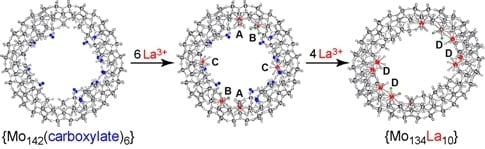
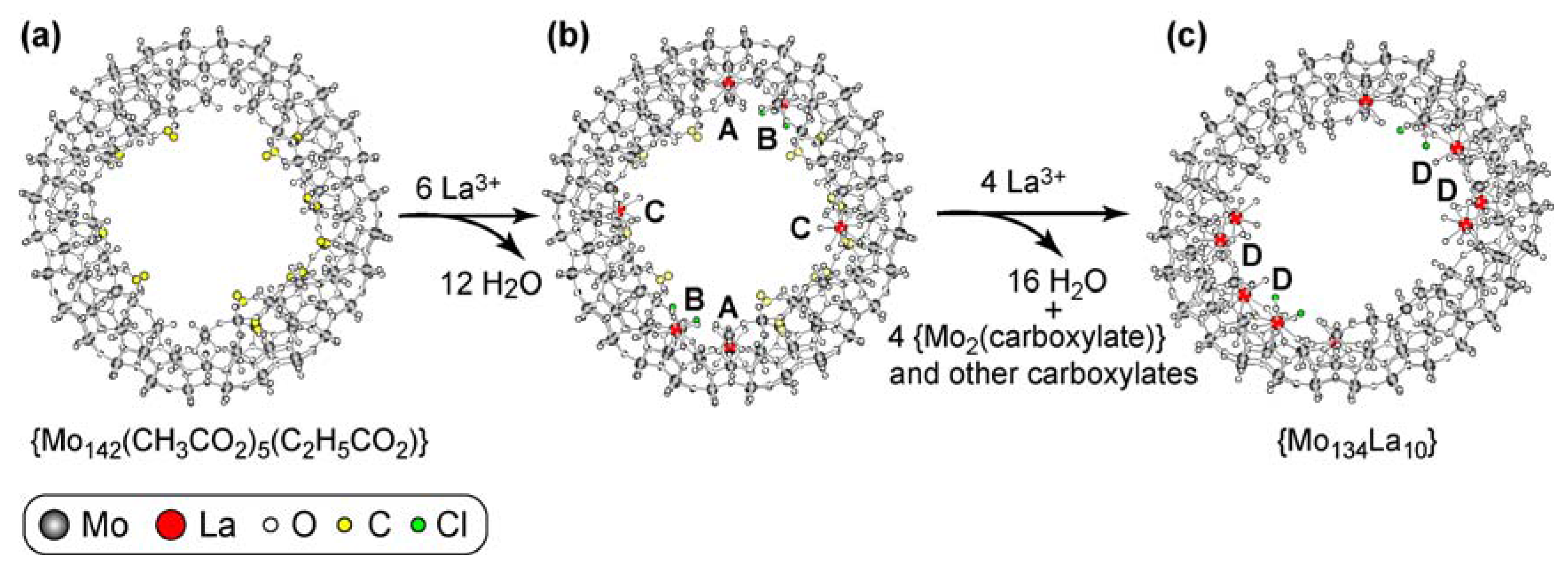
Coordination of {Mo142} Ring to La3+ Provides Elliptical {Mo134La10} Ring with a Variety of Coordination Modes
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Na10[Mo134O416H20(H2O)46{La(H2O)5}4{La(H2O)7}4{LaCl2(H2O)5}2]·144H2O
2.2. Crystal Data for Na10[Mo134O416H20(H2O)46{La(H2O)5}4{La(H2O)7}4{LaCl2(H2O)5}2]·144H2O
2.3. Isothermal Titration Calorimetry (ITC)
2.4. NMR Measurements
3. Results and Discussion
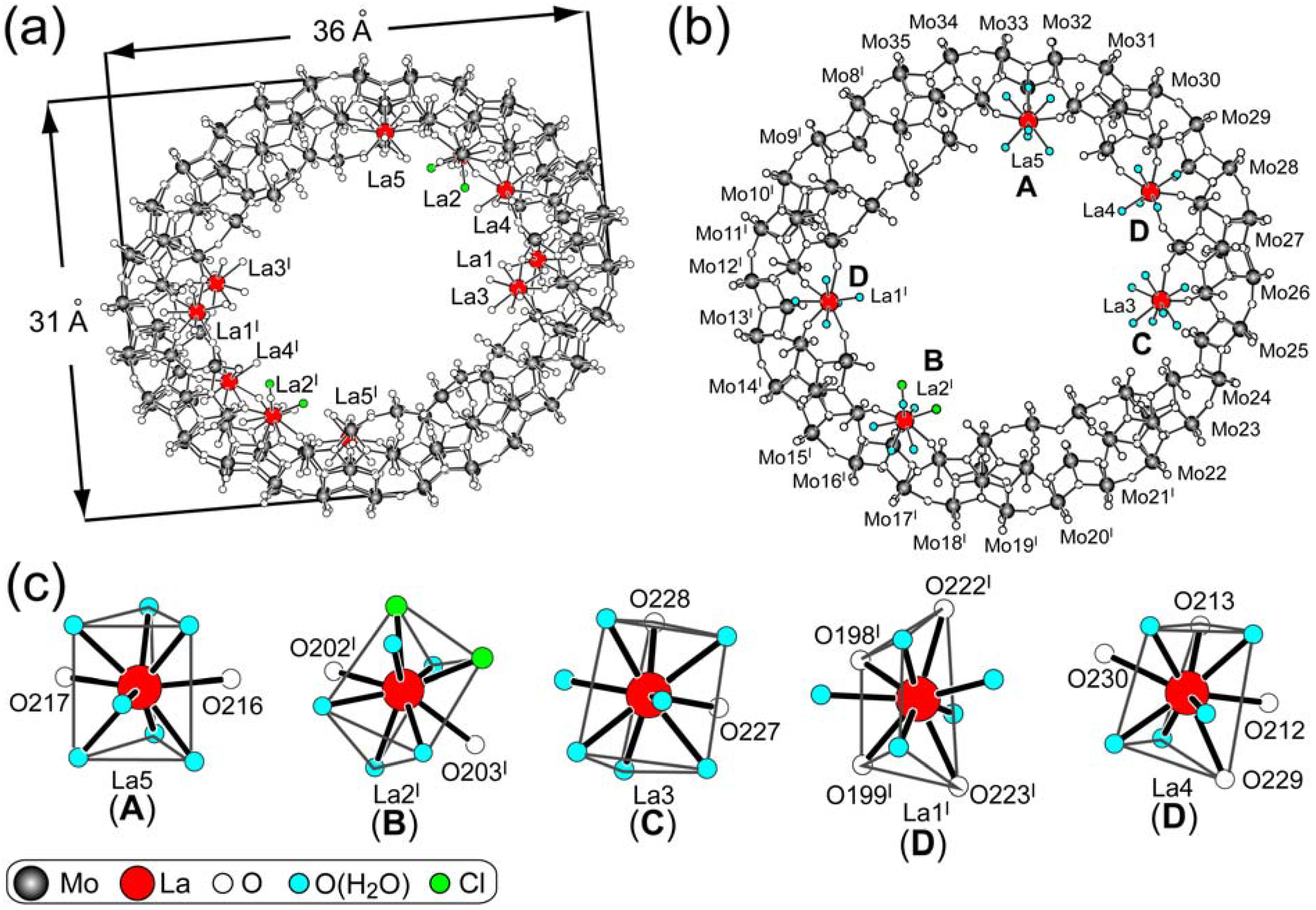
3.1. Crystal Structure of {Mo134(La)10}
3.2. Calorimetry for the Coordination of {Mo142(CH3CO2)5(C2H5CO2)} to La3+

| Temperature (K) | 278 | 293 | 313 | 333 |
| n | 5.6 | 6.1 | 6.2 | 5.8 |
| K (M-1) | 8.7 × 104 | 9.9 × 104 | 3.0 × 105 | 6.1 × 105 |
| ΔH (kJ∙mol-1) | 19 | 22 | 22 | 23 |
| ΔS (J·mol−1·K−1) | 164 | 172 | 175 | 182 |
| ΔG (kJ∙mol-1) | −28 | −28 | −33 | −37 |
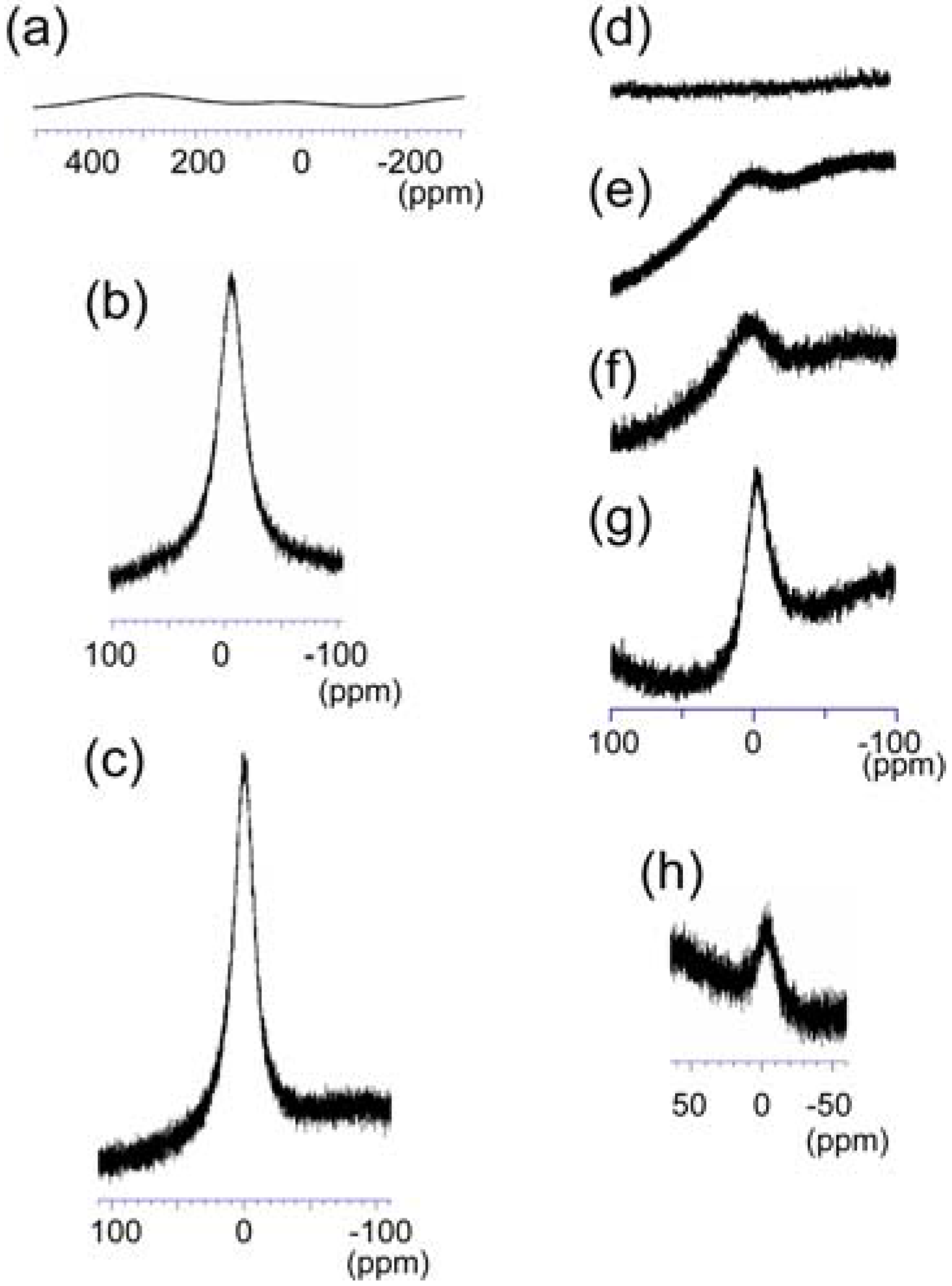
3.3. 139La-NMR Spectrometry
4. Conclusions
Acknowledgement
Supporting Information
References and Notes
- Müller, A.; Beugholt, C.; Bögge, H.; Schmidtmann, M. Influencing the size of giant rings by manipulating their curvatures: Na6[Mo120O366(H2O)48H12{Pr(H2O)5}6]⋅(~200 H2O) with open shell metal centers at the cluster surface. Inorg. Chem. 2000, 39, 3112–3113. [Google Scholar] [CrossRef]
- Yamase, T.; Ishikawa, E.; Abe, Y.; Yano, Y. Photoinduced self-assembly to lanthanide-containing molybdenum-blue superclusters and molecular design. J. Alloys Compd. 2006, 408–412, 693–700. [Google Scholar] [CrossRef]
- Cronin, L.; Beugholt, C.; Krickemeyer, E.; Schmidtmann, M.; Bögge, H.; Kögerler, P.; Kim, T.; Luong, K.; Müller, A. “Molecular symmetry breakers” generating metal-oxide-based nanoobject fragments as synthons for complex structures: [{Mo128Eu4O388H10(H2O)81}2]20-, a giant-cluster dimer. Angew. Chem. Int. Ed. Engl. 2002, 42, 2805–2808. [Google Scholar]
- Müller, A.; Das, S.K.; Fedin, V.P.; Krickemeyer, E.; Beugholt, C.; Bögge, H.; Schmidtmann, M.; Hauptfleisch, B. Rapid and simple isolation of the crystalline molybdenum-blue compounds with discrete and linked nanosized ring-shaped anions: Na15[MoVI126MoV28O462H14(H2O)70]0.5 [MoVI124MoV28O457H14(H2O)68]0.5⋅ca.400H2O and Na22[MoVI118MoV28O442H14(H2O)58]⋅ca.250H2O. Z. Anorg. Allg. Chem. 1999, 625, 1187–1192. [Google Scholar]
- Müller, A.; Beugholt, C.; Koop, M.; Das, S.K.; Schmidtmann, M.; Bögge, H. Facile and optimized syntheses and structures of crystalline molybdenum blue compounds including one with an interesting high degree of defects: Na26[Mo142O432(H2O)58H14]⋅ca.300H2O and Na16[(MoO3)176(H2O)63(CH3OH)17H16]⋅ca.600H2O⋅ca.6CH3OH. Z. Anorg. Allg. Chem. 1999, 625, 1960–1962. [Google Scholar]
- Müller, A.; Krickemeyer, E.; Bögge, H.; Schmidtmann, M.; Beugholt, C.; Kögerler, P.; Lu, C. Formation of a ring-shaped reduced “metal oxide” with the simple composition[(MoO3)176(H2O)80H32]. Angew. Chem. Int. Ed. Engl. 1998, 37, 1220–1223. [Google Scholar]
- Yamase, T.; Prokop, P. Photochemical formation of tire-shaped molybdenum blues: Topology of a defect anion, [Mo142O432H28(H2O)58]12-. Angew. Chem. Int. Ed. Engl. 2002, 37, 466–469. [Google Scholar]
- Yamase, T.; Prokop, P.; Arai, Y. Photochemical studies of alkylammonium molybdates. Part 12. O → Mo charge-transfer triplet-states-initiated self-assembly to {Mo154} ring- and tube-molybdenum-blues. J. Mol. Struct. 2003, 656, 107–117. [Google Scholar] [CrossRef]
- Yamase, T.; Prokop, P. Unpublished work.
- Yamase, T.; Yano, Y.; Ishikawa, E. Photoreductive self-assembly from [Mo7O24]6- to carboxylates-coordinated {Mo142} Mo-blue nanoring in the presence of carboxylic acids. Langmuir 2005, 21, 7823–7832. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.D.; Wu, K.K. Empirical parameters for calculating cation-oxygen bond valences. Acta Crystallogr. Sect. B 1976, 32B, 1957–1959. [Google Scholar] [CrossRef]
- ITC Data Analysis in Origin, Tutorial Guide, Version 5.0; MicroCal Incorporated: Northampton, MA, USA, 1998; p. 69.
- Tarasov, V.P.; Kirakosyan, G.A.; Trots, S.V.; Buslaev, Yu.A.; Panyushkin, V.T. Investigation of Aqueous Solutions of Lanthanum(III) and Scandium(III) Salts by 139La and 45Sc NMR. Koord. Khim. 1983, 9, 205–209. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ishikawa, E.; Yano, Y.; Yamase, T. Coordination of {Mo142} Ring to La3+ Provides Elliptical {Mo134La10} Ring with a Variety of Coordination Modes. Materials 2010, 3, 64-75. https://doi.org/10.3390/ma3010064
Ishikawa E, Yano Y, Yamase T. Coordination of {Mo142} Ring to La3+ Provides Elliptical {Mo134La10} Ring with a Variety of Coordination Modes. Materials. 2010; 3(1):64-75. https://doi.org/10.3390/ma3010064
Chicago/Turabian StyleIshikawa, Eri, Yutaka Yano, and Toshihiro Yamase. 2010. "Coordination of {Mo142} Ring to La3+ Provides Elliptical {Mo134La10} Ring with a Variety of Coordination Modes" Materials 3, no. 1: 64-75. https://doi.org/10.3390/ma3010064