Transformation of Tri-Titanium(IV)-Substituted α-Keggin Polyoxometalate (POM) into Tetra-Titanium(IV)-Substituted POMs : Reaction Products of Titanium(IV) Sulfate with the Dimeric Keggin POM Precursor under Acidic Conditions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instrumentation/Analytical Procedures
2.3. Synthesis
2.3.1. K5H[[{Ti(H2O)3}2(μ-O)](α-PW9Ti2O38)]2·9H2O (K-1)
2.3.2. K3[{Ti4(μ-O)3(SO4)2(H2O)8}(α-PW9O34)]·6H2O (K-2)
2.4. X-Ray Crystallography
2.4.1. Crystal data for K-1
2.4.2. Crystal data for K-2
3. Results and Discussion
3.1. Synthesis and Compositional Characterization
→ [[{Ti(H2O)3}2(μ-O)](α-PW9Ti2O38)]26- (1) + 4SO42- + 2H+
→ 2[{Ti4(μ-O)3(SO4)2(H2O)8}(α-PW9O34)]3- (2)
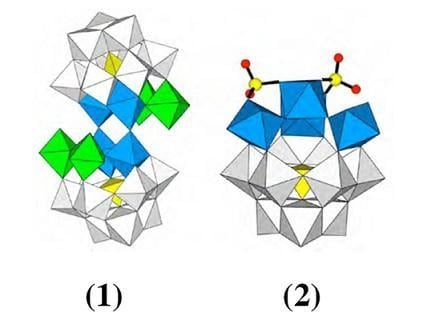
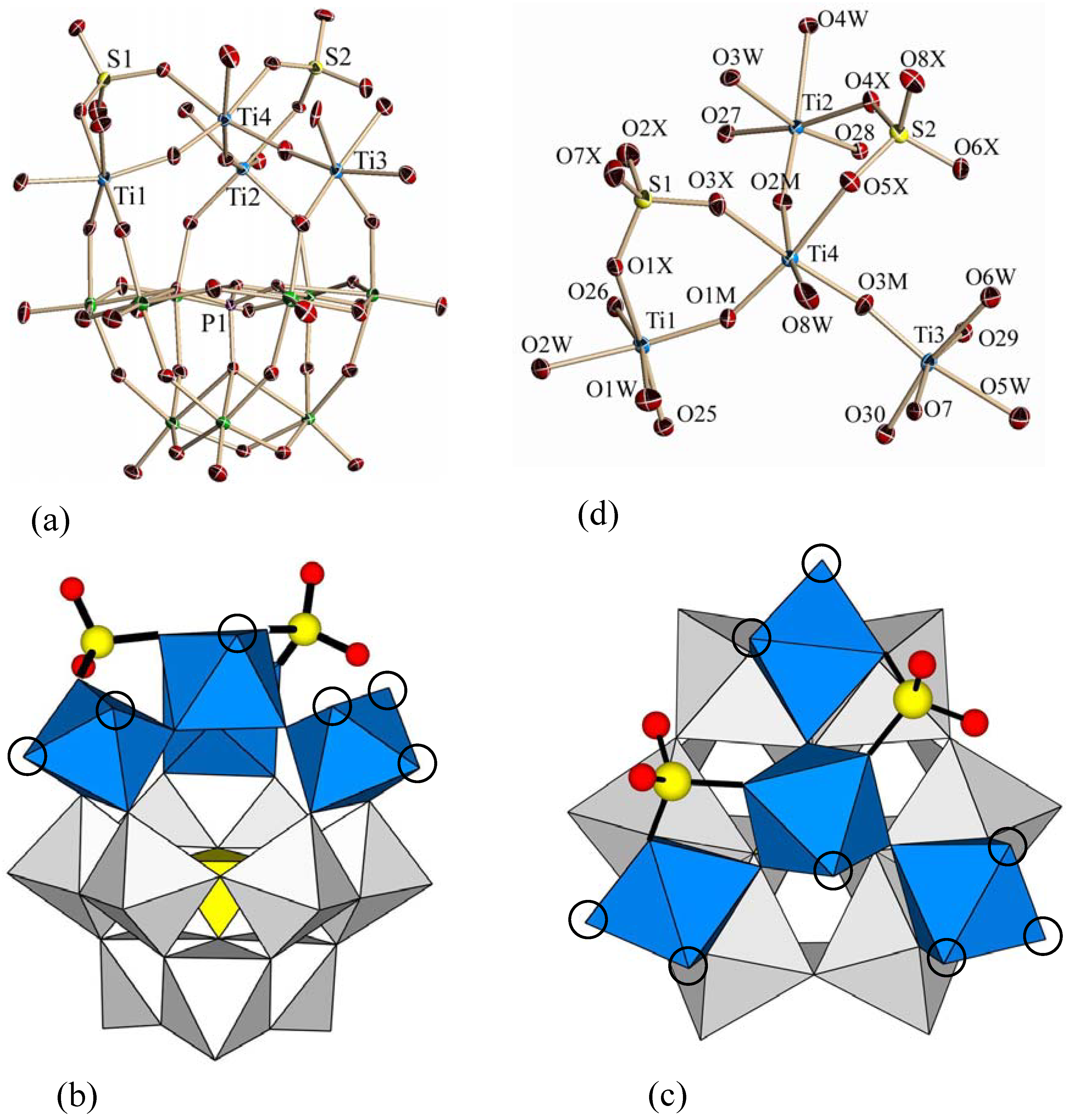
3.2. Molecular Structures of 1 and 2

| lengths | K-1 | K-2 | |
| Ti(1)-O(25) | 2.014(9) | Ti(1)-O(25) | 1.871(6) |
| Ti(1)-O(26) | 2.001(9) | Ti(1)-O(26) | 1.813(6) |
| Ti(1)-O(31) | 1.823(9) | Ti(1)-O(1M) | 1.823(6) |
| Ti(1)-O(32) | 1.912(9) | Ti(1)-O(1W) | 2.154(6) |
| Ti(1)-O(34) | 2.382(9) | Ti(1)-O(2W) | 2.100(6) |
| Ti(1)-O(38) | 1.806(9) | Ti(1)-O(1X) | 2.069(6) |
| average | 1.990 | average | 1.972 |
| Ti(2)-O(29) | 2.053(9) | Ti(2)-O(27) | 1.898(5) |
| Ti(2)-O(30) | 1.974(9) | Ti(2)-O(28) | 1.888(6) |
| Ti(2)-O(31) | 1.803(9) | Ti(2)-O(2M) | 1.745(5) |
| Ti(2)-O(33) | 1.915(9) | Ti(2)-O(3W) | 2.097(6) |
| Ti(2)-O(36) | 2.294(9) | Ti(2)-O(4W) | 2.139(5) |
| Ti(2)-O(38)i | 1.832(9) | Ti(2)-O(4X) | 2.053(5) |
| average | 1.9785 | average | 1.970 |
| Ti(3)-O(27) | 1.907(10) | Ti(3)-O(29) | 1.873(6) |
| Ti(3)-O(32) | 1.753(9) | Ti(3)-O(30) | 1.860(6) |
| Ti(3)-O(1X) | 1.897(10) | Ti(3)-O(3M) | 1.749(6) |
| Ti(3)-O(1W) | 2.080(11) | Ti(3)-O(5W) | 2.135(6) |
| Ti(3)-O(2W) | 2.179(10) | Ti(3)-O(6W) | 2.124(6) |
| Ti(3)-O(3W) | 2.101(11) | Ti(3)-O(7W) | 2.166(7) |
| average | 1.9862 | average | 1.9845 |
| Ti(4)-O(28) | 1.871(10) | Ti(4)-O(1M) | 1.791(6) |
| Ti(4)-O(33) | 1.747(9) | Ti(4)-O(2M) | 1.862(6) |
| Ti(4)-O(1X) | 1.864(10) | Ti(4)-O(3M) | 1.860(6) |
| Ti(4)-O(4W) | 2.119(11) | Ti(4)-O(8W) | 2.088(6) |
| Ti(4)-O(5W) | 2.162(9) | Ti(4)-O(3X) | 2.082(6) |
| Ti(4)-O(6W) | 2.097(9) | Ti(4)-O(5X) | 2.102(5) |
| average | 1.9767 | average | 1.9642 |
| angles | K-1 | K-2 | |
| Ti(1)-O(31)-Ti(2) | 155.7(5) | Ti(1)-O(1M)-Ti(4) | 153.0(4) |
| Ti(1)-O(32)-Ti(3) | 169.8(6) | Ti(2)-O(2M)-Ti(4) | 153.0(3) |
| Ti(2)-O(33)-Ti(4) | 165.9(6) | Ti(3)-O(3M)-Ti(4) | 164.8(4) |
| Ti(3)-O(1X)-Ti(4) | 136.9(5) | ||
| Ti(1)-O(38)-Ti(2)i | 136.0(5) |
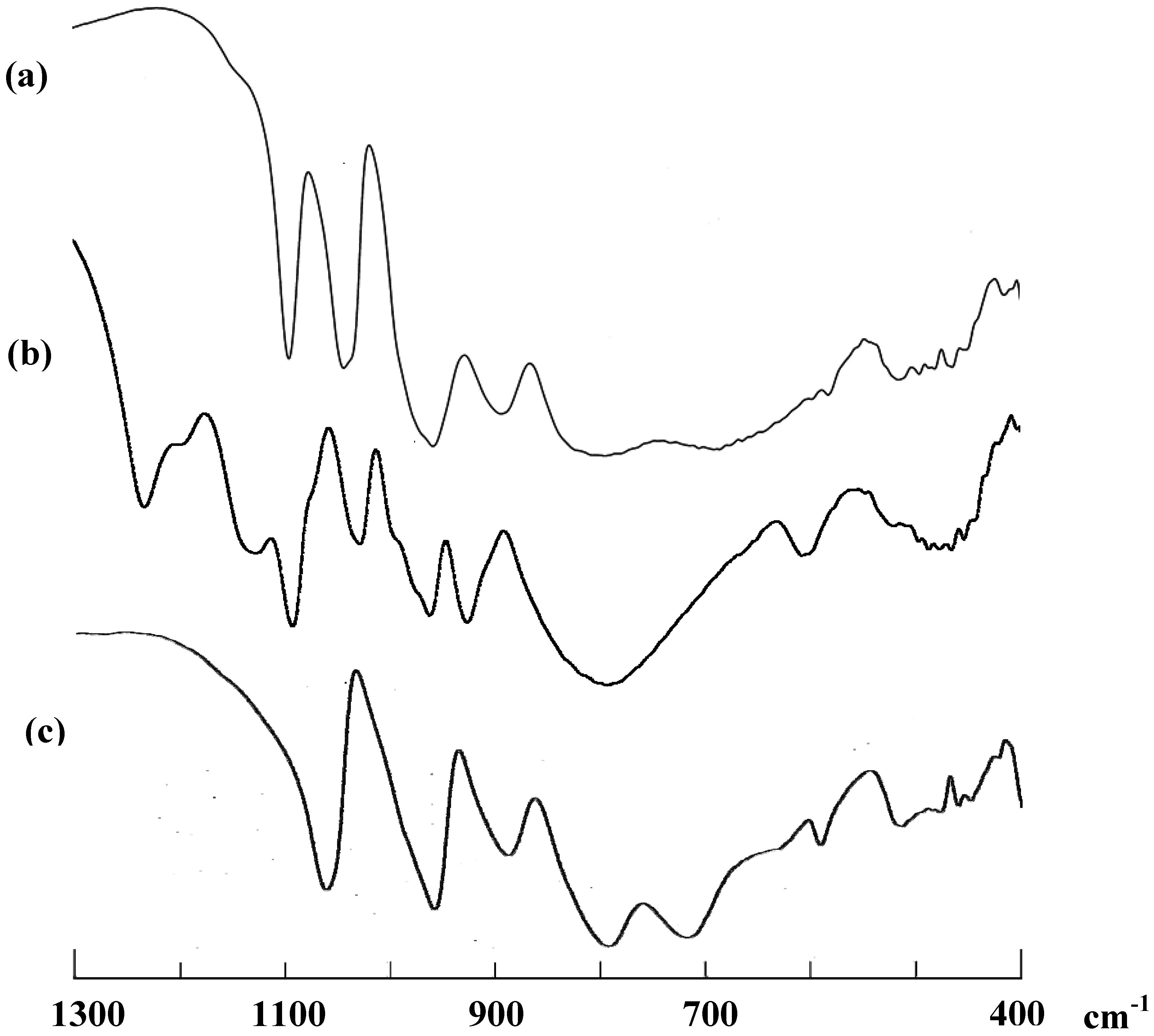
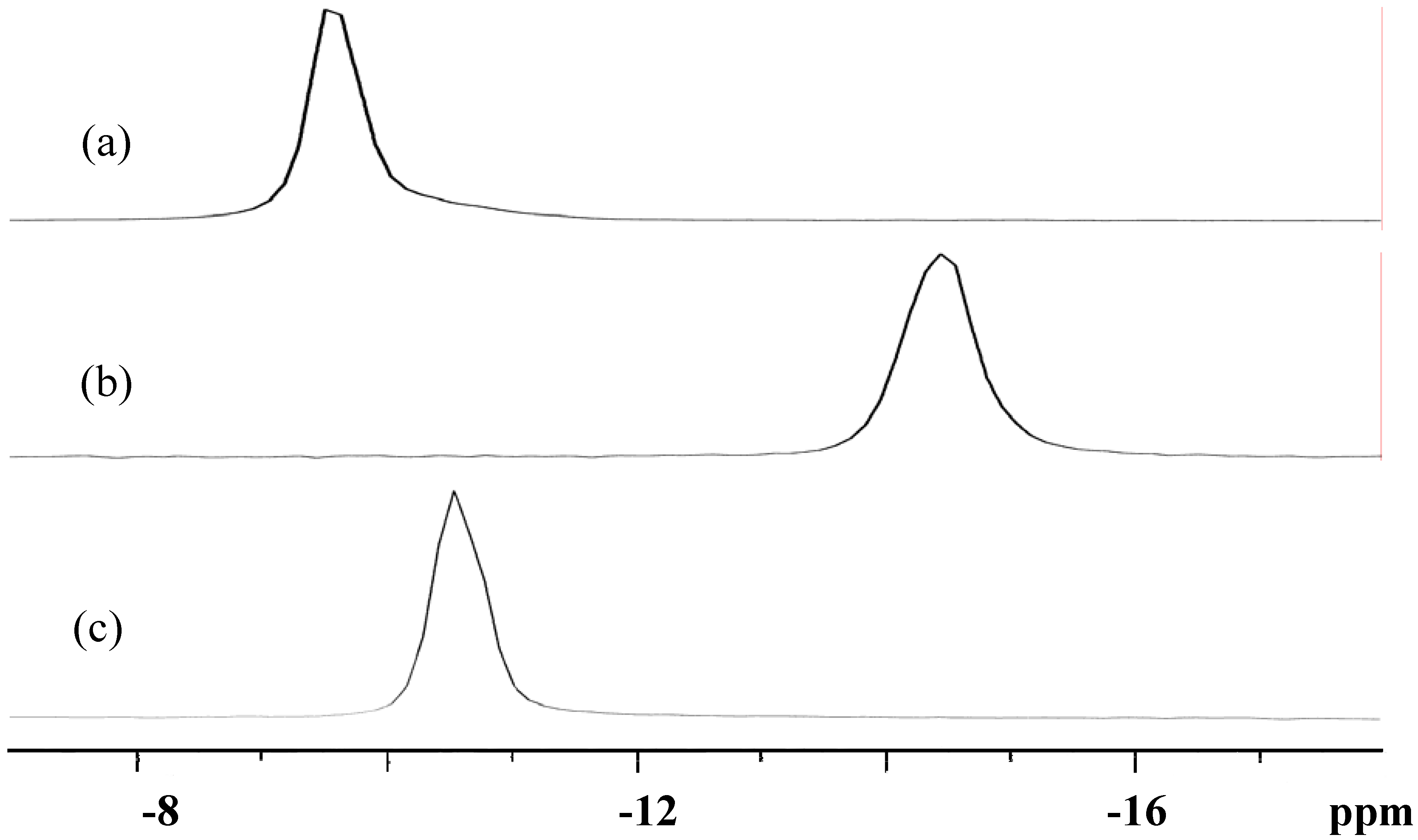
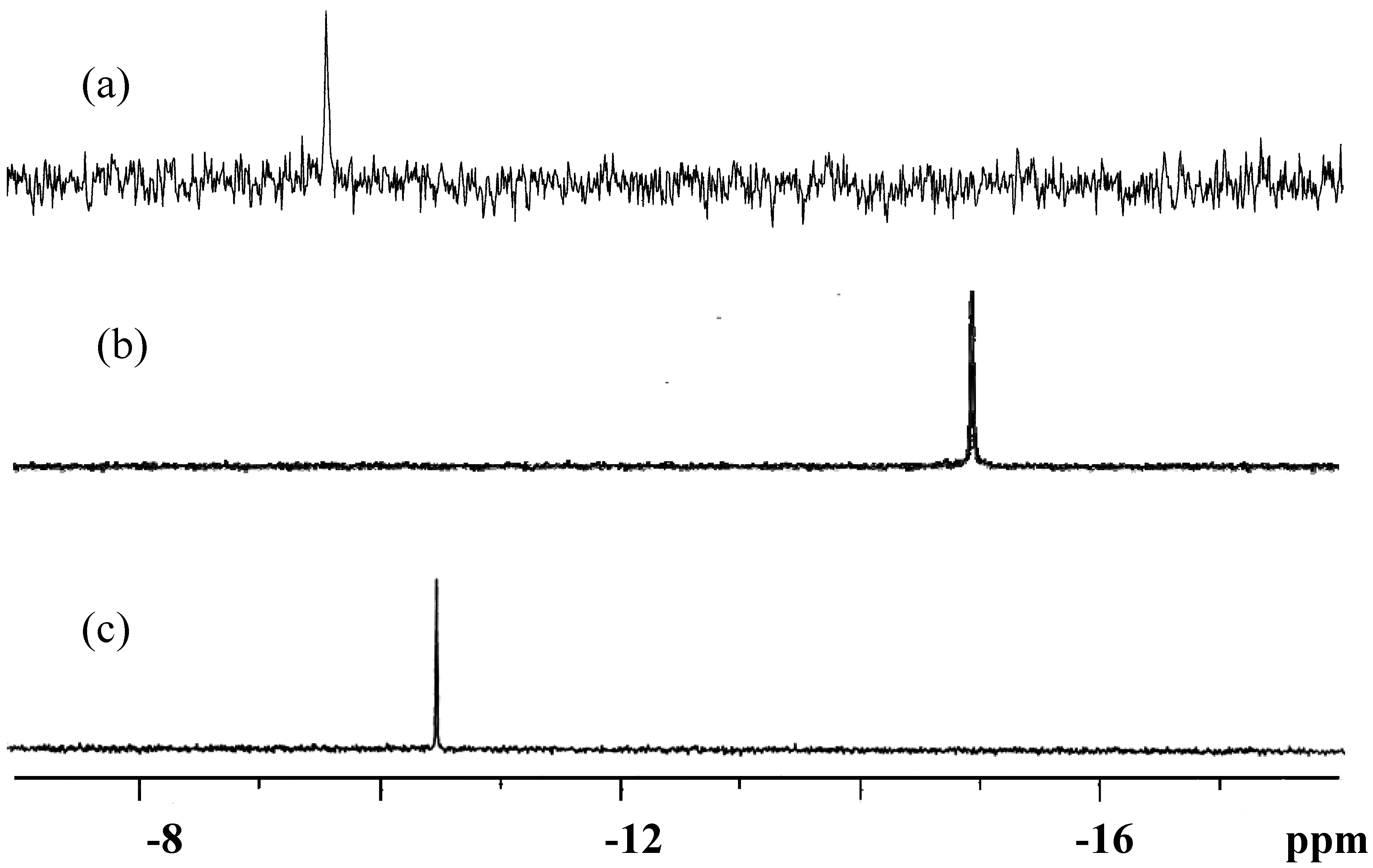
3.3. Solid-State and Solution NMR
4. Conclusions
Acknowledgements
Supplementary Materials
Supplementary File 1Supplementary File 2References and Notes
- Pope, M.T.; Müller, A. Polyoxometalate chemistry: An old field with new dimensions in several disciplines. Angew. Chem. Int. Ed. Engl. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer-Verlag: New York, NY, USA, 1983. [Google Scholar]
- Day, V.W.; Klemperer, W.G. Metal oxide chemistry in solution: The early transition metal polyoxoanions. Science 1985, 228, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L. Introduction: Polyoxometalates-multicomponent molecular vehicles to probe fundamental issues and practical problems. Chem. Rev. 1998, 98, 1–2. [Google Scholar] [CrossRef]
- Okuhara, T.; Mizuno, N.; Misono, M. Catalytic chemistry of heteropoly compounds. Adv. Catal. 1996, 41, 113–252. [Google Scholar]
- Hill, C.L.; Prosser-McCartha, C.M. Homogeneous catalysis by transition-metal oxygen anion clusters. Coord. Chem. Rev. 1995, 143, 407–455. [Google Scholar] [CrossRef]
- Pope, M.T. Polyoxo anions: Synthesis and structure. In Comprehensive Coordination Chemistry II; Wedd, A.G., Ed.; Elsevier Science: New York, NY, USA, 2004; Volume 4, pp. 635–678. [Google Scholar]
- Hill, C.L. Polyoxometalates: Reactivity. In Comprehensive Coordination Chemistry II; Wedd, A.G., Ed.; Elsevier Science: New York, NY, USA, 2004; Volume 4, pp. 679–759. [Google Scholar]
- Lin, Y.; Weakley, T.J.R.; Rapko, B.; Finke, R.G. Polyoxoanions derived from A-β-SiW9O3410- : Synthesis, single-crystal structural determination, and solution structural characterization by 183W NMR and IR of A-β-Si2W18Ti6O7714-. Inorg. Chem. 1993, 32, 5095–5101. [Google Scholar] [CrossRef]
- Yamase, T.; Ozeki, T.; Sakamoto, H.; Nishiya, S.; Yamamoto, A. Structure of hexatitanooctadecatungstodigermanate. Bull. Chem. Soc. Jpn. 1993, 66, 103–108. [Google Scholar] [CrossRef]
- Yamase, T.; Cao, X.O.; Yazaki, S. Structure of double Keggin-Ti/W-mixed polyanion [(A-β-GeTi3W9O37)2O3]14- and multielectron-transfer-based photocatalyic H2-generation. J. Mol. Catal., A Chem. 2007, 262, 119–127. [Google Scholar] [CrossRef]
- Nomiya, K.; Takahashi, M.; Ohsawa, K.; Widegren, J.A. Synthesis and characterization of tri-titanium(IV)-1,2,3-substituted α-Keggin polyoxotungstates with heteroatoms P and Si. Crystal structure of the dimeric, Ti-O-Ti bridged anhydride form K10H2[α,α-P2W18Ti6O77]·17H2O and confirmation of dimeric forms in aqueous solution by ultracentrifugation molecular weight measurements. J. Chem. Soc. Dalton Trans. 2001, 2872–2878. [Google Scholar] [CrossRef]
- Nomiya, K.; Takahashi, M.; Widegren, J.A.; Aizawa, T.; Sakai, Y.; Kasuga, N.C. Synthesis and pH-variable ultracentrifugation molecular weight measurements of the dimeric, Ti-O-Ti bridged anhydride form of a novel di-TiIV-1,2-substituted α-Keggin polyoxotungstate. Molecular structure of the [(α-1,2-PW10Ti2O39)2]10- polyoxoanion. J. Chem. Soc. Dalton Trans. 2002, 3679–3685. [Google Scholar] [CrossRef]
- Goto, Y.; Kamata, K.; Yamaguchi, K.; Uehara, K.; Hikichi, S.; Mizuno, N. Synthesis, structural characterization, and catalytic performance of dititanium-substituted γ-Keggin silicotungstate. Inorg. Chem. 2006, 45, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.X.; Li, D.L.; Wu, H.B.; Zhang, C.L.; Wang, X.H. Synthesis and structure of dititanium-containing 10-tungstogermanate [{γ-GeTi2W10O36(OH)2}2(μ-O)2]8-. Inorg. Chem. Commun. 2008, 11, 835–836. [Google Scholar] [CrossRef]
- Hussain, F.; Bassil, B.S.; Bi, L.H.; Reicke, M.; Kortz, U. Structural control on the nanomolecular scale: Self-assembly of the polyoxotungstate wheel [{β-Ti2SiW10O39}]24-. Angew. Chem. Int. Ed. 2004, 43, 3485–3488. [Google Scholar] [CrossRef]
- Al-Kadamany, G.A.; Hussain, F.; Mal, S.S.; Dickman, M.H.; Leclerc-Laronze, N.; Marrot, J.; Cadot, E.; Kortz, U. Cyclic Ti9 Keggin trimers with tetrahedral (PO4) or octahedral (TiO6) capping groups. Inorg. Chem. 2008, 47, 8574–8576. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.H.; Liu, S.X.; Cao, R.G.; Zhao, X.Y.; Cao, J.F.; Gao, C.Y. Two trimeric tri-TiIV-substituted Keggin tungstogermanates based on tetrahedral linkers. Inorg. Chem. Commun. 2008, 11, 1320–1322. [Google Scholar] [CrossRef]
- Kato, C.N.; Negishi, S.; Yoshida, K.; Hayashi, K.; Nomiya, K. The strong influence of structures around titanium centers in dimeric mono-, di-, and tri-titanium(IV)-substituted Keggin polyoxotungstates on the catalytic epoxidation of alkenes with H2O2. Appl. Catal. A Gen. 2005, 292, 97–104. [Google Scholar] [CrossRef]
- Hill, C.L. (Ed.) A series of 34 papers in a volume devoted to polyoxoanions in catalysis. J. Mol. Catal. A Chem. 1996, 114, 1–359. [CrossRef]
- Hill, C.L. (Ed.) A series of 32 recent papers in a volume devoted to Polyoxometalates in Catalysis. J. Mol. Catal. A Chem. 2007, 262, 1–242. [CrossRef]
- Hayashi, K.; Murakami, H.; Nomiya, K. Novel Ti-O-Ti bonding species constructed in a metal-oxide cluster: Reaction products of bis(oxalato)oxotitanate(IV) with the dimeric, 1,2-dititanium(IV)-substituted Keggin polyoxotungstate. Inorg. Chem. 2006, 45, 8078–8085. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Takahashi, M.; Nomiya, K. Ti-O-Ti bonding species constructed in a metal-oxide cluster. Dalton Trans. 2005, 3751–3756. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Crystallogr. Sect. A 1990, 46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97 Program for Crystal Structure Refinement; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SADABS; University of Gottingen: Gottingen, Germany, 1996. [Google Scholar]
- Thouvenot, R.; Fournier, M.; Franck, R.; Rocchiccioli-Deltcheff, C. Vibrational investigations of polyoxometalates. 3. Isomerism in molybdenum(VI) and tungsten(VI) compounds related to the Keggin structure. Inorg. Chem. 1984, 23, 598–605. [Google Scholar] [CrossRef]
- Brown, I.D.; Shannon, R.D. Empirical bond-strength-bond-length curves for oxides. Acta Crystallogr., Sect. A 1973, 29, 266–282. [Google Scholar] [CrossRef]
- Brown, I.D. VALENCE: A program for calculating bond valences. J. Appl. Crystallogr. 1996, 29, 479–480. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mouri, Y.; Sakai, Y.; Kobayashi, Y.; Yoshida, S.; Nomiya, K. Transformation of Tri-Titanium(IV)-Substituted α-Keggin Polyoxometalate (POM) into Tetra-Titanium(IV)-Substituted POMs : Reaction Products of Titanium(IV) Sulfate with the Dimeric Keggin POM Precursor under Acidic Conditions. Materials 2010, 3, 503-518. https://doi.org/10.3390/ma3010503
Mouri Y, Sakai Y, Kobayashi Y, Yoshida S, Nomiya K. Transformation of Tri-Titanium(IV)-Substituted α-Keggin Polyoxometalate (POM) into Tetra-Titanium(IV)-Substituted POMs : Reaction Products of Titanium(IV) Sulfate with the Dimeric Keggin POM Precursor under Acidic Conditions. Materials. 2010; 3(1):503-518. https://doi.org/10.3390/ma3010503
Chicago/Turabian StyleMouri, Yuki, Yoshitaka Sakai, Yoshitaka Kobayashi, Shoko Yoshida, and Kenji Nomiya. 2010. "Transformation of Tri-Titanium(IV)-Substituted α-Keggin Polyoxometalate (POM) into Tetra-Titanium(IV)-Substituted POMs : Reaction Products of Titanium(IV) Sulfate with the Dimeric Keggin POM Precursor under Acidic Conditions" Materials 3, no. 1: 503-518. https://doi.org/10.3390/ma3010503