Calcium Phosphate Bone Cements Including Sugar Surfactants: Part One—Porosity, Setting Times and Compressive Strength
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sample preparation, mixing and sterilization
| Surfactant | Cementek + surfactant without sterilization | Cementek + surfactant sterilized after mixture |
| none | 26 | 18 |
| SE11S 0.5% | 23 | 20 |
| SE11S 1% | 27 | 22 |
| SE11S 1.5% | 30 | 25 |
| SE11S 2% | 56 | 29 |
| SE16P 1% | 31 | 24 |
| M68EC 1% | 32 | 24 |
| Cementek + surfactant without sterilization | Cementek + surfactant sterilized before mixture | |
| SE16P 1% | 31 | 29 |
| M68EC 1% | 32 | 30 |
| Cementek + surfactant sterilized before mixture | Cementek + surfactantsterilized after mixture | |
| SE16P 2% | 61 | 32 |
| M68EC 2% | 63 | 29 |
| Surfactant | Cementek + surfactant without sterilization | Cementek + surfactantsterilized after mixture |
| none | 46 | |
| SE16P 0.5% | 50 | 50 |
| SE16P 1% | 53 | 51 |
| SE16P 1.5% | 55 | 51 |
| SE16P 2% | 57 | 52 |
| SE11S 2% | 58 | 54 |
| M68EC 0.5% | 49 | 48 |
| M68EC 1% | 51 | 50 |
| M68EC 1.5% | 52 | 51 |
2.2. Setting time
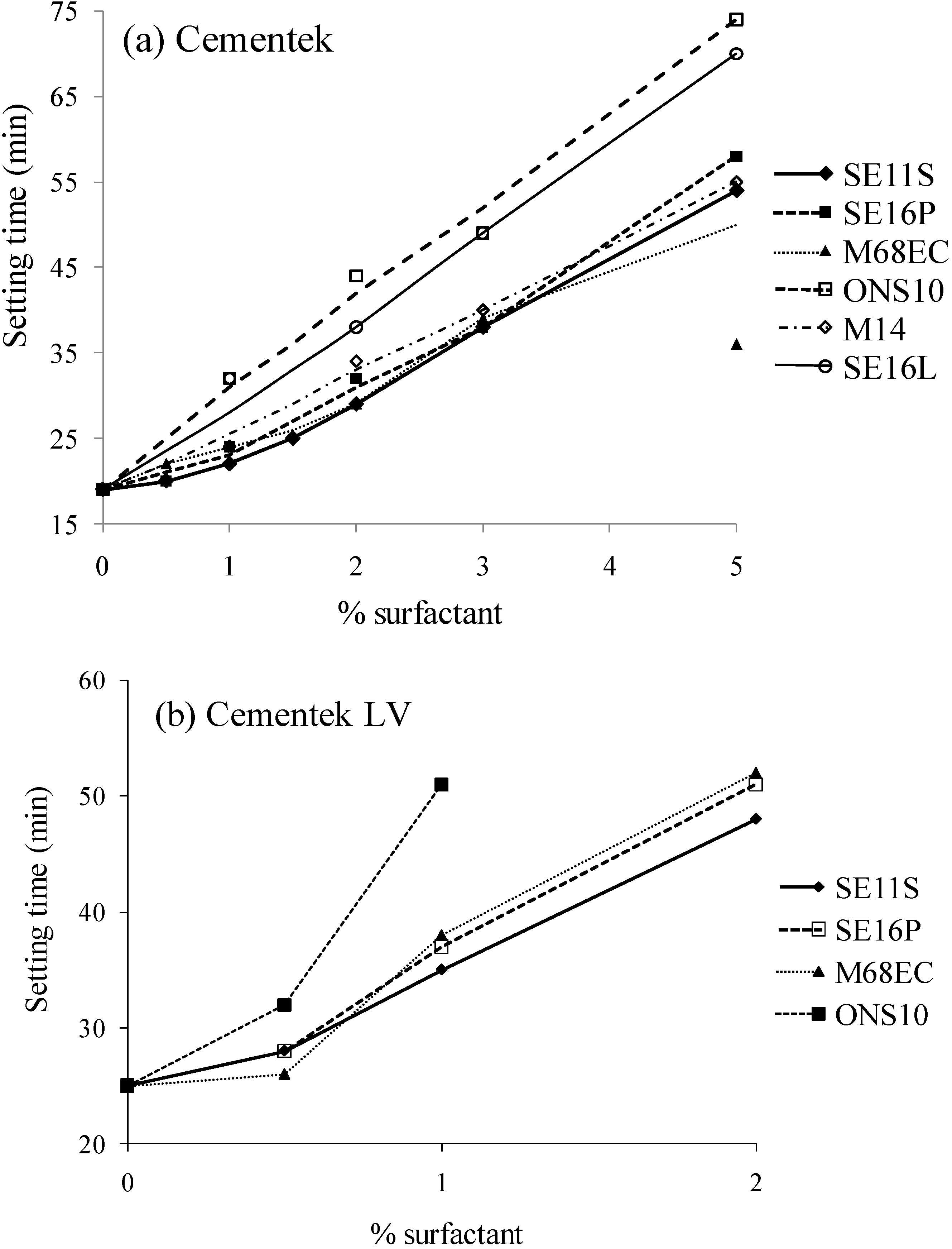
2.3. Porosity
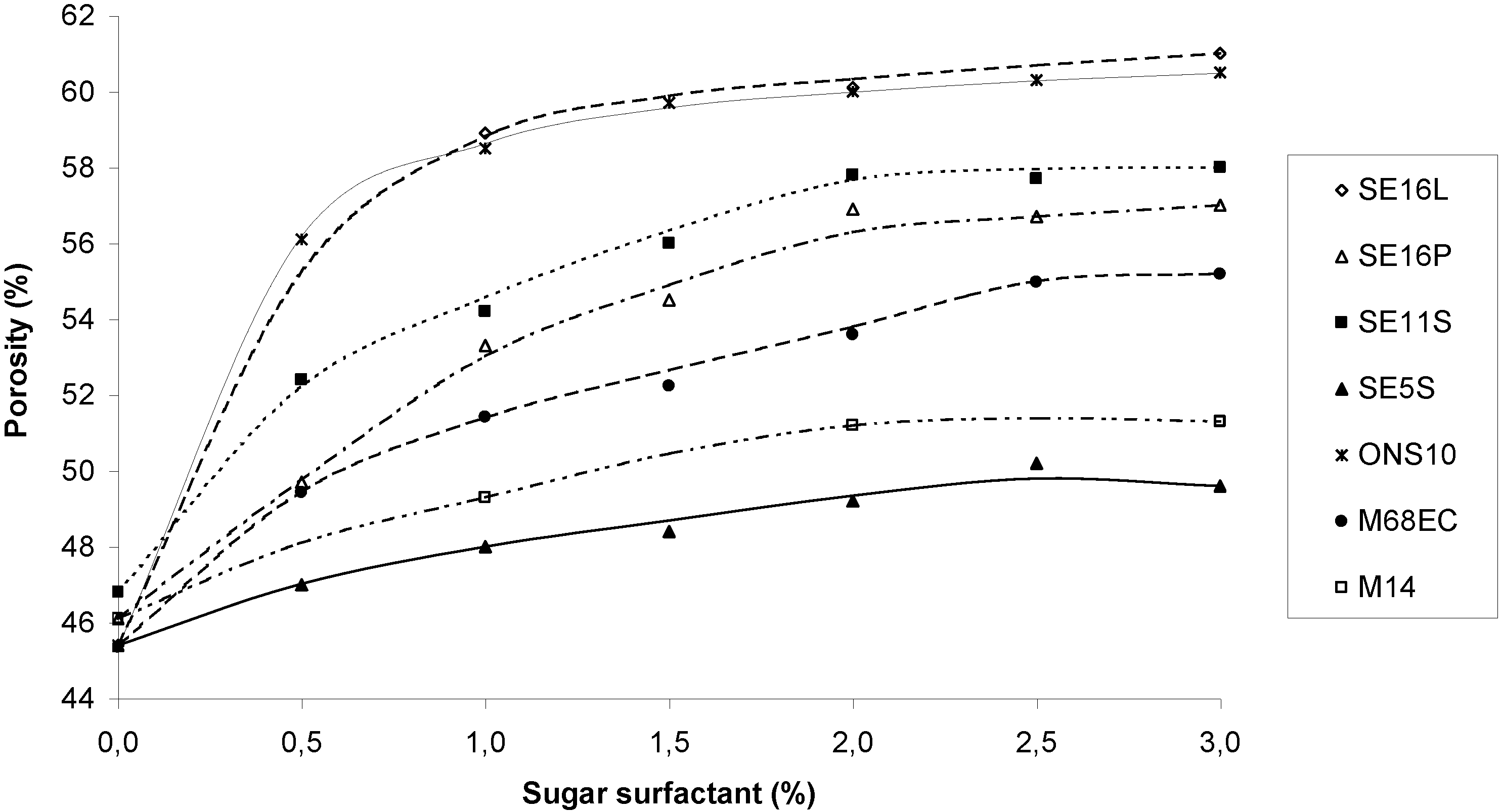

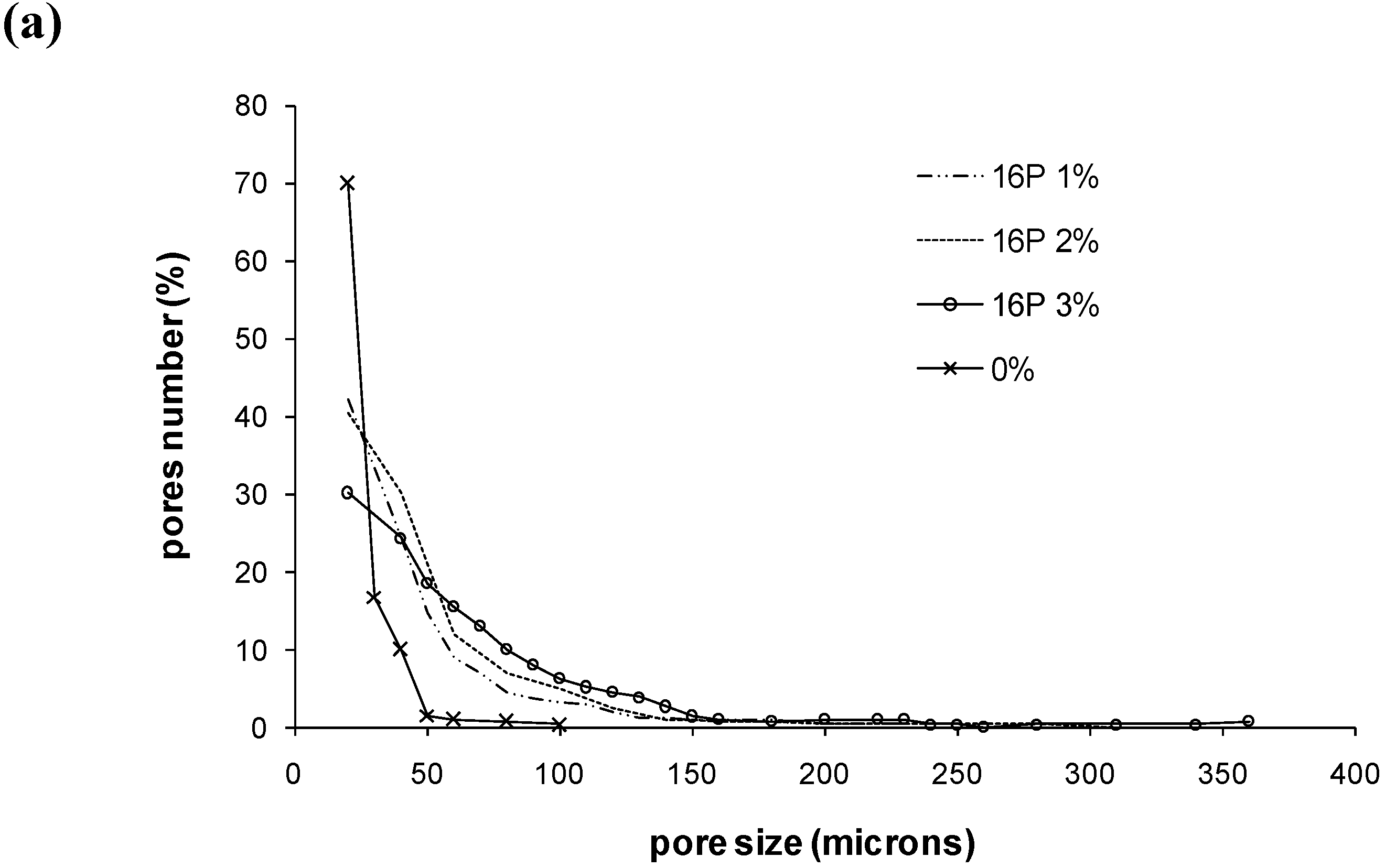

| mean pore diameter | diameter of bigger pores | pores with diameter <50µm (%) | pores with diameter ≥100 µm (%) | Number of pores for a surface 1.8 × 1.3 mm2 | |
| 0% | 26 µm | 100 µm | 96.6 | 0.3 | 71 |
| 11S 1% | 49 µm | 220 µm | 66.8 | 11.4 | 241 |
| 11S 2% | 56 µm | 230 µm | 63.1 | 12.4 | 343 |
| 11S 3% | 66 µm | 270 µm | 48.4 | 24.4 | 283 |
| 16P 1% | 48 µm | 225 µm | 65.8 | 9.4 | 231 |
| 16P 2% | 54 µm | 280 µm | 60.7 | 15.0 | 309 |
| 16P 3% | 92 µm | 360 µm | 27.9 | 33.1 | 206 |
2.4. Compressive strength

2.5. Cohesion of the pastes
| Cementek | SE11S | SE16P | SE16L | M14 | M68EC | ONS10 |
|---|---|---|---|---|---|---|
| 0% | --- | |||||
| 1% | --- | --- | + | --- | --- | +++ |
| 2% | --- | --- | + | -- | --- | +++ |
| 3% | -- | --- | ++ | + | --- | +++ |
| 5% | + | --- | ++ | -- | --- | n.d. |
| 10% | n.d. | - | n.d. | n.d. | n.d. | n.d. |
| 20% | n.d. | + (swelling) | n.d. | n.d. | n.d. | n.d. |
| Cementek LV | SE11S | SE16P | M68EC | ONS10 | |
|---|---|---|---|---|---|
| 0% | --- | ||||
| 0.5% | S | --- | --- | -- | +++ |
| NS | -- | - | - | +++ | |
| 1% | S | --- | --- | -- | n.d. |
| NS | - | - | + | n.d. | |
| 1.5% | S | --- | --- | - | n.d. |
| NS | - | - | + | n.d. | |
| 2% | S | -- | --- | + | n.d. |
| NS | + | + | +++ | n.d. | |
3. Experimental Section
4. Conclusions
Acknowledgements
References and Notes
- Dorozhkin, S.V. Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 2008, 43, 3028–3057. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury, Int. J. Care Injured 2005, 36S, S20–S27. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Gbureck, U.; Barralet, J.E. Technological issues for the development of more efficient calcium phosphate bone cements: A critical assessment. Biomaterials 2005, 26, 6423–6429. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. Injectable bone cements for use in vertebroplasty and kyphoplasty: state-of-the-art review. J. Biomed. Mater. Res.-Part B 2006, 76, 456–468. [Google Scholar] [CrossRef]
- Habib, M.; Baroud, G.; Gitzhofer, F.; Bohner, M. Mechanisms underlying the limited injectability of hydraulic calcium phosphate paste. Acta Biomater. 2008, 4, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Biresaw, G. Surfactants in lubrication. In Lubricant Additives: Chemistry and Applications, 2nd ed.; Rudnick, L.R., Ed.; CRC Press: Broken Sound Parkway, NW, USA, 2009; p. 407. [Google Scholar]
- Ginebra, M.P.; Boltong, M.G.; Fernandez, E.; Planell, J.A.; Driessens, F.C.M. Effect of various additives and temperature on some properties of an apatitic calcium phosphate cement. J. Mater. Sci.: Mater. Med. 1995, 6, 612–616. [Google Scholar] [CrossRef]
- Sarda, S.; Fernandez, E.; Nilsson, M.; Planell, J.A. Influence of air-entraining agent on bone cement macroporosity. Key Eng. Mater. 2002, 218–220, 335–338. [Google Scholar] [CrossRef]
- Sarda, S.; Nilsson, M.; Balcells, M.; Fernandez, E. Influence of surfactant molecules as air-entraining agent for bone cement macroporosity. J. Biomed. Mater. Res.-Part A 2003, 65, 215–221. [Google Scholar] [CrossRef]
- Ginebra-Molins, M.P.; Planell-Estany, J.A.; Gil-Mur, F.X. Injectable, self-setting calcium phosphate foam. Patent WO2006/030054, 2006. [Google Scholar]
- Ginebra, M.P.; Delgado, J.A.; Harr, I.; Almirall, A.; Del Valle, S.; Planell, J.A. Factors affecting the structure and properties of an injectable self-setting calcium phosphate foam. J. Biomed. Mater. Res. Part A 2007, 80, 351–361. [Google Scholar] [CrossRef]
- Delgado, J.A.; Harr, I.; Almirall, A.; del Valle, S.; Planell, J.A.; Ginebra, M.P. Injectability of a macroporous calcium phosphate cement. Key Eng. Mater. 2005, 284–286, 157–160. [Google Scholar] [CrossRef]
- Hesaraki, S.; Nemati, R. Cephalexin-loaded injectable macroporous calcium phosphate bone cement. J. Biomed. Mater. Res.-Part B 2009, 89, 342–352. [Google Scholar] [CrossRef]
- Bohner, M. Calcium phosphate emulsions: possible applications. Key Eng. Mater. 2001, 192–195, 765–768. [Google Scholar] [CrossRef]
- Muller, A.S.; Gagnaire, J.; Queneau, Y.; Karaoglanian, M.; Maître, J.P.; Bouchu, A. Winsor behaviour of sucrose fatty acid esters: Choice of the cosurfactant and effect of the surfactant composition. Colloids Surf. A 2002, 203, 55–66. [Google Scholar] [CrossRef]
- Fitremann, J.; Queneau, Y.; Maître, J.P.; Bouchu, A. Co-melting of solid sucrose and multivalent cations soaps for solvent-free synthesis of sucrose esters. Tetrahedron Lett. 2007, 48, 4111–4114. [Google Scholar] [CrossRef]
- Datsyuk, V.; Landois, P.; Fitremann, J.; Peigney, A.; Galibert, A.M.; Soula, B.; Flahaut, E. Double-walled carbon nanotubes dispersion via surfactant substitution. J. Mater. Chem. 2009, 19, 2729–2736. [Google Scholar] [CrossRef] [Green Version]
- Von Rybinski, W.; Hill, K. Alkyl polyglycosides: properties and applications of a new class of surfactants. Angew. Chem. Int. Ed. 1998, 37, 1328–1345. [Google Scholar]
- Lacout, J.L.; Hatim, Z.; Frèche-Botton, M. Procédé de préparation d'un biomatériau à base d'hydroxyapatite, biomatériau obtenu et application chirurgicale ou dentaire. Patent FR 2776282, 1998. [Google Scholar]
- Lacout, J.L.; Frèche, M.; Gonçalves, S.; Rodriguez, F. Procédé de préparation d'un matériau pâteux phosphocalcique injectable etc. Patent 2000. [Google Scholar]
- Zahraoui, C.; Sharrock, P. Influence of sterilization on injectable bone biomaterials. Bone 1999, 25, 63S–65S. [Google Scholar] [CrossRef] [PubMed]
- Takechi, M.; Miyamoto, Y.; Momota, Y.; Yuasa, T.; Tatehara, S.; Nagayama, M.; Ishikawa, K. Effects of various sterilization methods on the setting and mechanical properties of apatite cement. J. Biomed. Mater. Res. Part B 2004, 69, 58–63. [Google Scholar] [CrossRef]
- Garci Juenger, M.C.; Jennings, H.M. New insights into the effects of sugar on the hydration and micostructure of cement pastes. Cem. Concr. Res. 2002, 32, 393–399. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Ishikawa, K.; Takechi, M.; Toh, T.; Yuasa, T.; Nagayama, M.; Suzuki, K. Histological and compositional evaluations of three types of calcium phosphate cements when implanted in subcutaneous tissue immediately after mixing. J. Biomed. Mater. Res. Part B 1999, 48, 36–42. [Google Scholar] [CrossRef]
- Bohner, M.; Doebelin, N.; Baroud, G. Theoretical and experimental approach to test the cohesion of calcium phosphate pastes. Eur. Cell. Mater. 2006, 12, 26–35. [Google Scholar] [PubMed]
- Khairoun, I.; Driessens, F.C.M.; Boltong, M.G.; Planell, J.A.; Wenz, R. Addition of cohesion promotors to calcium phosphate cements. Biomaterials 1999, 20, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Pioletti, D.P.; Takei, H.; Lin, T.; Van Landuyt, P.; Ma, Q.J.; Kwon, S.Y.; Paul Sung, K.L. The effects of calcium phosphate cement particles on osteoblast functions. Biomaterials 2000, 21, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, O.; Bouler, J.M.; Weiss, P.; Bosco, J.; Aguado, E.; Daculsi, G. Short-term effects of mineral particle sizes on cellular degradation activity after implantation of injectable calcium phosphate biomaterials and the consequences for bone substitution. Bone 1999, 25, 71S–74S. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bercier, A.; Gonçalves, S.; Lignon, O.; Fitremann, J. Calcium Phosphate Bone Cements Including Sugar Surfactants: Part One—Porosity, Setting Times and Compressive Strength. Materials 2010, 3, 4695-4709. https://doi.org/10.3390/ma3104695
Bercier A, Gonçalves S, Lignon O, Fitremann J. Calcium Phosphate Bone Cements Including Sugar Surfactants: Part One—Porosity, Setting Times and Compressive Strength. Materials. 2010; 3(10):4695-4709. https://doi.org/10.3390/ma3104695
Chicago/Turabian StyleBercier, Ariane, Stéphane Gonçalves, Olivier Lignon, and Juliette Fitremann. 2010. "Calcium Phosphate Bone Cements Including Sugar Surfactants: Part One—Porosity, Setting Times and Compressive Strength" Materials 3, no. 10: 4695-4709. https://doi.org/10.3390/ma3104695





