2.1. <111>-Textured BaTiO3
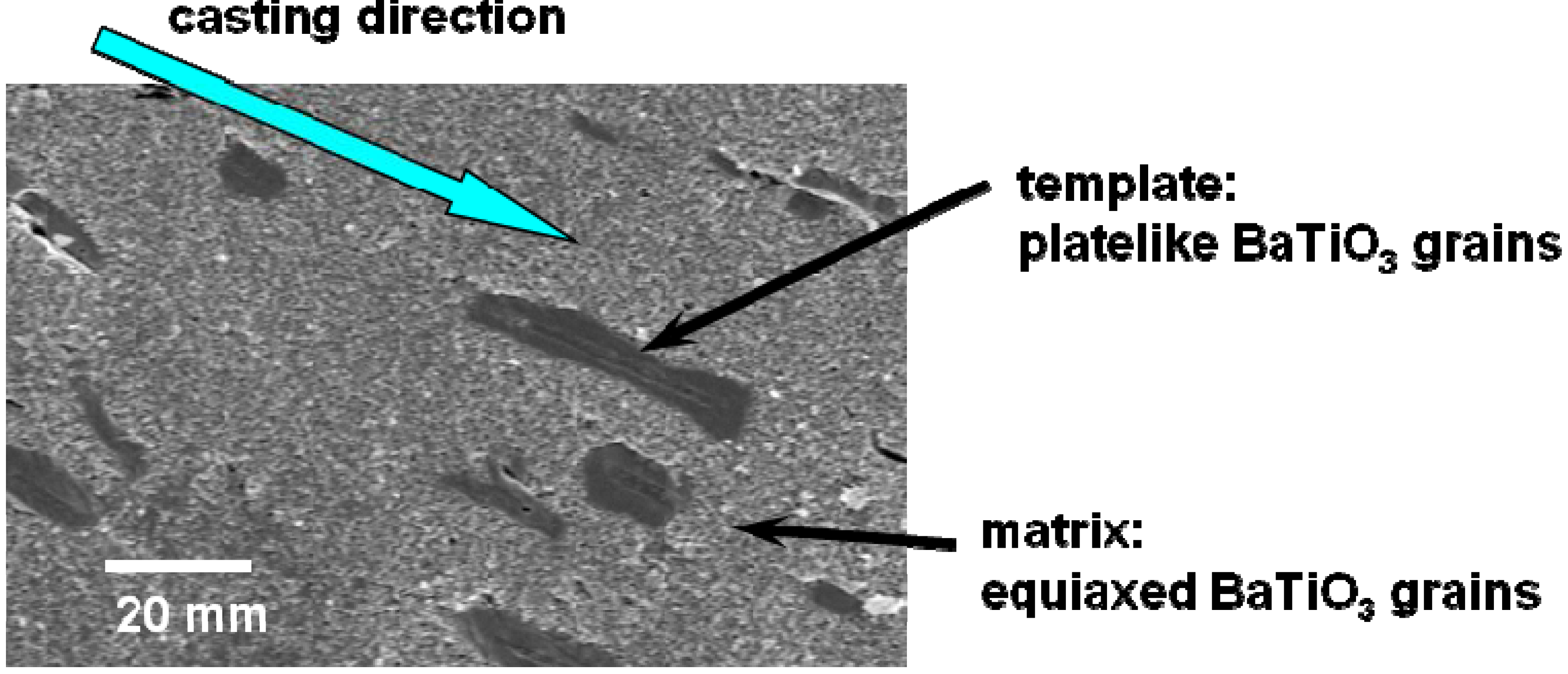
Figure 1 shows the microstructure of a calcined compact for <111>-textured BaTiO
3. The template grains were dispersed in the matrix of the matrix grains and aligned with their plate face parallel to the casting direction.
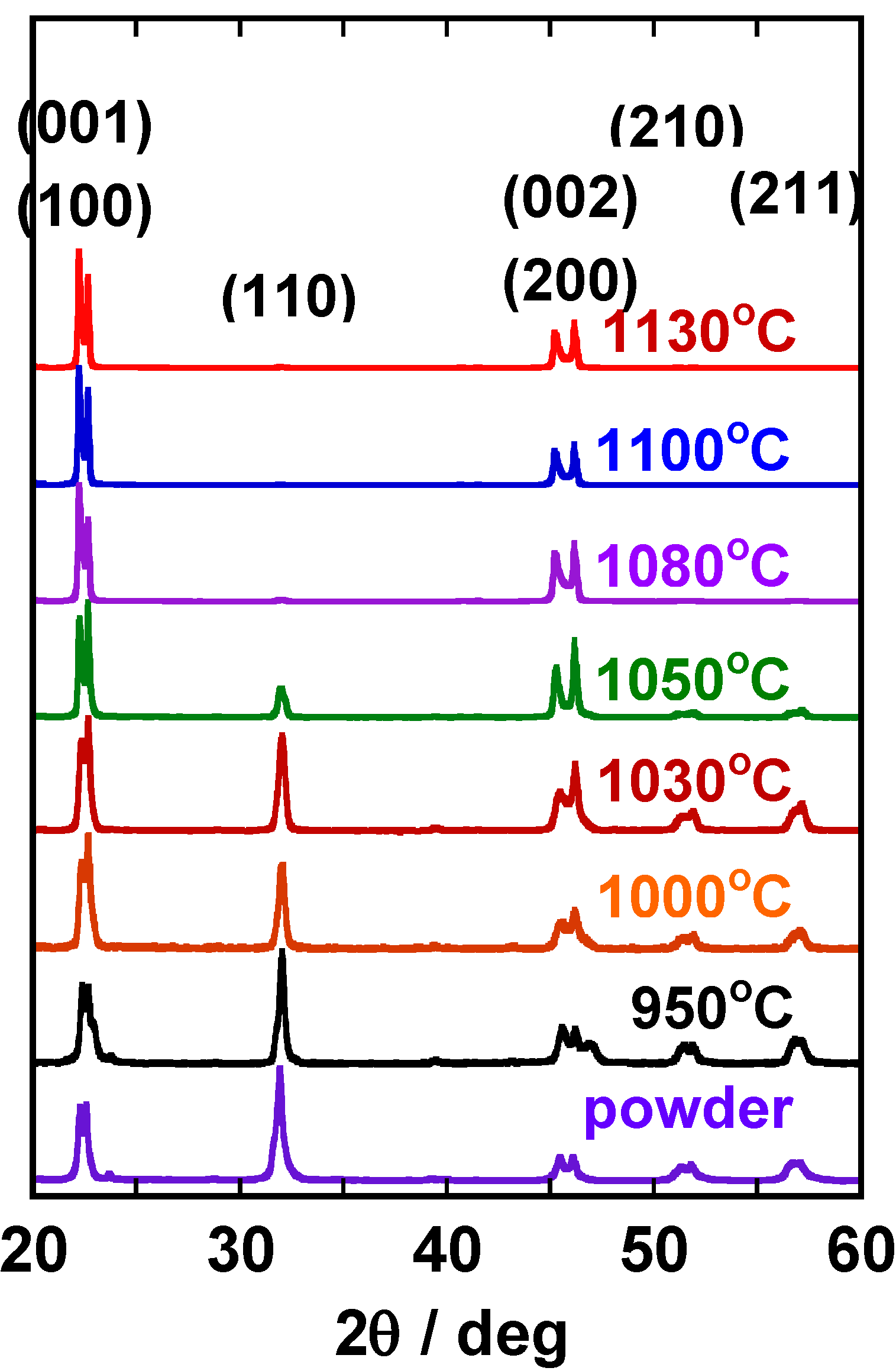
Figure 2 shows the X-ray diffraction patterns of the compacts heated at various temperatures for 2 or 5 h. For the compacts heated at temperatures below 1,300 °C, various diffraction lines were recognized, and the most intense line was (110). The relative intensity of the (111) line increased as the heating temperature was increased and the (111) line became the most intense in the specimens heated at and above 1,350 °C for 5 h.
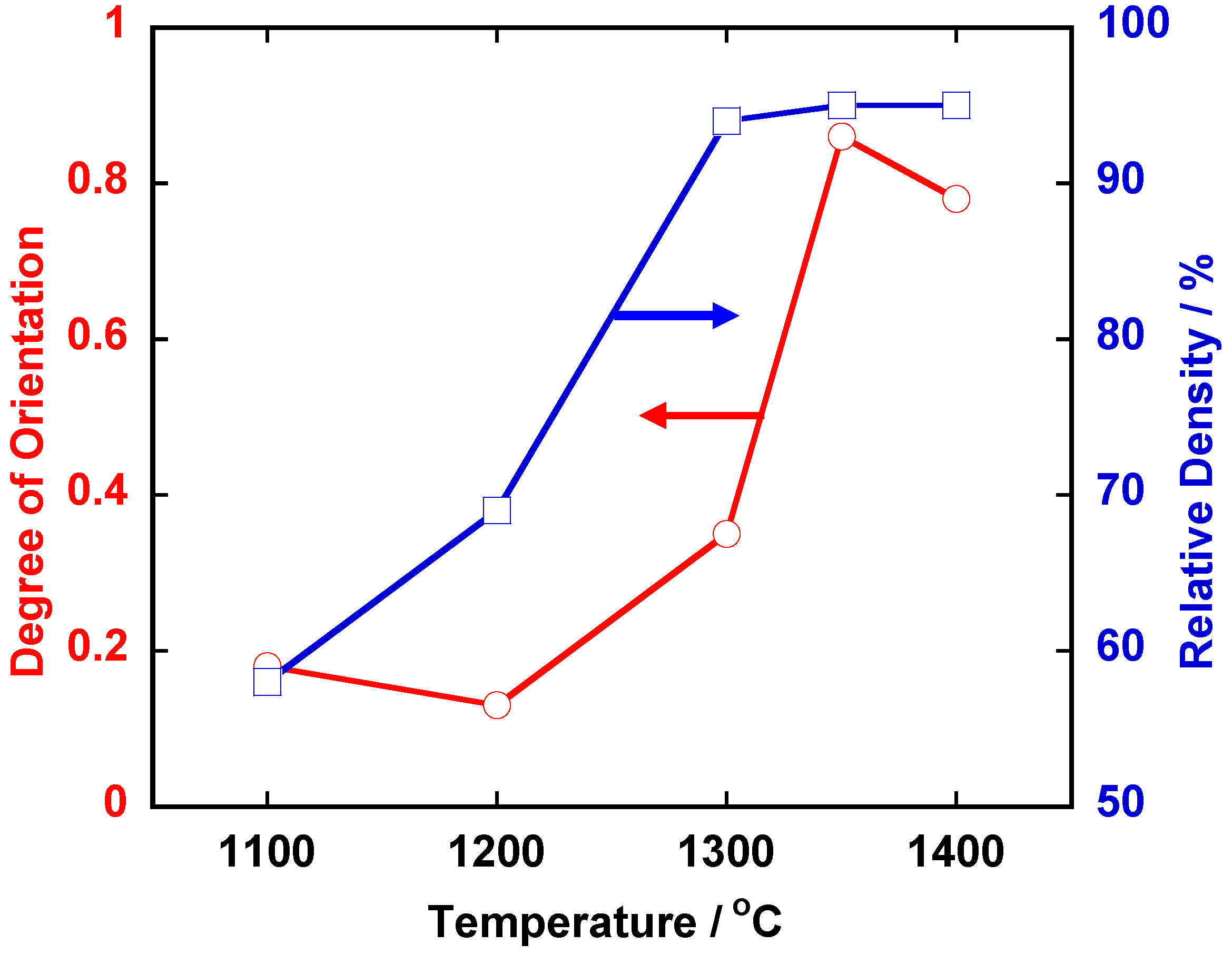
Figure 3 shows the temperature dependence of relative density and the degree of orientation of the compacts heated for 5 h. The compacts with density more than 90% and the degree of orientation of about 0.8 were obtained by heating at and above 1,350 °C.
Figure 1.
Microstructure of the BaTiO3 calcined compact containing platelike template grains dispersed in the equiaxed matrix grains. The plate faces of template grains aligned parallel to the casting direction. The <111> direction of template grains is perpendicular to the plate face.
Figure 1.
Microstructure of the BaTiO3 calcined compact containing platelike template grains dispersed in the equiaxed matrix grains. The plate faces of template grains aligned parallel to the casting direction. The <111> direction of template grains is perpendicular to the plate face.
Figure 2.
X-ray diffraction patterns of the BaTiO3 compacts heated at various temperatures. The heating condition (temperature and duration) is indicated in the figure.
Figure 2.
X-ray diffraction patterns of the BaTiO3 compacts heated at various temperatures. The heating condition (temperature and duration) is indicated in the figure.
Figure 3.
Temperature dependence of relative density and the degree of orientation in <111>-textured BaTiO3.
Figure 3.
Temperature dependence of relative density and the degree of orientation in <111>-textured BaTiO3.
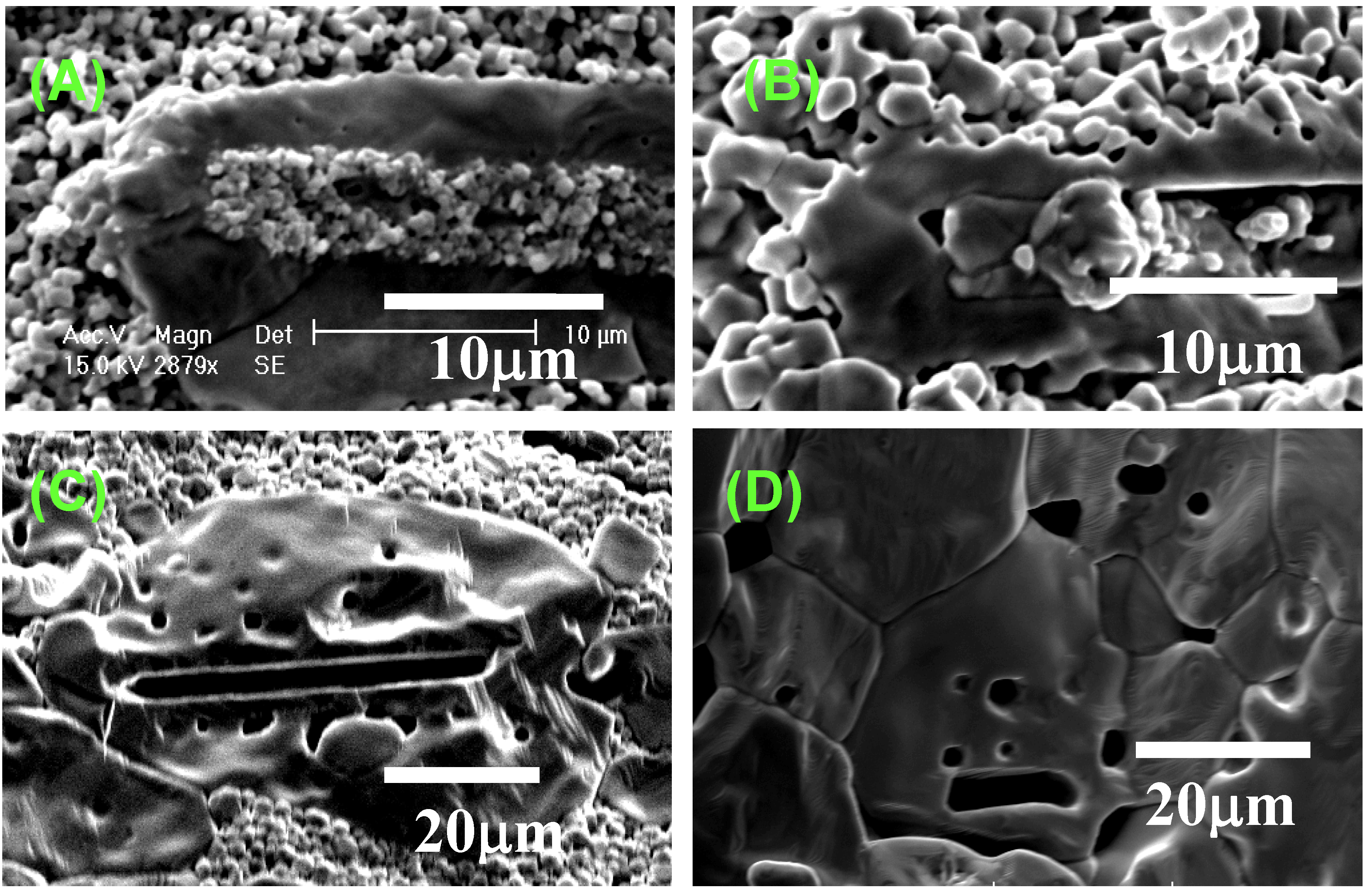
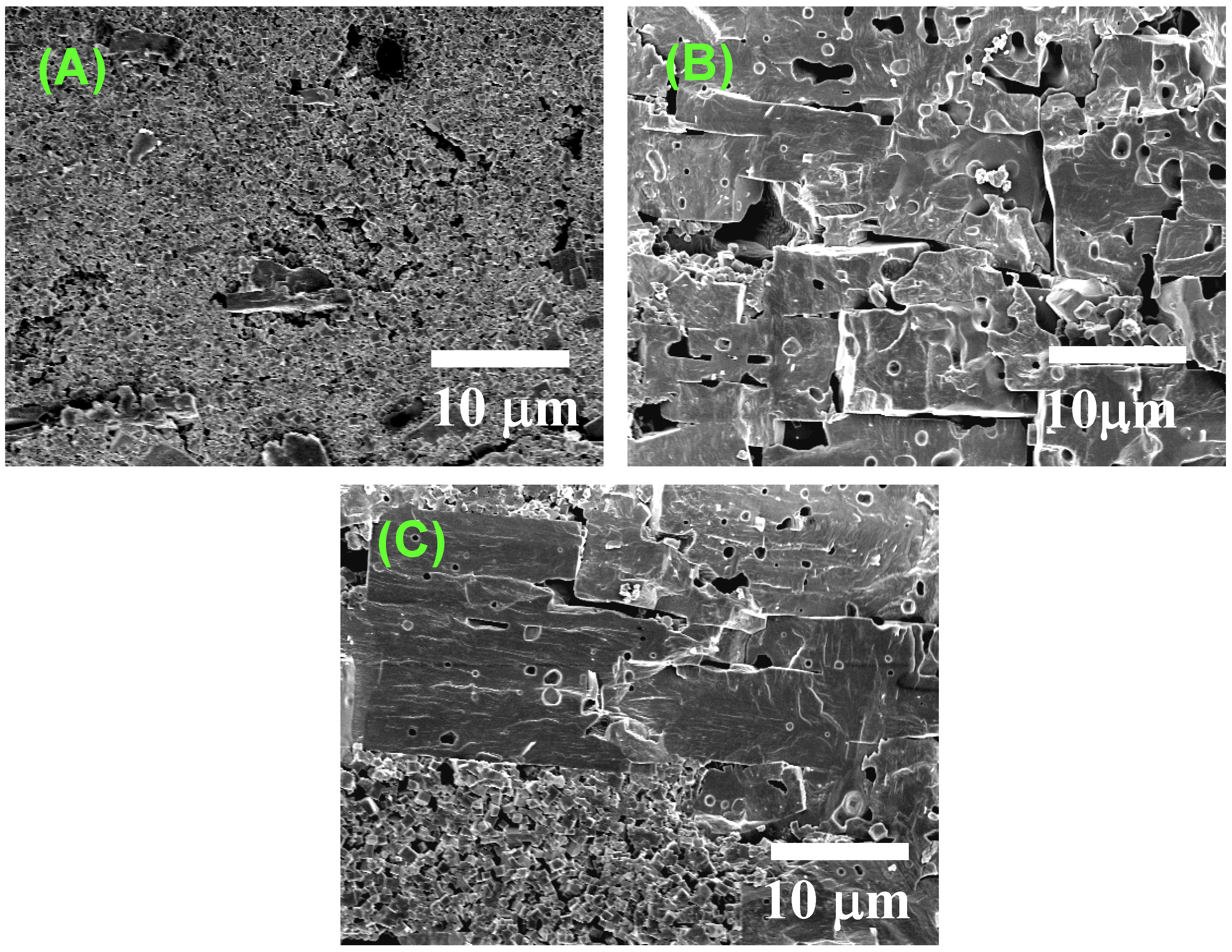
Figure 4 shows the microstructural change in <111>-textured BaTiO
3. At first, the matrix grains adhered to the surface of the template grain (
Figure 4(A)). The adhered matrix grains were integrated into the template grain (
Figure 4(B)). The size of template grains increased (
Figure 4(C)) and impinged on each other, resulting in the microstructure consisting of large equiaxed grains (
Figure 4(D)). The X-ray diffraction profiles shown in
Figure 2 indicate that each template grain is oriented with the <111> direction perpendicular to the compact surface.
The microstructures shown in
Figure 4 reveal that BaTiO
3 is textured by the growth of template grains at the expense of matrix grains. The microstructure change shown in
Figure 4(A) and
Figure 4(B) suggests that the mechanism of the growth of template grains is the migration of the boundary between template and matrix grains.
Figure 4(A) indicates the adherence of matrix grains to the template grain. The adhered matrix grains are integrated into the template grain (
Figure 4(B)). The surface of this template grain is rugged, indicating that the surface shape of the integrated matrix grains remained. This morphological change suggests the boundary migration shown in
Figure 5. The grain boundary develops between the template and adhered matrix grains (
Figure 5(A)). The balance of surface and grain boundary tension bends the grain boundary. The curved boundary migrates toward the center of curvature, resulting in the integration of the matrix grain into the template grain. The surface of the template grain just after the integration of the matrix grain is rugged and the shape of the matrix grain remained on the surface of the template grain (
Figure 5(D)). The rugged surface of the template grain becomes smooth by surface diffusion as shown in
Figure 4(C). Thus,
Figure 4 and
Figure 5 conclude that the mechanism of texture development in the present BaTiO
3 system is the growth of template grains at the expense of matrix grains by the migration of the boundary between the template and matrix grains.
Figure 4.
Microstructures of BaTiO3 compacts heated (A) at 1,200 °C for 5 h; (B) at 1,300 °C for 5 h; (C) at 1,350 °C for 2 h; and (D) at 1,400 °C for 5 h.
Figure 4.
Microstructures of BaTiO3 compacts heated (A) at 1,200 °C for 5 h; (B) at 1,300 °C for 5 h; (C) at 1,350 °C for 2 h; and (D) at 1,400 °C for 5 h.
Figure 5.
Schematic diagram of grain boundary migration through the neck region.
Figure 5.
Schematic diagram of grain boundary migration through the neck region.
Figure 4(A) shows the cross section of the template grain. The template grain is composed of two parts; the center is composed of many grains and the circumference has a smooth surface. The circumference might be a single crystal with its <111> direction perpendicular to the plate face. The structure of this template grain is not a main point of this paper but the origin of the formation of the central part is discussed here. The platelike BaTiO
3 grain is formed by the reaction of a Ba
6Ti
17O
40 (B6T17) grain with BaCO
3 by the unidirectional diffusion of BaO [
9]. At first the surface of the B6T17 grain changes to a BaTiO
3 layer surrounding remnant B6T17 and the reaction continues by the diffusion of BaO through the BaTiO
3 layer. Because the volume of product BaTiO
3 is 23%, as large as that of reactant B6T17, the stresses develop in BaTiO
3 at the central part of the platelike grain. These stresses result in the formation of polycrystalline particles at the center of the platelike grain. Furthermore, stressed BaTiO
3 grains have high energy and migrate to the surface of the template grain at high temperatures, resulting in the formation of a rectangular void at the center of the template grain (
Figure 4(C)).
2.2. <100>-Textured (K,Na,Li)(Nb,Ta)O3 Solid Solution
Figure 6 shows the X-ray diffraction patterns of the (K,Na,Li)(Nb,Ta)O
3 compacts heated at various temperatures for 1 h. The most intense line was (110) in the compact heated at 950 °C. The intensity of (001), (100), (002), and (200) increased as the heating temperature was increased, and finally diffraction lines other than (001), (100), (002), and (200) were not recognized. This means that platelike NaNbO
3 template grains develop <100>-texture in the (K,Na,Li)(Nb,Ta)O
3 matrix.
Figure 7 shows the temperature dependence of the degree of orientation. An abrupt change in the degree of orientation occurred between 1,030 °C and 1,050 °C.
Figure 7 also shows the same dependence for Bi
0.5(Na
0.5K
0.5)TiO
3 (BNKT) [
7]. In the case of BNKT, the temperature dependence is rather gentle. In BNKT, the mechanism of texture development is the growth of template grains by solid state spreading, as will be mentioned in
Section 2.3. The steep temperature dependence in (K,Na,Li)(Na,Ta)O
3 suggests another mechanism for texture development.
Figure 6.
X-ray diffraction patterns of the (K,Na,Li)(Na,Ta)O3 compacts heated at various temperatures for 1 h. The heating temperature is indicated in the figure.
Figure 6.
X-ray diffraction patterns of the (K,Na,Li)(Na,Ta)O3 compacts heated at various temperatures for 1 h. The heating temperature is indicated in the figure.
Figure 7.
Temperature dependence of the degree of orientation of (K,Na,Li)(Na,Ta)O3 and Bi0.5(Na0.5K0.5)0.5TiO3 (BNKT).
Figure 7.
Temperature dependence of the degree of orientation of (K,Na,Li)(Na,Ta)O3 and Bi0.5(Na0.5K0.5)0.5TiO3 (BNKT).
Figure 8.
Microstructures of (K,Na,Li)(Na,Ta)O3 compacts heated at 1,040 °C for 0 min (A) and for 15 min (B, C).
Figure 8.
Microstructures of (K,Na,Li)(Na,Ta)O3 compacts heated at 1,040 °C for 0 min (A) and for 15 min (B, C).
Figure 8 shows typical microstructures of the compacts heated at a temperature between 1,030 °C and 1,050 °C.
Figure 8(A) shows the microstructure of the specimen heated at 1,040 °C for 0 min (the specimen was quenched just after the furnace temperature reached 1,040 °C). The microstructure was almost the same as that of the calcined compact.
Figure 8(B) shows the microstructure of the specimen heated at 1,040 °C for 15 min and then quenched. The major part of the compact was composed of large brick-like grains. The specimens heated at the temperature between 1,030 °C and 1,050 °C for 0 to 30 min had either microstructure and it was difficult to prepare a specimen having the brick-like and matrix grains with almost the same volume. The area, which was composed of small matrix grains and surrounded by several brick-like grains, was found by a close examination of the specimen heated at 1,040 °C for 15 min (
Figure 8(C)). The coexistence of large grains with flat surfaces and small grains is a typical microstructure formed by abnormal grain growth. The presence of many intragrain pores is additional evidence of abnormal grain growth. These characteristics,
i.e., an abrupt increase in the degree of orientation, the formation of large brick-like grains in the matrix of small grains and the presence of the intragrain pores in the brick-like grains, are quite similar to those observed in BaTiO
3 textured by platelike Ba
6Ti
17O
40 hetero-template grains, in which abnormal grain growth is the dominant mechanism of texture development [
10]. When the compacts without template grains were heated at a temperature between 1,030 °C and 1,050 °C, the grains grew to about 10 μm with mono-modal grain size distribution. The addition of the template grains changed the grain size distribution to bi-modal (
Figure 8(C)). This indicates that the abnormal grain growth in the present system is nucleation-controlled [
11] but not diffusion-controlled [
12,
13].
2.3. Mechanisms of Texture Development
BaTiO
3, BNKT, and (K,Na,Li)(Na,Ta)O
3 have the same crystal structure (perovskite), but the mechanism of texture development is different. The mechanisms are (1) the growth of template grains by the migration of the boundary between template and matrix grains, (2) the growth of template grains by the solid state spreading of matrix grains, and (3) abnormal grain growth. Mechanisms (1) and (3) are explained in
Section 2.1 and
Section 2.2, respectively. Here, mechanism (2) is briefly reviewed.
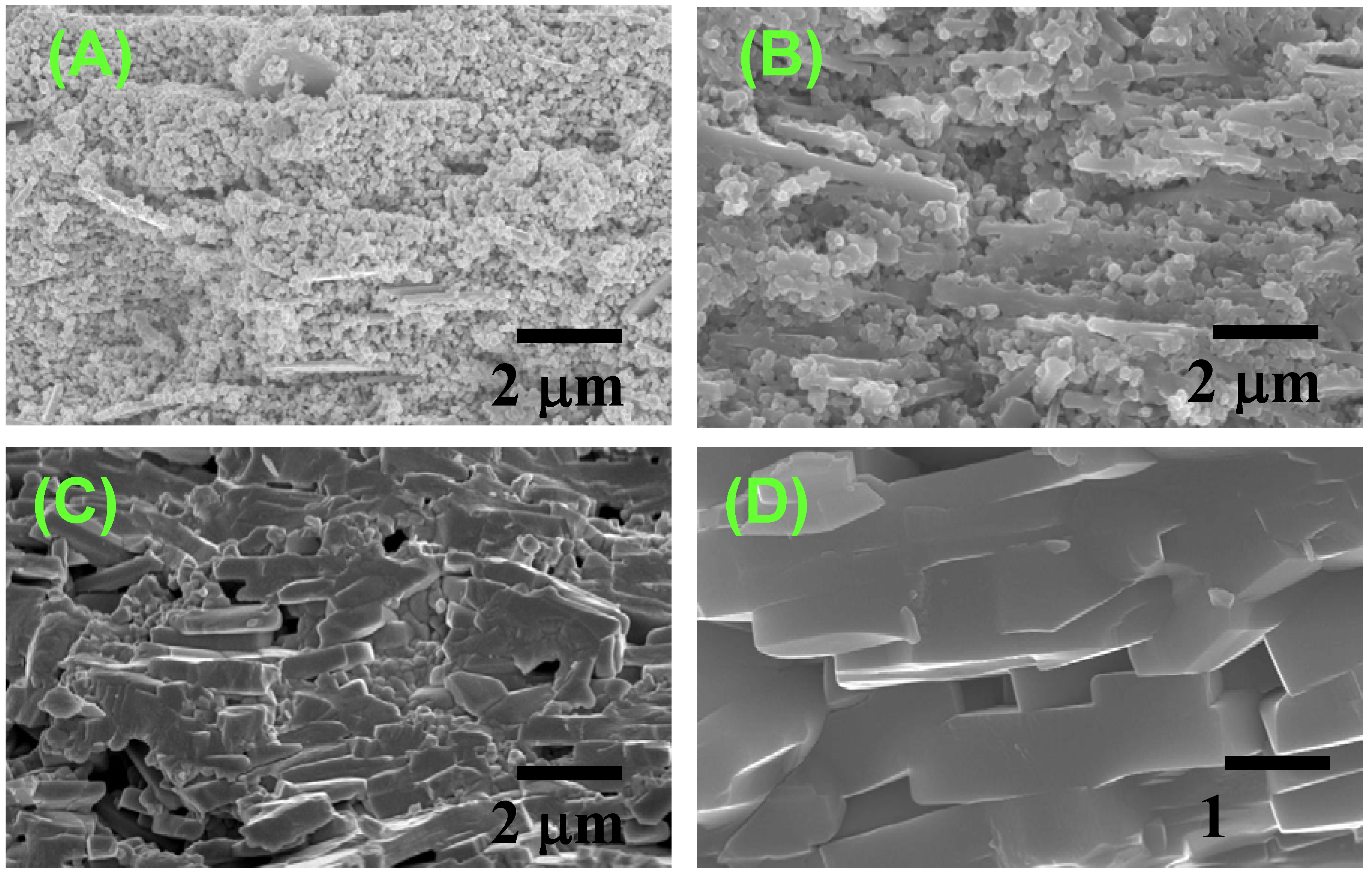
Figure 9 shows the microstructure development in the BNKT system [
7]. The specimen shown in
Figure 9(A) is composed of aligned template grains and randomly oriented matrix grains. The size of matrix grains and the thickness of template grains increase up to 1,000 °C (
Figure 9(B)). The growth of template grains continues, whereas the volume of matrix grains is decreased (
Figure 9(C)), and finally, the specimen is composed of only platelike template grains (
Figure 9(D)). This microstructure development indicates that the texture is developed by the growth of template grains.
Figure 10 shows the morphological change of matrix grains at an early stage [
7]. The matrix grains adhere to the template grain (
Figure 10(A)) and spread over the surface of the template grain (
Figure 10(B)). A close look at
Figure 10(B) reveals the presence of a groove on the surface of the matrix grains. The groove suggests the formation of a third grain between the template and matrix grains. To confirm the formation of the third grain, a composite composed of a SrTiO
3 single crystal substrate and Bi
0.5Na
0.5TiO
3 particles on the substrate was heated as a model experiment [
8]. The specimen was prepared by dropping a suspension containing Bi
0.5Na
0.5TiO
3 particles (average particle size of about 0.5 μm) in 2-methoxyethanol and drying. Almost a single layer of Bi
0.5Na
0.5TiO
3 particles was formed on the (100) surface of SrTiO
3.
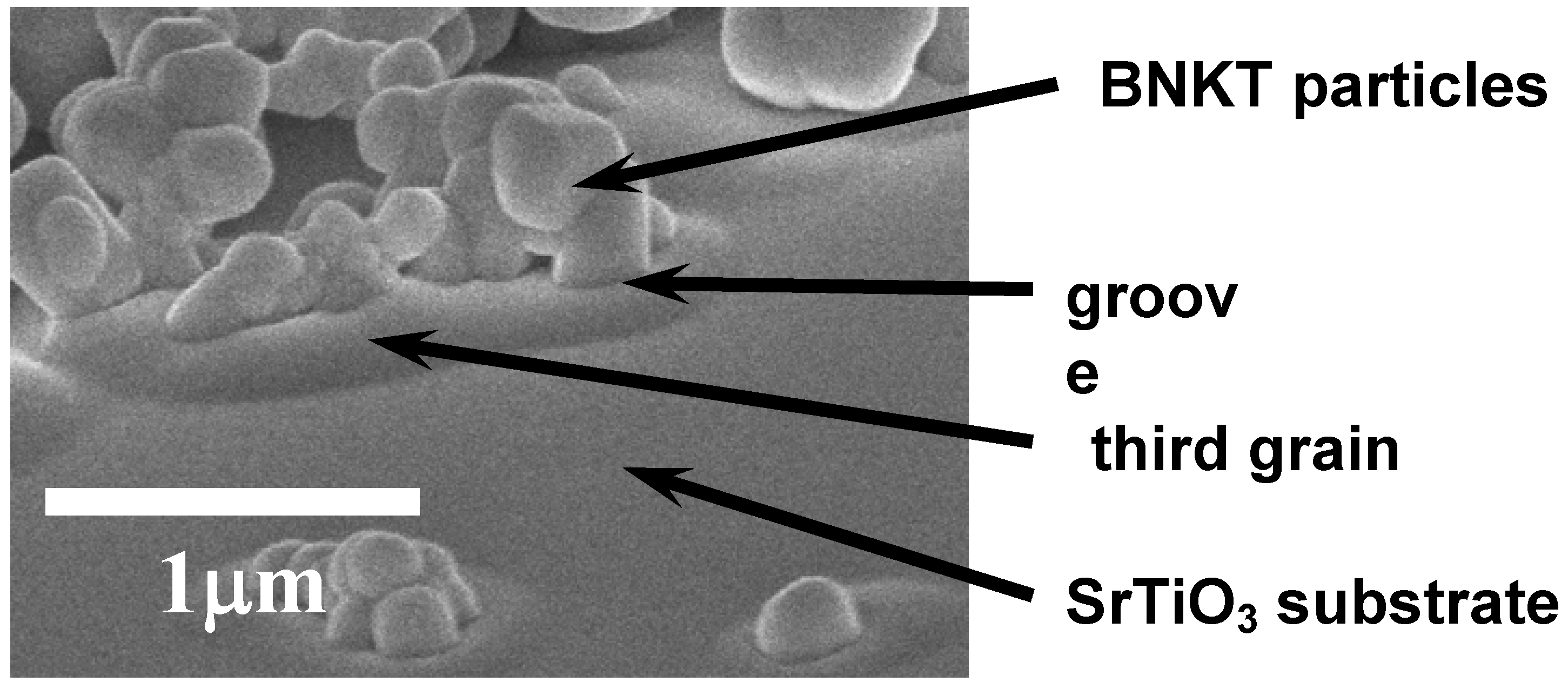
Figure 11 shows the microstructure of the composite heated at 900 °C for 2 h. The positions of SrTiO
3 and Bi
0.5Na
0.5TiO
3 are shown in the figure. The third grains were formed between the SrTiO
3 substrate and Bi
0.5Na
0.5TiO
3 particles, and the grooves were observed between Bi
0.5Na
0.5TiO
3 particles and third grains. This microstructure is quite similar to that shown in
Figure 10(B). The mechanism of the formation of the third grain is not neck growth but solid stage spreading [
14].
Figure 9.
Microstructures of Bi0.5(Na0.5K0.5)0.5TiO3 compacts heated at (A) 900 °C; (B) 1,000 °C; (C) 1,050 °C; and (D) 1,100 °C for 2 h.
Figure 9.
Microstructures of Bi0.5(Na0.5K0.5)0.5TiO3 compacts heated at (A) 900 °C; (B) 1,000 °C; (C) 1,050 °C; and (D) 1,100 °C for 2 h.
Figure 10.
Microstructures at different positions of Bi0.5(Na0.5K0.5)0.5TiO3 compact heated at 1,000 °C for 2 h.
Figure 10.
Microstructures at different positions of Bi0.5(Na0.5K0.5)0.5TiO3 compact heated at 1,000 °C for 2 h.
The texture development is closely related to the grain growth behavior.
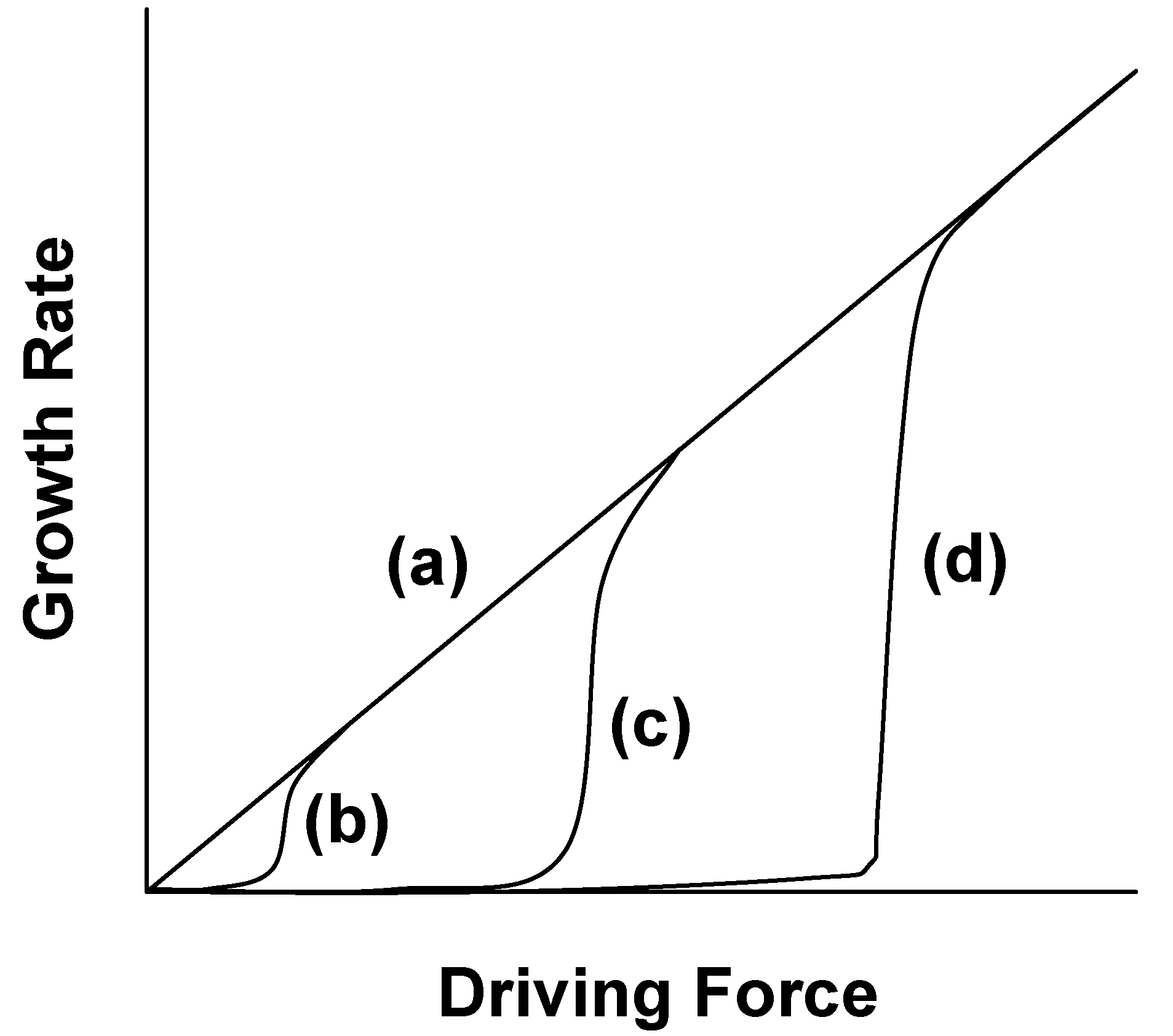
Figure 12 shows the relation between the grain growth rate and driving force [
15]. The grain growth behavior is roughly divided into two groups depending on the surface structure. When the surface structure is atomically rough, the growth rate is proportional to the driving force, as shown by curve (a) in
Figure 12. When the surface structure is atomically smooth, the growth rate is not proportional to the driving force, as shown by curves (b), (c), and (d) in
Figure 12. The driving force at which the growth rate abruptly increases is called the critical driving force. The value of the critical driving force is dependent on the degree of smoothness of the surface; a smoother surface has a larger critical driving force.
Figure 11.
Microstructure of composite composed of a SrTiO3 single crystal substrate and Bi0.5Na0.5TiO3 (BNT) particles, heated at 900 °C for 2 h.
Figure 11.
Microstructure of composite composed of a SrTiO3 single crystal substrate and Bi0.5Na0.5TiO3 (BNT) particles, heated at 900 °C for 2 h.
Figure 12.
Relation between grain growth rate and driving force.
Figure 12.
Relation between grain growth rate and driving force.
Figure 13 shows the microstructures of BaTiO
3, BNKT, and (K,Na,Li)(Na,Ta)O
3 obtained by sintering of the compacts of equiaxed powders. The grain shape of BaTiO
3 is irregular, whereas that of BNKT and (K,Na,Li)(Na,Ta)O
3 is cubic. In the BNKT and (K,Na,Li)(Na,Ta)O
3 cases, the presence of flat (100) faces indicates that the surface is atomically smooth. The degree of smoothness is judged from the shape of the edges. (K,Na,Li)(Na,Ta)O
3 has pointed edges, whereas BNKT has round edges, indicating that the degree of smoothness is higher for (K,Na,Li)(Na,Ta)O
3 than BNKT. It is reported that the surface structure of BaTiO
3 heated in air is atomically smooth [
16], but
Figure 13(A) shows that the grain boundaries are curved. The origin of the curved grain boundaries is either that the grains have {111} surfaces with high surface energy or that the boundaries are composed of facets with a hill-and-valley structure at an atomic level [
15]. High energy surfaces and the hill-and-valley structure might provide growing steps for grain boundary migration. Therefore, it is suggested that the degree of smoothness is low for present BaTiO
3. The relation between the growth rate and driving force is qualitatively expressed by curves (b), (c), and (d) in
Figure 12 for BaTiO
3, BNKT, and (K,Na,Li)(Na,Ta)O
3, respectively.
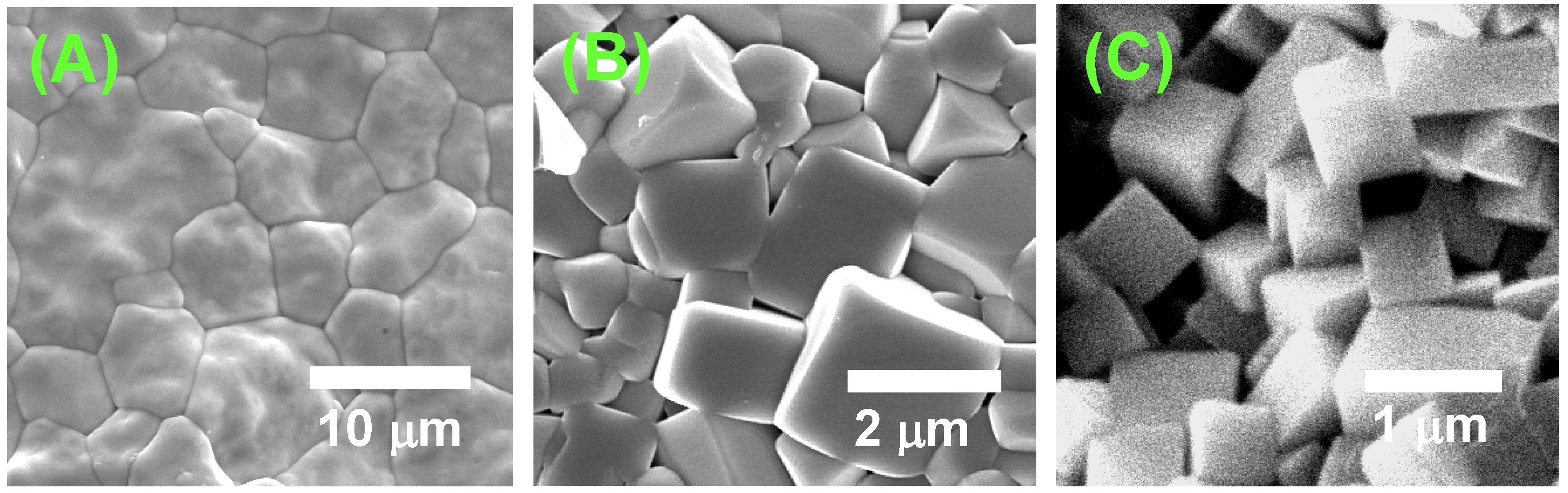
Figure 13.
Microstructures of sintered compacts of (A) BaTiO3, (B) Bi0.5(Na0.5K0.5)0.5TiO3, and (C) (K,Na,Li)(Na,Ta)O3. The compacts were prepared by sintering of matrix grains.
Figure 13.
Microstructures of sintered compacts of (A) BaTiO3, (B) Bi0.5(Na0.5K0.5)0.5TiO3, and (C) (K,Na,Li)(Na,Ta)O3. The compacts were prepared by sintering of matrix grains.
In the case of BaTiO
3, the driving force for grain growth (determined by the curvature of the grain boundary) exceeds the critical value, and the grains can grow with a rate proportional to the driving force. Thus, the boundary between template and matrix grains migrates towards the center of curvature as shown in
Figure 5. In BNKT, the critical driving force lies in a medium region. In this case, the driving force for grain boundary migration is lower than the critical value, and the grains cannot grow by the grain boundary migration. The spreading of matrix grains on the template grains becomes the dominant mechanism of microstructure development. In the (K,Na,Li)(Na,Ta)O
3 case, a high critical value inhibits the normal grain growth. The material transport along atomically flat boundaries is also restricted [
17]. Thus, the grain growth by grain boundary migration and solid state spreading is sluggish and a small number of grains grow abnormally at a high temperature [
15]. The above discussion concludes that the mechanism of texture development is determined by the surface structure at an atomic level.
A liquid phase gives a profound effect on the grain boundary structure and grain growth behavior. In the present experiment, the obvious presence of a liquid phase was not confirmed. However, the detection of a small amount of liquid phase is difficult, and the possible presence of a liquid phase is undeniable. The examination of the effects of a liquid phase remains on the growth behavior in the TGG process.