The Effect of (Ag, Ni, Zn)-Addition on the Thermoelectric Properties of Copper Aluminate
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of samples
2.2. Characterization
3. Results and Discussion
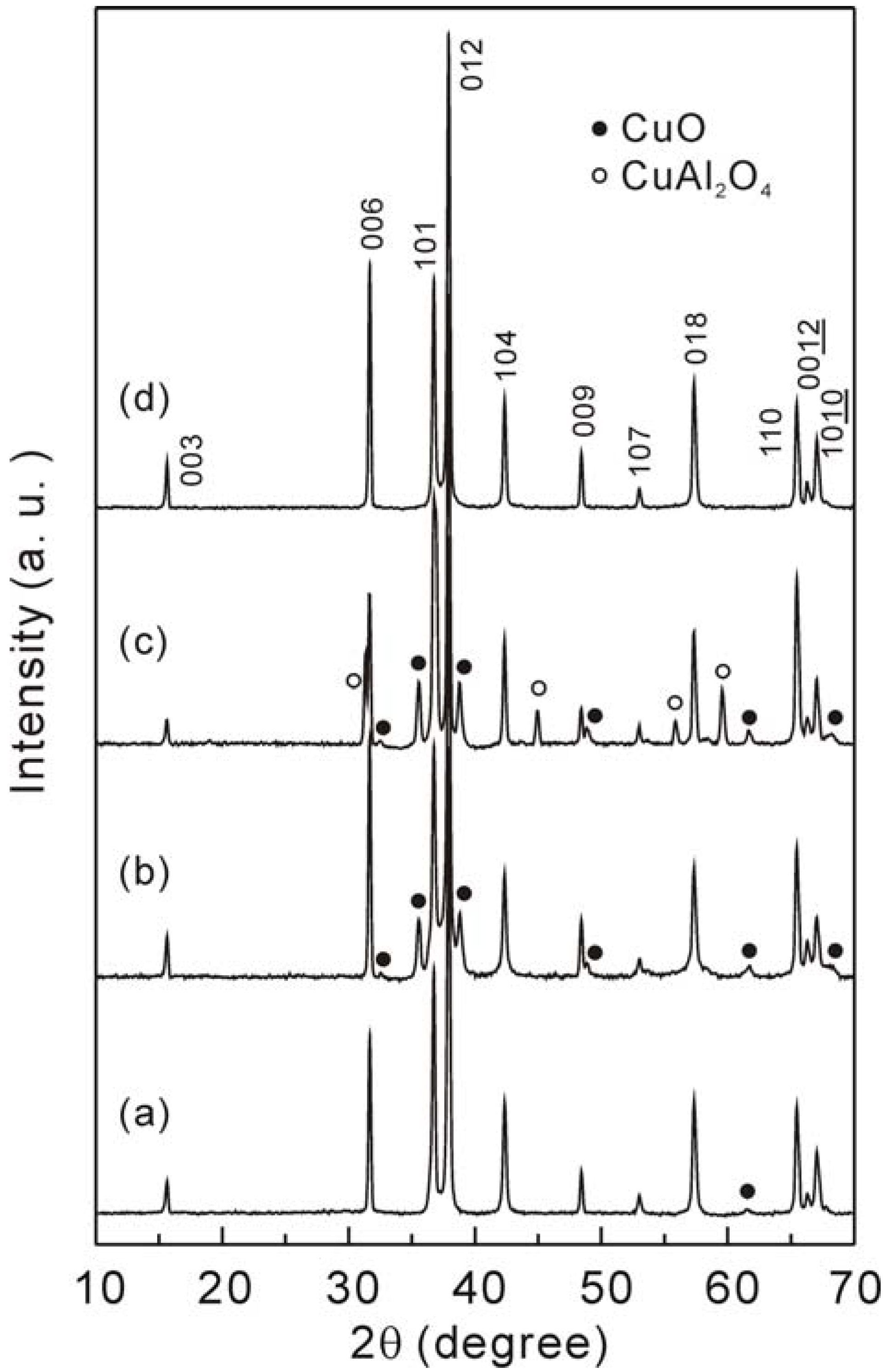
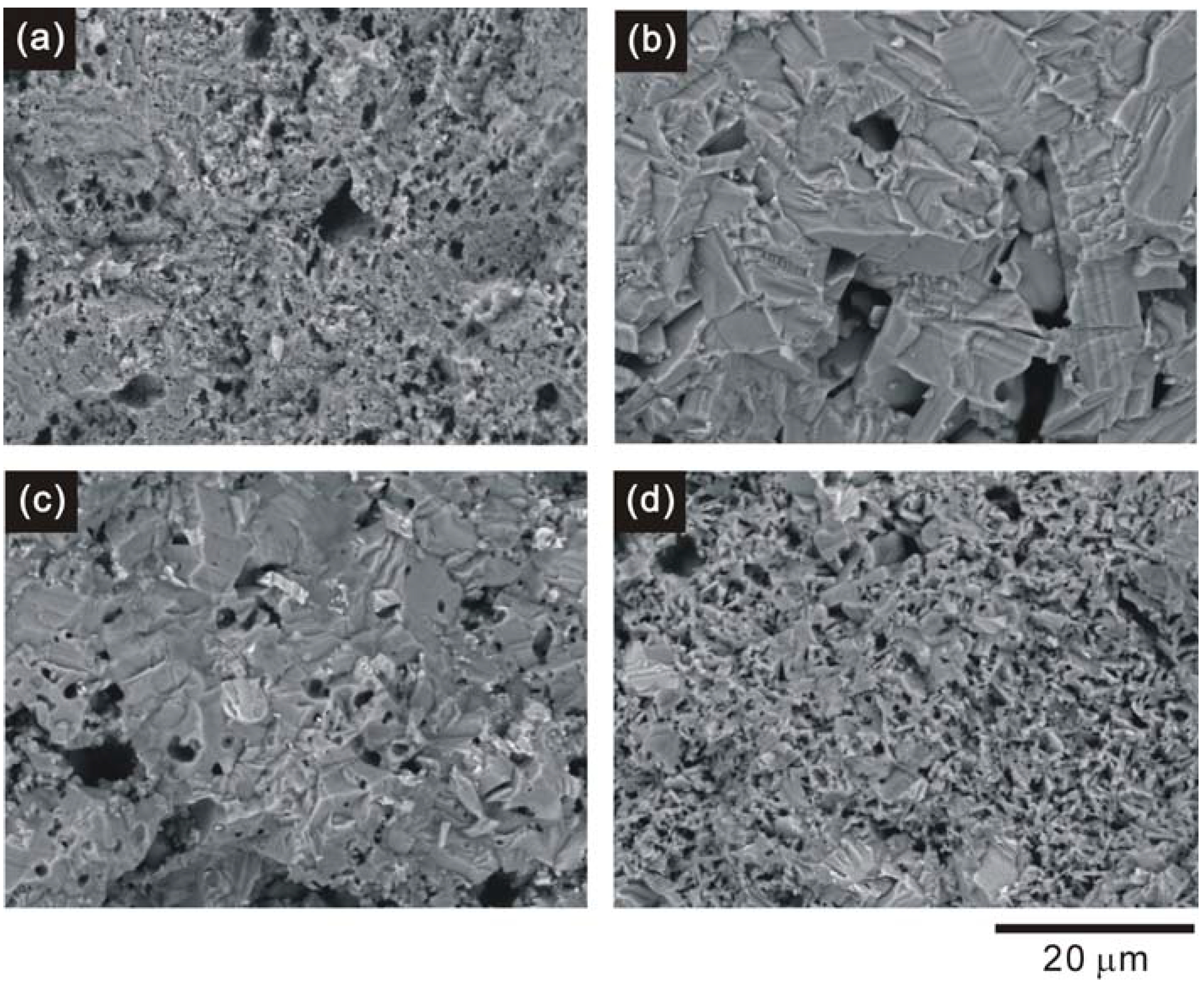
3.1. Crystal structure and microstructure
- (1)
- CuO + Al2O3 → CuAl2O4, and
- (2)
- CuAl2O4 + CuO → 2CuAlO2 + 1/2O2↑.
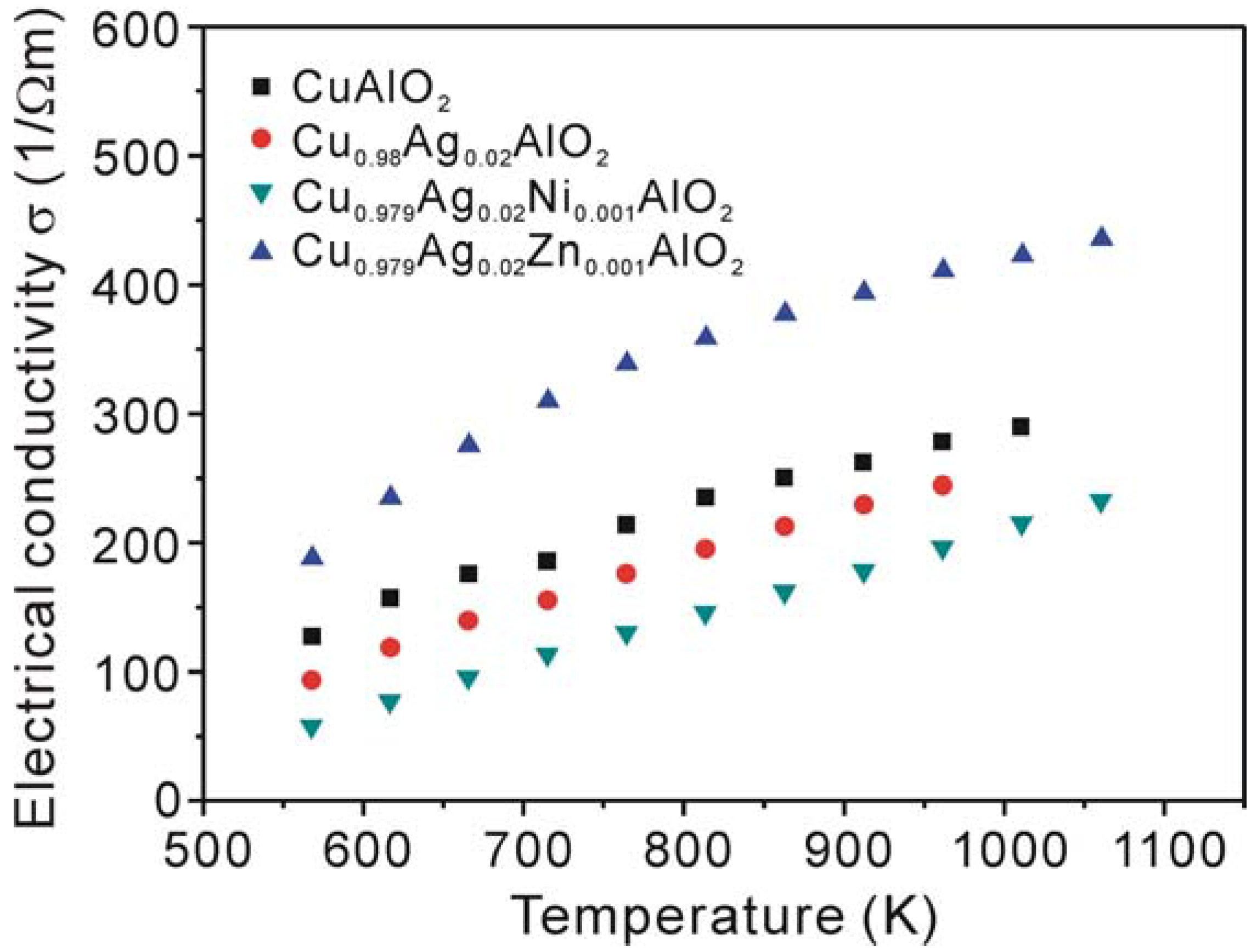
3.2. Thermoelectric properties

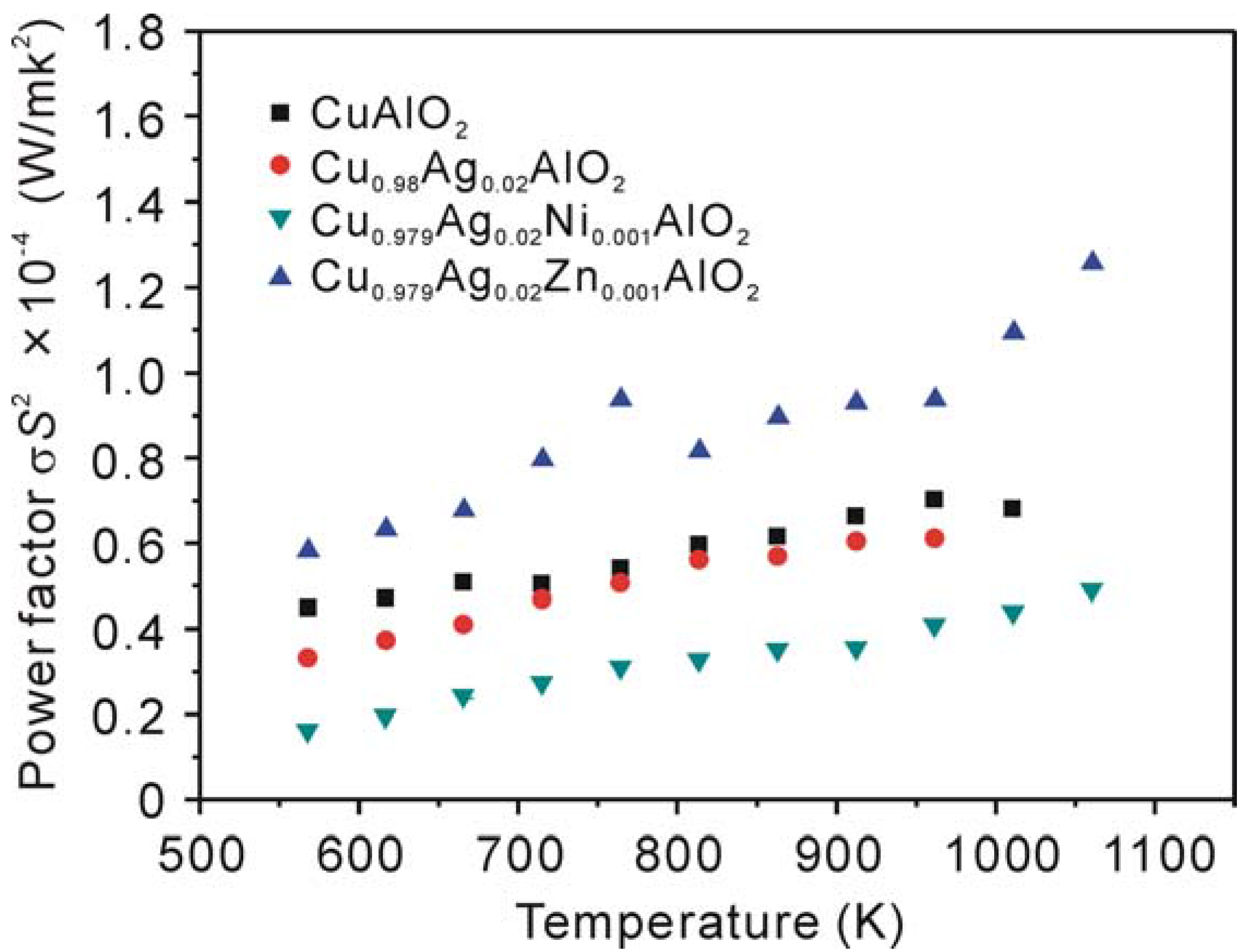
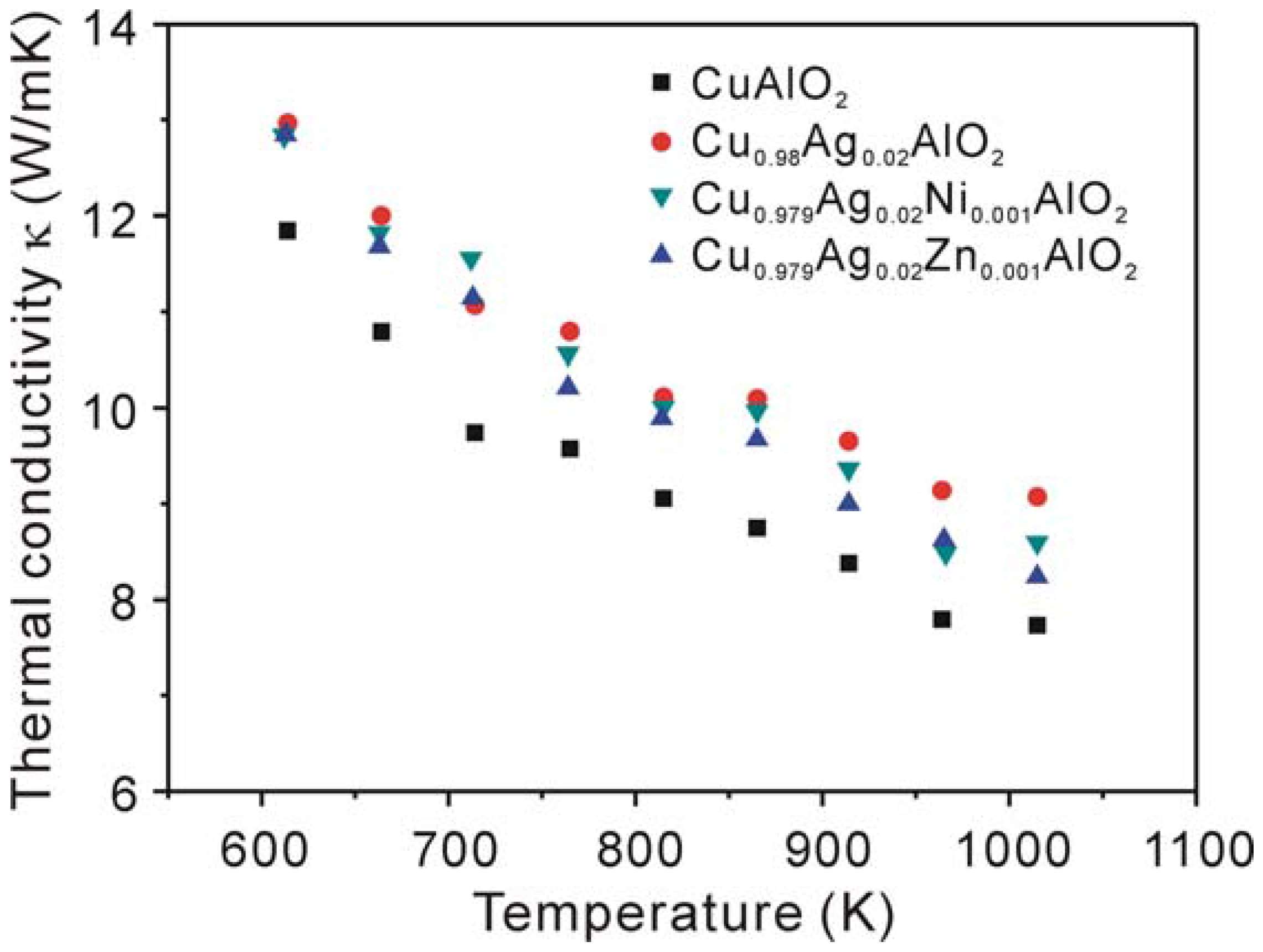
4. Conclusions
Acknowledgments
References
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, C.M.; Rowe, D.M. Optimization of carrier concentration. In CRC Handbook of Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 43–53. [Google Scholar]
- Ohtaki, M.; Araki, K.; Yamamoto, K. High thermoelectric performance of dually doped ZnO ceramics. J. Electron. Mater. 2009, 38, 1234–1238. [Google Scholar] [CrossRef]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; Chen, X.; Liu, J.; Dresselhaus, M.S.; Chen, G.; Ren, Z. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, H.; Scherrer, S. Thermoelectric properties of bismuth antimony telluride solid solutions. In Thermoelectrics Handbook: Macro to Nano; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2006; p. 27. [Google Scholar]
- Hsu, K.F.; Loo, S.; Guo, F.; Chen, Y.; Dyck, J.S.; Uher, C.; Hogan, T.; Polychroniadis, E.K.; Kanatzidis, M.G. Cubic AgPbmSbTe2+m: bulk thermoelectric materials with high figure of merit. Science 2004, 303, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.M.; Shukla, V.S. The effect of phonon-grain boundary scattering on the lattice thermal conductivity and thermoelectric conversion efficiency of heavily doped fine-grained, hot-pressed silicon germanium alloy. J. Appl. Phys. 1981, 52, 7421–7426. [Google Scholar] [CrossRef]
- Zhang, Q.; He, J.; Zhu, T.J.; Zhang, S.N.; Zhao, X.B.; Tritt, T.M. High figures of merit and natural nanostructures in Mg2Si0.4Sn0.6 based thermoelectric materials. Appl. Phys. Lett. 2008, 93, 102109. [Google Scholar] [CrossRef]
- Caillat, T.; Fleurial, J.-P.; Borshchevsky, A. Preparation and thermoelectric properties of semiconducting Zn4Sb3. J. Phys. Chem. Solids 1997, 58, 1119–1125. [Google Scholar] [CrossRef]
- Shi, X.; Kong, H.; Li, C.-P.; Uher, C.; Yang, J.; Salvador, J.R.; Wang, H.; Chen, L.; Zhang, W. Low thermal conductivity and high thermoelectric figure of merit in n-type BaxYbyCo4Sb12 double-filled skutterudites. Appl. Phys. Lett. 2008, 92, 182101. [Google Scholar] [CrossRef]
- Sakurada, S.; Shutoh, N. Effect of Ti substitution on the thermoelectric properties of (Zr,Hf)NiSn half-Heusler compounds. Appl. Phys. Lett. 2005, 86, 082105. [Google Scholar] [CrossRef]
- Kim, J.H.; Okamoto, N. L.; Kishida, K.; Tanaka, K.; Inui, H. High thermoelectric performance of type-III clathrate compounds of the Ba-Ge-Ga system. Acta. Mater. 2006, 54, 2057–2062. [Google Scholar] [CrossRef]
- Terasaki, I.; Sasago, Y.; Uchinokura, K. Large thermoelectric power in NaCo2O4 single crystals. Phys. Rev.B 1997, 56, R12685–R12687. [Google Scholar] [CrossRef]
- Masset, A.C.; Michel, C.; Maignan, A.; Hervieu, M.; Toulemonde, O.; Studer, F.; Raveau, B.; Hejtmanek, J. Misfit-layered cobaltite with an anisotropic giant magnetoresistance: Ca3Co4O9. Phys. Rev. B 2000, 62, 166–175. [Google Scholar] [CrossRef]
- Ohtaki, M.; Tsubota, T.; Eguchi, K.; Arai, H. High-temperature thermoelectric properties of (Zn1–xAlx )O. J. Appl.Phys. 1996, 79, 1816–1818. [Google Scholar] [CrossRef]
- Ohta, S.; Ohta, H.; Koumoto, K. Grain size dependence of thermoelectric performance of Nb-doped SrTiO3 polycrystals. J. Ceram. Soc. Jpn. 2006, 114, 102–105. [Google Scholar] [CrossRef]
- Koumoto, K.; Koduka, H.; Seo, W.S. Thermoelectric properties of single crystal CuAlO2 with a layered structure. J. Mater. Chem. 2001, 11, 251–252. [Google Scholar] [CrossRef]
- Park, K.; Ko, K.Y.; Seo, W.-S. Thermoelectric properties of CuAlO2. J. Euro. Ceram. Soc. 2005, 25, 2219–2222. [Google Scholar] [CrossRef]
- Tsuboi, N.; Takahashi, Y.; Kobayashi, S.; Shimizu, H.; Kato, K.; Kaneko, F. Delafossite CuAlO2 films prepared by reactive sputtering using Cu and Al targets. J. Phys. Chem. Solids 2003, 64, 1671–1674. [Google Scholar] [CrossRef]
- Cai, J.; Gong, H. The influence of Cu/Al ratio on properties of chemical-vapor-deposition-grown p-type Cu-Al-O transparent semiconducting films. J. Appl. Phys. 2005, 98, 033707. [Google Scholar] [CrossRef]
- Yanagi, H.; Inoue, S.; Ueda, K.; Kawazoe, H.; Hosono, H.; Hamada, N. Electronic structure and optoelectronic properties of transparent p-type conducting CuAlO2. J. Appl. Phys. 2000, 88, 4159–4163. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ishizawa, N.; Mizutani, N.; Kato, M.; Tanaka, K.; Marumo, F. Charge-density distribution in crystals of CuAlO2 with d-s hybridization. Acta Cryst. 1983, B39, 564–569. [Google Scholar] [CrossRef]
- Kawazoe, H.; Yasukawa, M.; Hyodo, H.; Kurita, M.; Yanagi, H.; Hosono, H. P-type electrical conduction in transparent thin films of CuAlO2. Nature 1997, 389, 939–942. [Google Scholar] [CrossRef]
- Park, K.; Ko, K.Y.; Seo, W.-S. Effect of partial substitution of Ca for Al on the microstructure and high-temperature thermoelectric properties of CuAlO2. Mater. Sci. Eng. B 2006, 129, 1–7. [Google Scholar] [CrossRef]
- Park, K.; Ko, K.Y.; Seong, J.K.; Nahm, S. Microstructure and high-temperature thermoelectric properties of polycrystalline CuAl1-xMgxO2 ceramics. J. Euro. Ceram. Soc. 2007, 27, 3735–3738. [Google Scholar] [CrossRef]
- Park, K.; Ko, K.Y.; Kwon, H.-C.; Nahm, S. Improvement in thermoelectric properties of CuAlO2 by adding Fe2O3. J. Alloys Compd. 2007, 437, 1–6. [Google Scholar] [CrossRef]
- Lali, M.V.; Mestnik-Filho, J.; Carbonari, A.W.; Saxena, R.N. Changes induced by the presence of Zn or Ni impurity at Cu sites in CuAlO2 delafossite. Solid State Commun. 2003, 125, 175–178. [Google Scholar] [CrossRef]
- Shibasaki, S.; Kobayashi, W.; Terasaki, I. Transport properties of the delafossite Rh oxide Cu1−xAgxRh1−yMgyO2: Effect of Mg substitution on the resistivity and Hall coefficient. Phys. Rev. B 2006, 74, 235110. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Cryst. 1969, B25, 925–946. [Google Scholar] [CrossRef]
- Hamada, I.; Katayama-Yoshida, H. Energetics of native defects in CuAlO2. Physica B 2006, 376–377, 808–811. [Google Scholar] [CrossRef]
- Ingram, B.J.; González, G.B.; Mason, T.O.; Shahriari, D.Y.; Barnabè, A.; Ko, D.; Poeppelmeier, K.R. Transport and defect mechanisms in cuprous delafossites. 1. Comparison of hydrothermal and standard solid-state synthesis in CuAlO2. Chem. Mater. 2004, 16, 5616–5622. [Google Scholar] [CrossRef]
- Wongcharoen, N.; Gaewdang, T. Thermoelectric properties of Ni-doped CuAlO2. Physica Procedia 2009, 2, 101–106. [Google Scholar] [CrossRef]
- Ingram, B.J.; Mason, T.O.; Asahi, R.; Park, K.T.; Freeman, A.J. Electronic structure and small polaron hole transport of copper aluminate. Phys. Rev. B 2001, 64, 155114. [Google Scholar] [CrossRef]
- Rowe, D.M. General principles and basic considerations. In Thermoelectrics handbook: Macro to Nano; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2006; p. 1. [Google Scholar]
- Xu, G.; Funahashi, R.; Shikano, M.; Pu, Q.; Liu, B. High temperature transport properties of Ca3-xNaxCo4O9 system. Solid State Commun. 2002, 124, 73–76. [Google Scholar] [CrossRef]
- Liu, M.-S.; Lin, M.C.-C.; Huang, I.-T.; Wang, C.-C. Enhancement of thermal conductivity with CuO for Nanofluids. Chem. Eng. Technol. 2006, 29, 72–77. [Google Scholar] [CrossRef]
© 2010 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yanagiya, S.-i.; Van Nong, N.; Xu, J.; Pryds, N. The Effect of (Ag, Ni, Zn)-Addition on the Thermoelectric Properties of Copper Aluminate. Materials 2010, 3, 318-328. https://doi.org/10.3390/ma3010318
Yanagiya S-i, Van Nong N, Xu J, Pryds N. The Effect of (Ag, Ni, Zn)-Addition on the Thermoelectric Properties of Copper Aluminate. Materials. 2010; 3(1):318-328. https://doi.org/10.3390/ma3010318
Chicago/Turabian StyleYanagiya, Shun-ichi, Ngo Van Nong, Jianxiao Xu, and Nini Pryds. 2010. "The Effect of (Ag, Ni, Zn)-Addition on the Thermoelectric Properties of Copper Aluminate" Materials 3, no. 1: 318-328. https://doi.org/10.3390/ma3010318



