography polymerized using ammonium peroxodisulfate as initiator. J. Sep. Sci. 2003, 26, 1623–1628. [Google Scholar] [CrossRef]
- Tan, A.; Benetton, S.; Henion, J.D. Chip-based solid-phase extraction pretreatment for direct electro-spray mass spectrometry analysis using an array of monolithic columns in a polymeric substrate. Anal. Chem. 2003, 75, 5504–5511. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Davey, M.; Svec, F.; Frechet, J.M.J. Monolithic porous polymer for on-chip solid-phase extraction and preconcentration prepared by photoinitiated in situ polymerization within a microfluidic device. Anal. Chem. 2001, 73, 5088–5096. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Armenta, J.M.; Lee, M.L. Preparation and evaluation of poly(polyethylene glycol methyl ether acrylate-co-polyethylene glycol diacrylate) monolith for protein analysis. J. Chromatogr. A 2005, 1079, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Safrany, A.; Beiler, B.; Laszlo, K.; Svec, F. Control of pore formation in macroporous polymers synthesized by single-step γ-radiation-initiated polymerization and cross-linking. Polymer 2005, 46, 2862–2871. [Google Scholar] [CrossRef]
- Beiler, B.; Vincze, A.; Svec, F.; Safrany, A. Poly(2-hydroxyethyl acrylate-co-ethyleneglycol dimethacrylate) monoliths synthesized by radiation polymerization in a mold. Polymer 2007, 48, 3033–3040. [Google Scholar] [CrossRef]
- Beiler, B.; Safrany, A. Polymer monoliths synthesized by radiation co-polymerization in solution. Rad. Phys. Chem. 2007, 76, 1351–1354. [Google Scholar] [CrossRef]
- Kanamori, Z.; Hasegawa, J.; Nakanishi, K.; Hanada, T. Facile synthesis of macroporous cross-linked methacrylate gels by atom transfer radical polymerization. Macromolecules 2008, 41, 7186–7193. [Google Scholar] [CrossRef]
- Phenomenex website for Onyx HPLC columns. http://www.phenomenex.com/phen/products/on-yx/p_order_info.htm/ accessed 20 March 2007.
- Svec, F.; Fréchet, J.M.J. Kinetic control of pore formation in macroporous polymers. The formation of "molded" porous materials with high flow characteristics for separation or catalysis. Chem. Mater. 1995, 7, 707–715. [Google Scholar] [CrossRef]
- Okay, O. Macroporous copolymer networks. Prog. Polym. Sci. 2000, 25, 711–779. [Google Scholar] [CrossRef]
- Svec, F.; Fréchet, J.M.J. Temperature, a simple and efficient tool for the control of pore size distribution in macroporous polymers. Macromolecules 1995, 28, 7580–7582. [Google Scholar] [CrossRef]
- Courtois, J.; Bystrom, E.; Irgum, K. Novel monolithic materials using poly(ethylene glycol) as porogen for protein separation. Polymer 2006, 2603–2611. [Google Scholar] [CrossRef]
- Aoki, H.; Kubo, T.; Ikegami, T.; Tanaka, N.; Hosoya, K.; Tokuda, D.; Ishizuka, N. Preparation of glycerol dimethacrylate-based polymer monolith with unusual porous proper-ties achieved via viscoelastic phase separation induced by monodisperse ultra high molecular weight poly(styrene) as a porogen. J. Chromatogr. A 2006, 1119, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Slabospitskaya, Yu.M.; Vlakh, E.G.; Saprykina, N.N.; Tennikova, T.B. Synthesis and investigation of a new macroporous monolithic material based on an N-hydroxyphthalimide ester of acrylic acid-co-glycidyl methacrylate-co-ethylene di-methacrylate terpolymer. J. Appl. Polym. Sci. 2009, 111, 692–700. [Google Scholar]
- Sinitsyna, E.S.; Sergeeva Yu, N.; Vlakh, E.G.; Saprikina, N.N.; Tennikova, T.B. New platforms for 3-D microarrays: synthesis of hydrophilic polymethacrylate monoliths using macromolecular porogens. React. Funct. Polym. 2009, 69, 385–392. [Google Scholar] [CrossRef]
- Hebb, A.; Senoo, K.; Cooper, A. Synthesis of porous cross-linked polymer monoliths using 1,1,1,2-tetrafluoroethane (R134a) as the porogen. Compos. Sci. Technol. 2003, 63, 2379–2387. [Google Scholar] [CrossRef]
- Svec, F. Preparation and HPLC applications of rigid macroporous organic polymer monoliths. J. Sep. Sci. 2004, 27, 747–766. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xu, M.; Svec, F.; Frechet, J.M.J. Preparation of monolithic polymers with controlled porous properties for microfluidic chip applications using photoinitiated free-radical polymerization. J. Polym. Sci. A Polym. Chem. 2000, 40, 755–769. [Google Scholar] [CrossRef]
- Li, Y.; Gu, B.; Tolley, H.D.; Lee, M. Preparation of polymeric monoliths by copolymerization of acrylate monomers with amine functionalities for anion-exchange capillary liquid chromatography of protein. J. Chromatogr. A 2009, 1216, 5525–5532. [Google Scholar] [CrossRef] [PubMed]
- Mayr, B.; Holzl, G.; Eder, K.; Buchmeiser, M.R.; Huber, C.G. New hydrophobic, pellicular, monolithic capillary columns based on crosslinked polynorbornene for biopolymer separations. Anal. Chem. 2002, 74, 6080–6087. [Google Scholar] [CrossRef] [PubMed]
- Mayr, B.; Tessadri, R.; Post, E.; Buchmeiser, M. Metathesis-based monoliths: influence of polymerization conditions on the separation of biomolecules. Anal. Chem. 2001, 73, 4071–4078. [Google Scholar] [CrossRef] [PubMed]
- Sinner, F.; Buchmeiser, M. A new class of continuous polymer supports prepared by ring-opening metathesis polymerization: a straightforward route to functionalized monoliths. Macromolecules 2000, 33, 5777–5786. [Google Scholar] [CrossRef]
- Scheibitz, B.; Prager, A.; Buchmeiser, M. Schrock catalyst triggered, ring-opening methatesis polymerization based synthesis of functional monolithic materials. Macromolecules 2009, 42, 3493–3499. [Google Scholar] [CrossRef]
- Ren, L.; Liu, Z.; Liu, Y.; Dou, P.; Chen, H.Y. Ring-opening polymerization with synergistic comonomers: Access to a boronate-functionalized polymeric monolith for the specific capture of cis-diol-containing biomolecules under neutral conditions. Angew. Chem. Int. Ed. 2009, 48, 6704–6707. [Google Scholar] [CrossRef]
- Ren, L.; Liu, Z.; Liu, Y.; Dong, M.; Ye, M.; Zou, H. Synthesis and characterization of a new boronate affinity monolithic capillary for specific capture of cis-diolcontaining compounds. J. Chromatogr. A 2009, 1216, 4768–4774. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.; Podgornik, A.; Merher, M.; Schallaun, E.; Jungbauer, A. Affinity monoliths generated by in situ polymerization of the ligand. Anal. Chem. 2001, 73, 5126–5132. [Google Scholar] [CrossRef] [PubMed]
- Bisjak, C.; Bakry, R.; Huck, C.; Bonn, G. Amino-functionalized monolithic poly(glycidyl methacrylate-co-divinylbenzene) ion-exchange stationary phases for the separation of oligonucleotides. Chromatographia 2005, 62, 31–36. [Google Scholar]
- Wiedner, W.; Bisjak, C.; Bakry, R.; Huck, C.; Bonn, G. Monolithic poly(glycidyl methacrylate-co-divinylbenzene) capillary columns functionalized to strong anion exchangers for nucleotide and oligonucleotide separation. J. Sep. Sci. 2006, 29, 2478–2484. [Google Scholar]
- Wei, Y.; Huang, X.; Liu, R.; Shen, Y.; Geng, X. Preparation of a monolithic column for WCX and its application in the separation of biopolymer. J. Sep. Sci. 2006, 29, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zou, H.; Xiao, X.; Guo, Z.; Kong, L.; Mao, X. Chromatographic separation of proteins on metal immobilized iminodiacetic acid-bound molded monolithic rods of macroporous poly(glycidyl methacrylate-co-ethylene dimethacrylate). J. Chromatogr. A 2001, 26, 255–264. [Google Scholar] [CrossRef]
- Luo, Q.; Wei, Y.; Liu, T.; Lei, G.; Geng, X. Poly [(chloromethyl) styrene-co-divinylbenzene] continuous rod column of weak cation exchange chromatography and its applications in the separation of biopolymers. Chinese Chem. Lett. 1999, 10, 215–218. [Google Scholar]
- Pan, Z.; Zou, H.; Mo, W.; Huang, X.; Wu, R. Protein A immobilized monolithic capillary column for affinity chromatography. Anal. Chim. Acta 2002, 466, 141–150. [Google Scholar] [CrossRef]
- Jiang, T.; Mallik, R.; Hage, D.S. Affinity monoliths for ultrafast immunoextraction. Anal. Chem. 2005, 77, 2362–2372. [Google Scholar] [CrossRef] [PubMed]
- Frechet, J.M.J. Design and preparation of novel particulate and continuous polymeric macroporous media for the separation of biological and synthetic molecules. Makromol. Chem. Macromol. Symp. 1993, 70/71, 289–301. [Google Scholar] [CrossRef]
- Svec, F.; Fréchet, J.M.J. Modified poly(glycidyl methacrylate-co-ethylene dimethacrylate) continuous rod columns for preparative scale ion-exchange chromatography of proteins. J. Chromatogr. A 1995, 702, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Preinerstorfer, B.; Bicker, W.; Lindner, W.; Lammerhorfer, M. Development of reactive thiol-modified monolithic capillaries and in-column surface functionalization by radical addition of a chromatographic ligand for capillary electrochromatography. J. Chromatogr. A 2004, 1044, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Gusev, I.; Huang, X.; Horváth, C. Capillary columns with in situ formed porous monolithic packing for micro high-performance liquid chromatography and capillary electrochromatography. J. Chromatogr. A 1999, 855, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Buchmeiser, M.R. New synthetic ways for the preparation of high-performance liquid chromatography supports. J. Chromatogr. A 2001, 918, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Buchmeiser, M.R. Polymeric monolithic materials: Syntheses, properties, functionalization and applications. Polymer 2007, 48, 2187–2198. [Google Scholar] [CrossRef]
- Hanora, A.; Savina, I.; Plieva, F.M.; Izumrudov, V.A.; Mattiasson, B.; Galaev, I. Direct capture of plasmid DNA from non-clarified bacterial lysate using polycation-grafted monoliths. J. Biotechnol. 2006, 123, 343–355. [Google Scholar]
- Tripp, J.; Svec, F.; Frechet, J.M.J. Grafted macroporous polymer monolithic disks: a new format of scavengers for solution-phase combinatorial chemistry. J. Comb. Chem. 2001, 3, 216–223. [Google Scholar]
- Kawai, T.; Saito, K.; Lee, W. Protein binding to polymer brush, based on ion-exchange, hydrophobic, and affinity interactions. J. Chromatogr. B 2003, 790, 131–142. [Google Scholar]
- Mayadunne, R.; Rizzardo, E.; Chiefari, J.; Kristina, J.; Moad, G.; Postma, A.; Thang, S. Living polymers by the use of trithiocarbonates as reversible addition−fragmentation chain transfer (RAFT) agents: ABA triblock copolymers by radical polymerization in two steps. Macromolecules 2000, 33, 243–245. [Google Scholar] [CrossRef]
- Fukuda, T.; Goto, A.; Ohno, K. Mechanisms and kinetics of living radical polymerizations. Macromol. Rapid Commun. 2000, 21, 151–165. [Google Scholar] [CrossRef]
- Savina, I.; Galaev, I.; Mattiasson, B. Anion exchange supermacroporous monolithic matrices with grafted polymer brushes of N,N-dimethylaminoethyl-methacrylate. J. Chromatogr. A 2005, 1092, 199–205. [Google Scholar]
- Peters, E.; Petro, M.; Svec, F.; Frechet, J.M.J. Molded rigid polymer monoliths as separation media for capillary electrochromatography. 1. Fine control of porous properties and surface chemistry. Anal. Chem. 1998, 70, 2288–2295. [Google Scholar]
- Gu, B.; Chen, Z.; Thulin, C.; Lee, M. Efficient polymer monolith for strong cation-exchange capillary liquid chromatography of peptides. Anal. Chem. 2006, 78, 3509–3518. [Google Scholar]
- Gu, B.; Li, Y.; Lee, M. Polymer monoliths with low hydrophobicity for strong cation-exchange capillary liquid chromatography of peptides and proteins. Anal. Chem. 2007, 79, 5848–5855. [Google Scholar] [CrossRef] [PubMed]
- Viklund, C.; Svec, F.; Frechet, J.M.J.; Irgum, K. Fast ion-exchange HPLC of proteins using porous poly(glycidyl methacrylate-co-ethylene dimethacrylate) monoliths grafted with poly(2-acrylamido-2-methyl-1-propanesulfonic acid). Biotechnol. Prog. 1997, 13, 597–600. [Google Scholar]
- Savina, I.; Galaev, I.; Mattiasson, B. Ion-exchange macroporous hydrophilic gel monolith with grafted polymer brushes. J. Mol. Recognit. 2006, 19, 313–321. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Zhou, X.; Tan, T. Preparation and characterization of polyethyleneimine modified ion-exchanger based on poly(methacrylate-co-ethylenedimethacrylate) monolith. J. Chromatogr. A 2007, 1147, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, J.; Zhou, X.; Tan, T. Modification with DEAE-dextran, an alternative way to prepare anion-exchange monolithic column with lower pressure drop. Biochem. Engin. J. 2007, 34, 76–81. [Google Scholar] [CrossRef]
- Bae, Y.; Okano, T.; Kim, S. Temperature dependence of swelling of crosslinked poly(N,N’-alkyl substituted acrylamides) in water. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 923–936. [Google Scholar] [CrossRef]
- Fujishige, S.; Kubota, K.; Ando, I. Phase transition of aqueous solutions of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide. J. Phys. Chem. 1989, 93, 3311–3313. [Google Scholar]
- Peters, E.; Svec, F.; Frechet, J.M.J. Thermally responsive rigid polymer monoliths. Adv. Mater. 1997, 9, 630–633. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, G.; Xin, P.; Qi, L.; Chen, Y. Preparation of poly(N-isopropylacrylamide)-grafted polymer monolith for hydrophobic interaction chromatography of proteins. J. Chromatog. A 2009, 1216, 2404–2411. [Google Scholar] [CrossRef]
- Rohr, T.; Hilder, E.; Donovan, J.; Svec, F.; Frechet, J.M.J. Photografting and the control of surface chemistry in three-dimensional porous polymer monoliths. Macromolecules 2003, 36, 1677–1684. [Google Scholar] [CrossRef]
- Stachowiak, T.; Svec, F.; Frechet, J.M.J. Patternable protein resistant surfaces for multifunctional microfluidic devices via surface hydrophilization of porous polymer monoliths using photografting. Chem. Mater. 2006, 5950–5957. [Google Scholar] [CrossRef]
- Meyer, U.; Svec, F.; Frechet, J.M.J.; Hawker, C.; Irgum, K. Use of stable free radicals for the sequential preparation and surface grafting of functionalized macroporous monoliths. Macromolecules 2000, 33, 7769–7775. [Google Scholar] [CrossRef]
- Wei, X.; Qi, L.; Yang, G.; Wang, F. Preparation and characterization of monolith column by grafting pH-responsive polymer. Talanta 2009, 79, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Sinner, F.; Buchmeiser, M.R. A new class of continuous polymer supports prepared by ring-opening metathesis polymerization: a straightforward route to functionalized monoliths. Macromolecules 2000, 33, 5777–5786. [Google Scholar] [CrossRef]
- Sinner, F.M.; Buchmeiser, M.R. Ring-opening metathesis polymerization: access to a new class of functionalized, monolithic stationary phases for liquid chromatography. Angew. Chem. Int. Ed. 2000, 39, 1433–1436. [Google Scholar]
- Buchmeiser, M.R. Stationary phases for chromatography prepared by ring opening metathesis polymerization. J. Sep. Sci. 2008, 31, 1907–1922. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.O.; Lubbad, S.; Nuyken, O.; Buchmeiser, M.R. Monolith-and silica-supported carboxylate-bassed Grubbs-Herrmann-type metathesis catalysts. Adv. Synth. Catal. 2003, 345, 996–1004. [Google Scholar] [CrossRef]
- Mayr, M.; Wang, D.; Kröll, R.; Schuler, N.; Prühs, S.; Fürstner, A.; Buchmeiser, M.R. Monolithic disk-supported metathesis catalysts for use in combinatorial chemistry. Adv. Synth. Catal. 2005, 347, 484–492. [Google Scholar] [CrossRef]
- Krause, J.O.; Lubbad, S.; Nuyken, O.; Buchmeiser, M.R. Heterogenization of a modified Grubbs-Hoveyda catalyst on a ROMP-derived monolithic support. Macromol. Rapid Commun. 2003, 24, 875–878. [Google Scholar] [CrossRef]
- Lubbad, S.; Mayr, B.; Huber, C.G.; Buchmeiser, M.R. Micropreparative fractionation of DNA fragments on metathesisbased monoliths: Influence of stoichiometry on separation. J. Chromatogr. A 2002, 959, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Ramstrom, O.; Mosbach, K. Synthesis and catalysis by molecularly imprinted materials. Curr. Opin. Chem. Biol. 1999, 3, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Striegler, S. Designing selective sites in templated polymers utilizing coordinative bonds. J. Chromatogr. B 2004, 804, 183–195. [Google Scholar] [CrossRef]
- Baggiani, C.; Trotta, F.; Giraudi, G.; Moraglio, G.; Vanni, A. Chromatographic characterization of a molecularly imprinted polymer binding theophylline in aqueous buffers. J. Chromatogr. A 1997, 786, 23–29. [Google Scholar]
- Turiel, E.; Martin-Esteban, A. Molecularly imprinted polymers: towards highly selective stationary phases in liquid chromatography and capillary electrophoresis. Anal. Bioanal. Chem. 2004, 378, 1876–1886. [Google Scholar] [CrossRef]
- Schweitz, L.; Andersson, L.; Nilsson, S. Rapid electrochromatographic enantiomer separations on short molecularly imprinted polymer monoliths. Anal. Chim. Acta 2001, 435, 43–47. [Google Scholar] [CrossRef]
- Schweitz, L.; Andersson, L.; Nilsson, S. Capillary electrochromatography with molecular imprint-based selectivity for enantiomer separation of local anaesthetics. J. Chromatogr. A 1997, 792, 401–409. [Google Scholar]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their used in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Blanco López, M.; Lobo Castanón, M.; Miranda Ordieres, A.; Tuñón Blanco, P. Electrochemical sensors based on molecularly imprinted polymers. Trends Anal. Chem. 2004, 23, 36–48. [Google Scholar] [CrossRef]
- Andersson, L. Molecular imprinting: developments and applications in the analytical chemistry field. J. Chromatogr. B 2000, 745, 3–13. [Google Scholar]
- Haginaka, J. Molecularly imprinted polymers for solid-phase extraction. Anal. Bioanal. Chem. 2004, 379, 332–334. [Google Scholar] [CrossRef]
- Lanza, F.; Sellergren, B. The application of molecular imprinting technology to solid phase extraction. Chromatographia 2001, 53, 599–611. [Google Scholar] [CrossRef]
- He, Ch.; Long, Y.; Pan, J.; Li, K.; Liu, F. Application of molecularly imprinted polymers to solid phase extraction of analytes from real samples. J. Biochem. Biophys. Methods 2007, 70, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Ulbricht, M. Enantio-selective MIP “nano-monolith” composite membranes. Desalination 2006, 199, 532–534. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, J.; Zhou, Q.; Chen, G.; Liu, Z. On-line solid-phase extraction of ceramides from yeast with ceramide III imprinted monolith. J. Chromatogr. A 2003, 984, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Özkara, S.; Say, R.; Andac, C.; Denizli, A. An ion-imprinted monolith for in vitro removal of iron out of human plasma with beta Thalassemia. Ind. Eng. Chem. Res. 2008, 47, 7849–7856. [Google Scholar] [CrossRef]
- Chirca, G.; Remcho, V. Novel monolithic columns with template porosity. J. Chromatogr. A 2001, 924, 223–232. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J.; Fu, Q.; He, L.; Li, Y. Concentration and extraction of sinomenine from herb and plasma using a molecularly imprinted polymer as the stationary phase. Anal. Chim. Acta 2006, 561, 178–182. [Google Scholar] [CrossRef]
- Matsui, J.; Kato, T.; Takeuchi, T.; Suzuki, M.; Yokoyama, K.; Tamiya, E.; Karube, I. Molecular recognition in continuous polymer rods prepared by a molecular imprinting technique. Anal. Chem. 1993, 65, 2223–2224. [Google Scholar] [CrossRef]
- Matsui, J.; Nicholls, I.A.; Takeuchi, T. Molecular recognition in cinchona alkaloid molecular imprinted polymer rods. Anal. Chim. Acta 1998, 365, 89–93. [Google Scholar] [CrossRef]
- Courtois, J.; Fischer, G.; Sellergren, B.; Irgum, K. Molecularly imprinted polymers grafted to flow through poly(trimethylolpropane trimethacrylate) monoliths for capillary-based solid-phase extraction. J. Chromatog. A 2006, 1109, 92–99. [Google Scholar] [CrossRef]
- Vlakh, E.G.; Tennikova, T.B. Applications of polymethacrylate-based monoliths in high-performance liquid chromatography. J. Chromatog. A 2009, 1216, 2637–2650. [Google Scholar] [CrossRef]
- Li, Y.; Tolley, D.; Lee, M.L. Preparation of polymer monoliths that exhibit size exclusion properties for proteins and peptides. Anal. Chem. 2009, 81, 4406–4413. [Google Scholar] [CrossRef] [PubMed]
- Isobe, K.; Kawakami, I. Preparation of convection interaction media isobutyl disc monolithic column and its application to purification of secondary alcohol dehydrogenase and alcohol oxidase. J. Chromatog. A 2007, 1144, 85–89. [Google Scholar] [CrossRef]
- Jiang, Z.; Smith, N.W.; Ferguson, P.D.; Taylor, M.R. Preparation and characterization of long alkyl chain methacrylate-based monolithic column for capillary chromatography. J. Biochem. Biophys. Meth. 2007, 70, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.; Podgornik, A.; Merhar, M.; Schallaun, E.; Jungbauer, A. Affinity monoliths generated by in situ polymerization of the ligand. Anal. Chem. 2001, 73, 5126–5132. [Google Scholar] [CrossRef] [PubMed]
- Uzun, L.; Yavuz, H.; Say, R.; Erso, A.; Denizli, A. Poly(ethylene dimethacrylate-glycidyl methacrylate) monolith as a stationary phase in dye-affinity chromatography. Ind. Eng. Chem. Res. 2004, 43, 6507–6513. [Google Scholar] [CrossRef]
- Cheeks, M.C.; Kamal, N.; Sorrell, A.; Darling, D.; Farzaneh, F.; Slater, N.K.H. Immobilized metal affinity chromatography of histidine-tagged lentiviral vectors using monolithic adsorbents. J. Chromatog. A 2009, 1216, 2705–2711. [Google Scholar] [CrossRef]
- Tripp, J.A.; Svec, F.; Fréchet, J.M.J. Solid-phase acylating reagents in new format: Macroporous polymer disks. J. Comb. Chem. 2001, 3, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Kunz, U.; Kirschning, A.; Wen, H.L.; Solodenko, W.; Cecilia, R.; Kappe, C.O.; Turek, T. Monolithic polymer/carrier materials: Versatile composites for fine chemical synthesis. Catalysis Today 2005, 105, 318–324. [Google Scholar] [CrossRef]
- Brown, J.F.; Krajnc, P.; Cameron, N.R. PolyHIPE supports in batch and flow-through suzuki cross-coupling reactions. Ind. Eng. Chem. Res. 2005, 44, 8565–8572. [Google Scholar] [CrossRef]
- Petro, M.; Svec, F.; Fréchet, J.M.J. Immobilization of trypsin onto “molded” macroporous poly(glycidyl methacrylate-co-ethylene dimethacrylate) rods and use of the conjugates as bioreactors and for affinity chromatography. Biotechnol. Bioeng. 1996, 49, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Svec, F.; Fréchet, J.M.J. Design of reactive porous polymer supports for high throughput bioreactors: Poly(2-vinyl-4,4-dimethylazlactone-co-acrylamide-co-ethylene dimethacrylate) monoliths. Biotechnol. Bioeng. 1999, 62, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.S.; Rohr, T.; Svec, F.; Fréchet, J.M.J. Enzymatic microreactor-on-a-chip: Protein mapping using trypsin immobilized on porous polymer monoliths molded in channels of microfluidic devices. Anal. Chem. 2002, 74, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Krenkova, J.; Lacher, N.A.; Svec, F. Highly efficient enzyme reactors containing trypsin and endoproteinase LysC immobilized on porous polymer monolith coupled to MS suitable for analysis of antibodies. Anal. Chem. 2009, 81, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Miletic, N.; Rohandi, R.; Vukovic, Z.; Nastasovic, A.; Loos, K. Surface modification of macroporous poly(glycidyl methacrylate-co-ethylene glycol dimethacrylate) resins for improved Candida antarctica lipase B immobilization. React. Funct. Polym. 2009, 69, 68–75. [Google Scholar] [CrossRef]
- Pontén, E.; Viklund, C.; Irgum, K.; Bogen, S.T.; Lindgren, Å.N. Solid phase chemiluminescence detection reactors based on in situ polymerized methacrylate materials. Anal. Chem. 1996, 68, 4389–4396. [Google Scholar]
- Steinke, J.H.G.; Dunkin, I.R.; Sherrington, D.C. Molecularly imprinted anisotropic polymer monoliths. Macromolecules 1996, 29, 407–415. [Google Scholar] [CrossRef]
- Xie, S.; Svec, F.; Fréchet, J.M.J. Porous polymer monoliths: preparation of sorbent materials with high-surface areas and controlled surface chemistry for high-throughput, online, solid-phase extraction of polar organic compounds. Chem. Mater. 1998, 10, 4072–4078. [Google Scholar] [CrossRef]
- Iannacone, J.M.; Ren, S.; Hatcher, N.G.; Sweedler, J.V. Collecting peptide release from the brain using porous polymer monolith-based solid phase extraction capillaries. Anal. Chem. 2009, 81, 5433–5438. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Benetton, S.; Henion, J.D. Chip-based solid-phase extraction pretreatment for direct electrospray mass spectrometry analysis using an array of monolithic columns in a polymeric substrate. Anal. Chem. 2003, 75, 5504–5511. [Google Scholar] [CrossRef] [PubMed]
- Korotchenko, M.V.; Gagné, M.R. Fluorophobic enhanced decontamination of a mustard simulant by porous monolithic flow-through columns. React. Funct. Polym. 2007, 67, 422–431. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arrua, R.D.; Strumia, M.C.; Alvarez Igarzabal, C.I. Macroporous Monolithic Polymers: Preparation and Applications. Materials 2009, 2, 2429-2466. https://doi.org/10.3390/ma2042429
Arrua RD, Strumia MC, Alvarez Igarzabal CI. Macroporous Monolithic Polymers: Preparation and Applications. Materials. 2009; 2(4):2429-2466. https://doi.org/10.3390/ma2042429
Chicago/Turabian StyleArrua, Ruben Dario, Miriam Cristina Strumia, and Cecilia Inés Alvarez Igarzabal. 2009. "Macroporous Monolithic Polymers: Preparation and Applications" Materials 2, no. 4: 2429-2466. https://doi.org/10.3390/ma2042429