Introduction
Recently, bulk metallic glasses [
1] of metal-metal and metal-metalloid multi-component systems with clear glass transitions have been attracting much interest with their excellent mechanical and physical properties, and in these glasses local atomic arrangements are considered to play a crucial role for the stabilization of glass-phases. A number of X-ray and neutron diffraction structure analyses have been performed for both the metal-metal and metal-metalloid metallic glasses [
2,
3]. Atomic short range order (SRO) with respect to the nearest neighbor structures has been elucidated through radial distribution function (RDF) analyses for the glass states. In addition to this, crystallization processes, especially in nanoscale, also become important for understanding changes of mechanical and physical properties [
4,
5]. Even for specimens with nanocrystallized structures, however, such average structural analyses have been performed by focusing on changes of the SRO structures [
6]. To understand inhomogeneity of nanoscale structures, including medium range order (MRO) structures or very small nanocrystals, we should carry out atomistic-scale local structural analyses by means of advanced transmission electron microscopy (TEM), taking the averaged structure information from the RDF analysis into consideration.
One of the advantages of using modern TEM is to obtain structural information from local regions as small as 1 nm by using nanobeam electron diffraction (NBED) technique. This technique enables us even to take crystalline symmetric diffraction patterns from 1 nm-sized atomic ordered regions in as‑formed glass structures, which directly proves a presence of extended MRO structures [
7,
8]. Moreover, average structural information can also be obtained precisely by selected area diffraction (SAED) patterns from wide areas (0.1~1 μm) of the specimens. The RDF analysis from SAED intensity and reverse Monte Carlo (RMC) simulation enables us to construct plausible structural models as average information [
9]. Owing to advanced technologies such as energy filtering and digital data processing with imaging-plates (IP), precise intensity analyses become available even by electron diffraction. A simultaneous observation in both real and reciprocal spaces for the same region of interest is a great advantage in the structure analyses using TEM. In other words, both of the imaging and diffraction techniques are concurrently available for identical regions in the specimens. Therefore, we can always monitor a degree of inhomogeneity of the microstructure in real space which corresponds to the RDF profile of averaged structure. Using these techniques, we have revealed both local and average atomic structures in the glass and nanocrystallized specimens. In this paper, we first briefly explain the analytical techniques developed by our group, and then introduce some examples of the analyses especially for Fe-based metallic glasses.
3. Results and Discussion
We have been systematically examining local atomic structures on Fe-based metallic glasses with special significance as magnetic materials, by using the above-mentioned advanced TEM techniques. As a first step, we researched the simple Fe-B binary system, for which there are fruitful and reliable data examined by neutron and X-ray diffraction methods. In this study, Fe-B alloys with three different compositions were used to examine a compositional dependence of the local atomic structure. Although bcc-like clusters extending to about 1 nm were frequently observed in high‑resolution electron microscope (HREM) images [
11], it was difficult to find any difference in the HREM images. From electron diffraction intensity analyses, however, we found detectable structural differences in these alloys. The obtained results are described below.
Plausible structure models for Fe
86B
14, Fe
83B
17, Fe
80B
20 alloys were constructed [
10] using the above mentioned RMC simulation technique. The coordination numbers and bond lengths are well consistent with those reported based on the neutron data [
12]. To understand the local atomic environments, a Voronoi polyhedral analysis [
13] was performed for the obtained structural models.
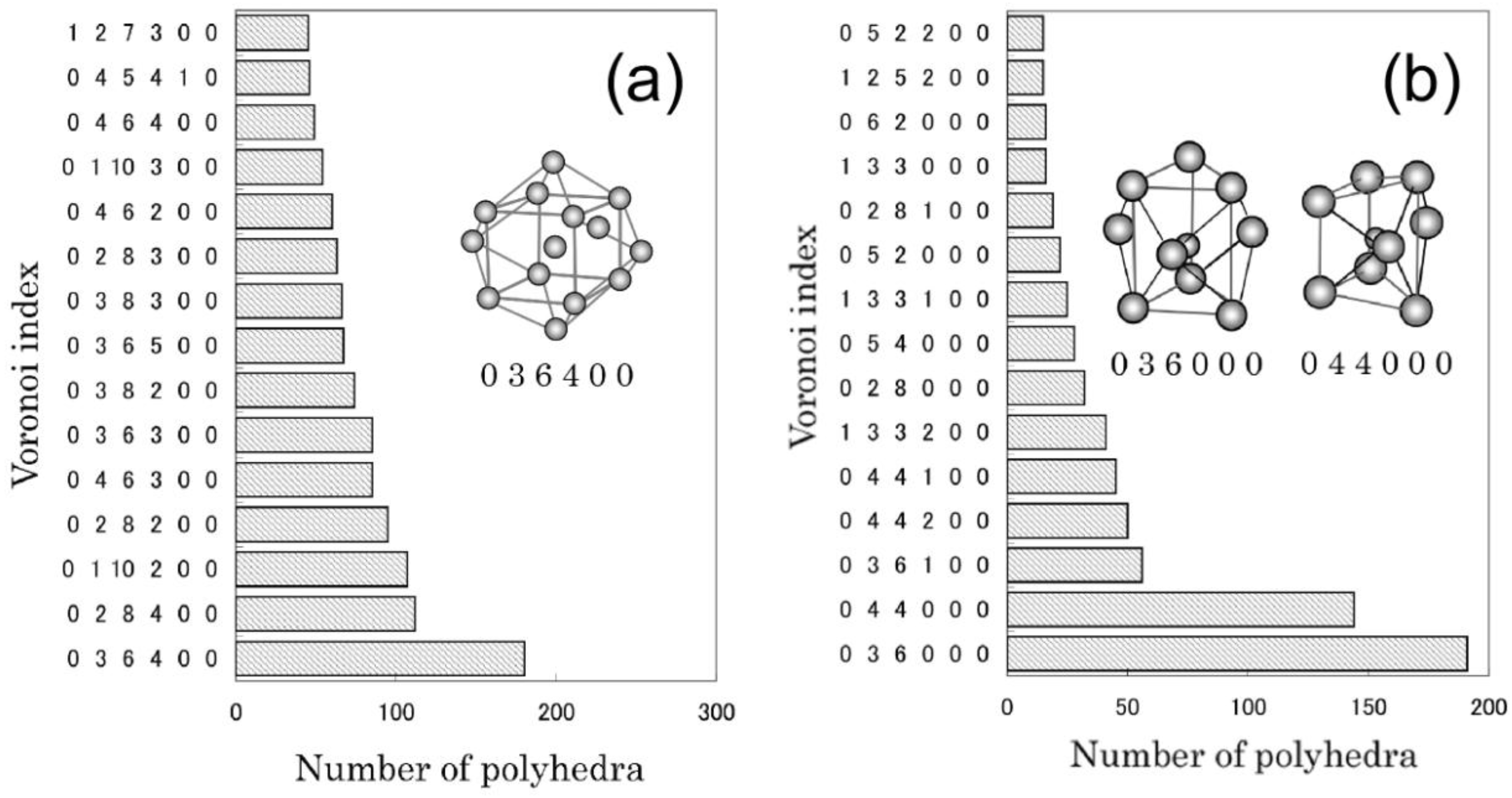
Figure 2 shows the analyzed results obtained from the model for Fe
80B
20. The bcc-Fe like polyhedra are frequently found around central Fe atoms, whereas the prism-type polyhedra are frequently formed around central B atoms. The trigonal prism, which is a structural unit of an Fe
3B compound, increases with the increment of B content, as well as Archimedean prism. In contrast, the bcc-Fe like polyhedra increase with the decrease of B content. It is interesting to note that the change of ratio of the bcc-Fe and prism type local structures with the B content basically corresponds to the change of moler ratio of bcc-Fe and Fe
3B compound with the B composition in the equilibrium Fe-B phase diagram. In each alloy, moreover, icosahedron (coordination number is 12) and polyhedra with large coordination numbers also exist.
Figure 2.
Voronoi polyhedral analyses for the reverse Monte Carlo (RMC) structure model which reproduces the interference function i(Q) obtained from Fe80B20. Voronoi polyhedra with central (a) Fe and (b) B atoms found in the final structural model are shown.
Figure 2.
Voronoi polyhedral analyses for the reverse Monte Carlo (RMC) structure model which reproduces the interference function i(Q) obtained from Fe80B20. Voronoi polyhedra with central (a) Fe and (b) B atoms found in the final structural model are shown.
It was confirmed that the precise electron diffraction intensity analyses can be performed for the Fe‑B binary system as above. We then moved our subjects to ternary Fe-B-Nb alloys in order to clarify a difference in local atomic structures of metallic glasses with various glass stabilities [
14]. We focused especially on ternary Fe
84Nb
7B
9 and Fe
70Nb
10B
20 glass structures [
15,
16], with a structural reference of Fe
80B
20. The Fe
70Nb
10B
20 glass has a high glass forming ability, showing a large supercooled liquid region. Structural models for the Fe
84Nb
7B
9 and Fe
70Nb
10B
20 glasses were also derived using the RMC simulation, and a reliability of the models was confirmed by checking the consistency of average coordination numbers between the simulation and experiments. Even for ternary alloys, experimental coordination numbers and bond lengths can be obtained by using an X-ray anomalous dispersion method or a neutron diffraction method [
17]. We actually obtained the RMC simulated model for ternary Fe
70Nb
10B
20, which is well consistent with the results from an X-ray anomalous dispersion method [
17]. Voronoi polyhedral analyses for these glasses revealed that local atomic structures have “nanoscale-phase-separated” features which basically consist of bcc-Fe like clusters, trigonal prisms, and compound-like polyhedra. Note that the fraction for each cluster strongly depends on the alloy composition: for example, the fraction of bcc-Fe clusters in Fe
70Nb
10B
20 is much smaller than that in the other glasses.
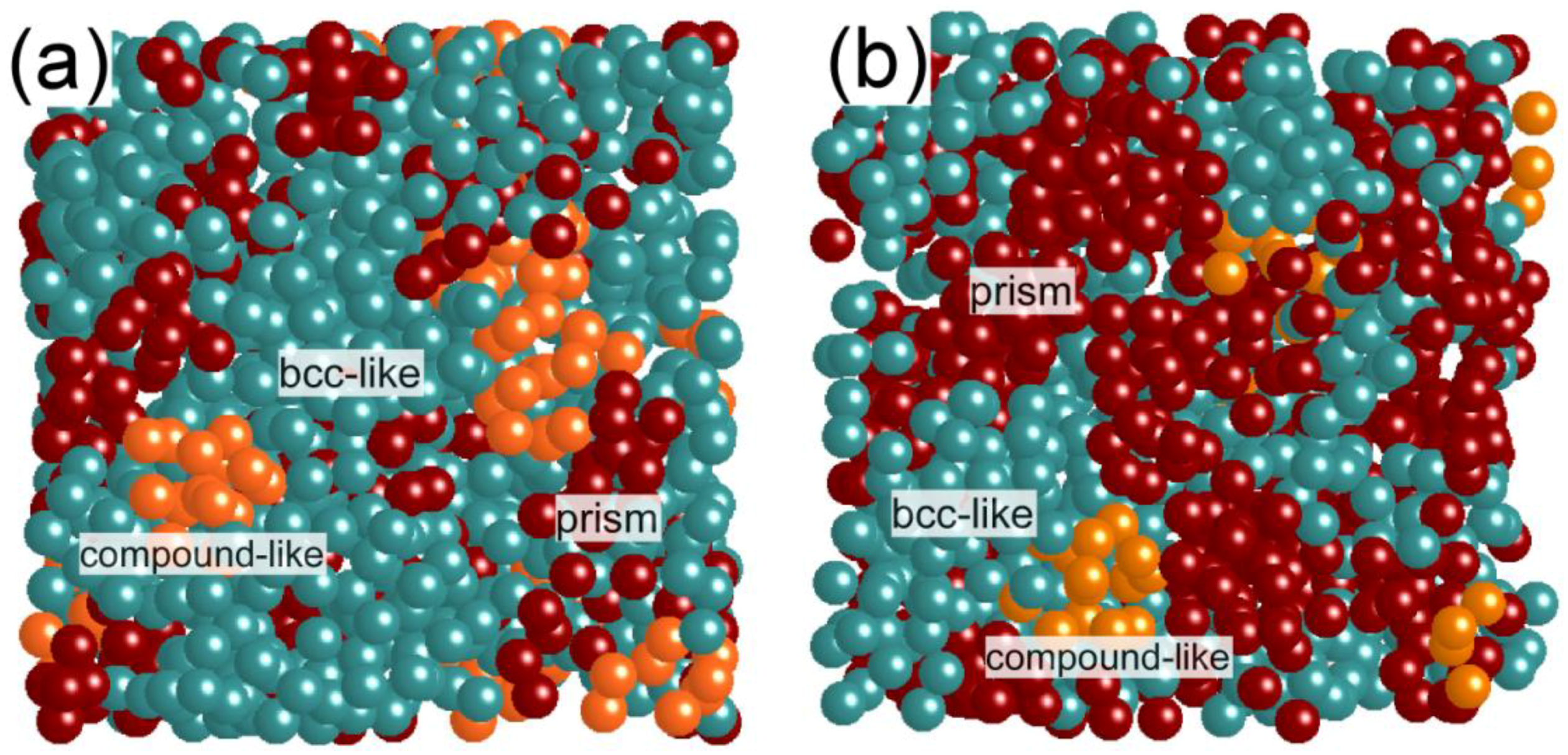
Figure 3 visually shows the atomic structures for Fe
84Nb
7B
9 and Fe
70Nb
10B
20 obtained from the RMC simulation. It can be seen that a fraction of the bcc-Fe like clusters in Fe
70Nb
10B
20 has a small proportion compared to Fe
84Nb
7B
9. Moreover, we can see trigonal prisms and compound-like polyhedra between aggregates of bcc-Fe like clusters, indicating a formation of the structures characterized by “nanoscale phase separation” [
8]. This kind of structural fluctuation probably gives a strong effect on the crystallization processes of these glasses [
18].
Figure 3.
Structure models of (a) Fe84Nb7B9 and (b) Fe70Nb10B20 metallic glasses obtained from the RMC simulation. A fraction of the bcc-Fe clusters in Fe70Nb10B20 is smaller than that in Fe84Nb7B9.
Figure 3.
Structure models of (a) Fe84Nb7B9 and (b) Fe70Nb10B20 metallic glasses obtained from the RMC simulation. A fraction of the bcc-Fe clusters in Fe70Nb10B20 is smaller than that in Fe84Nb7B9.
In addition to the local structural analyses, we have been trying to understand the glass stability from a viewpoint of the crystallization processes. We also examined multicomponent metallic glasses, such as Fe72Si4B20Nb4 and Fe36Co36Si4B20Nb4, with large bulk glass forming abilities, much larger than the above binary and ternary glasses. As a result, we found a distinct difference in crystallization processes between glasses with (multicomponent) and without (binary and ternary) bulk glass forming abilities, as follows. The complex intermetallic compound phases are directly formed from the glass states in the bulk metallic glasses, whereas the simple bcc-Fe phase is initially formed prior to the compound formation in the metallic glasses without bulk forming abilities. An example of the crystallization process in one of the multicomponent bulk metallic glasses, will be shown below.
We observed microstructures of as-quenched and annealed Fe
36Co
36Si
4B
20Nb
4 metallic glass [], which can form a bulk rod glass with a diameter as large as 4 mm [
21]. In the as-quenched specimen, HREM images indicate a homogeneous glass structure where extended MRO structures cannot be seen clearly.
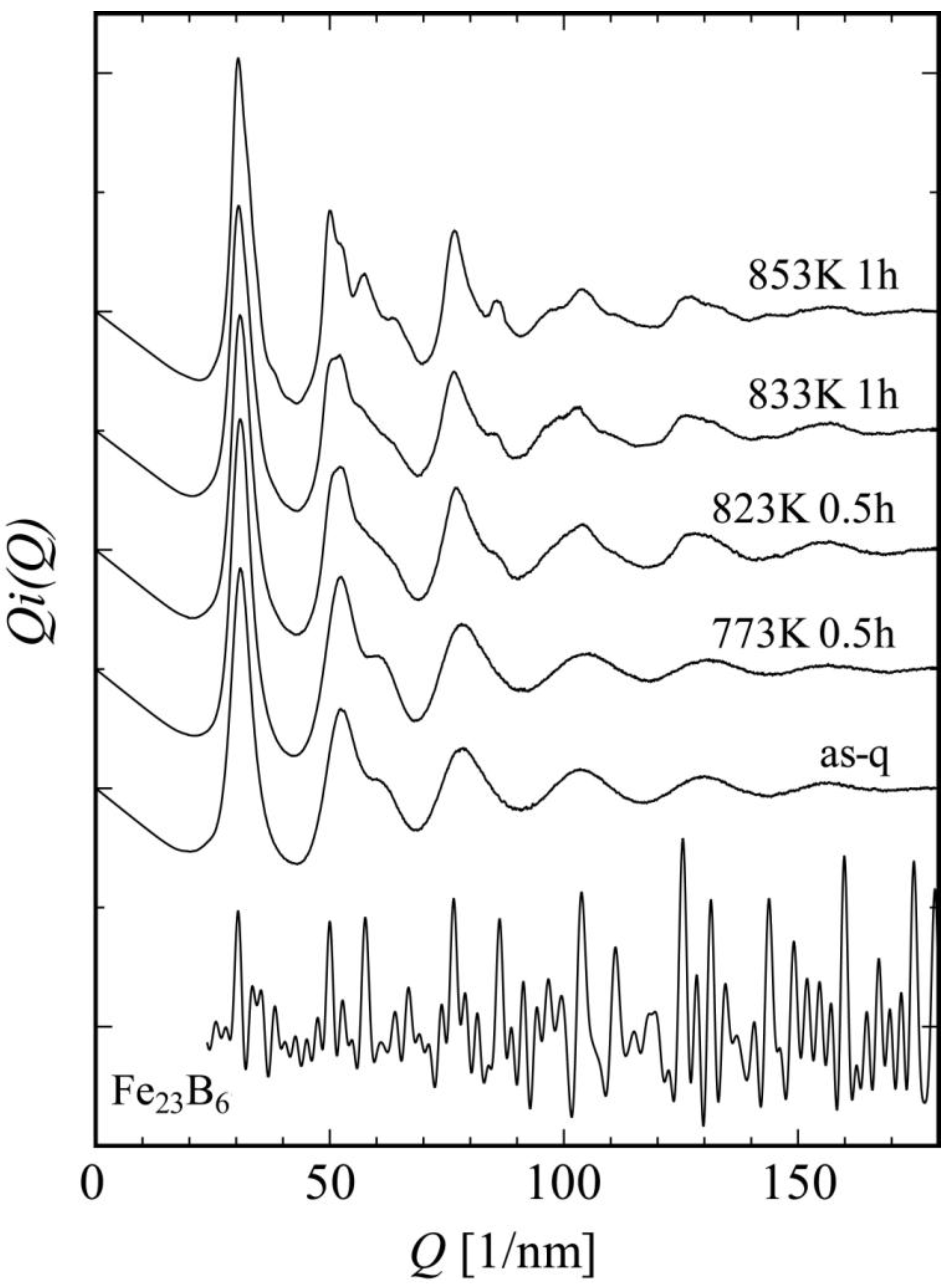
Figure 4 shows a change in interference function
i(Q) on the annealing process of Fe
36Co
36Si
4B
20Nb
4. In this alloy system, it was found that the glass structure directly transforms to Fe
23B
6 (cF116 Cr
23C
6-type) nanocrystals without a formation of bcc-Fe. To understand the nanoscale structural changes, we next took a lot of NBED patterns from a broad range of areas in each specimen. Diffraction spots with strong intensities are frequently observed just on the first- and second-halo-ring positions in NBED patterns, indicating a development of the MRO structures even in the as-quenched state (
Figure 5 (a)). Contrary to the metallic glasses with low glass forming abilities, crystal-like (including bcc-Fe) diffraction patterns cannot be obtained even by a careful inspection of a lot of NBED patterns. It seems that there are locally extended MRO structures to form NBED spots arranged on the halo-ring positions without any specific atomic structure with high symmetry detectable by NBED.
Figure 5 (b) shows an [110] NBED pattern from a metastable Fe
23B
6-type structure in the specimen annealed at 873 K, together with a corresponding calculated pattern in (b’). A NBED pattern, slightly deformed from the perfect Fe
23B
6 one, is shown in
Figure 5 (c). In the pattern, diffraction spots indicated by arrows form a pattern with pseudo-tenfold symmetry in which a proportion of Q values between spots 3 and 4 is about 1.62. This value is very close to the golden ratio (1.618), which characterizes quasicrystals, and is far from a normal value of 1.5 (proportion between spots 1 and 2) found in the perfect Fe
23B
6 structure. This means that, even in the Fe-based metallic glass, the quasicrystal-like structure can be found, as well as in the Zr-based metallic glasses. Moreover, we found the quasicrystal-like structure also in an Fe-Cr-Mo-C-B-Tm metallic glass with an extremely high glass forming ability [
22,
23]. It is therefore concluded that the glass nature to form a quasicrystal structure in crystallization stage is closely related to the glass formation as was previously discussed in the Zr-based systems [
24].
Figure 4.
Change of reduced interference functions obtained from Fe36Co36Nb4Si4B20 on heating. The glass state directly transforms into a metastable Fe23B6 crystal.
Figure 4.
Change of reduced interference functions obtained from Fe36Co36Nb4Si4B20 on heating. The glass state directly transforms into a metastable Fe23B6 crystal.
Figure 5.
(a) NBED patterns obtained from as-quenched Fe36Co36Nb4Si4B20 specimen. NBED patterns obtained from Fe36Co36Nb4Si4B20 specimens annealed at 873K for 1h are also shown in (b)-(d). (b) [110] pattern from a perfect Fe23B6 structure, (c) Fe23B6–like pseudo-tenfold pattern, and (d) almost tenfold pattern. The simulated [110] pattern of Fe23B6 is also shown in (b’).
Figure 5.
(a) NBED patterns obtained from as-quenched Fe36Co36Nb4Si4B20 specimen. NBED patterns obtained from Fe36Co36Nb4Si4B20 specimens annealed at 873K for 1h are also shown in (b)-(d). (b) [110] pattern from a perfect Fe23B6 structure, (c) Fe23B6–like pseudo-tenfold pattern, and (d) almost tenfold pattern. The simulated [110] pattern of Fe23B6 is also shown in (b’).
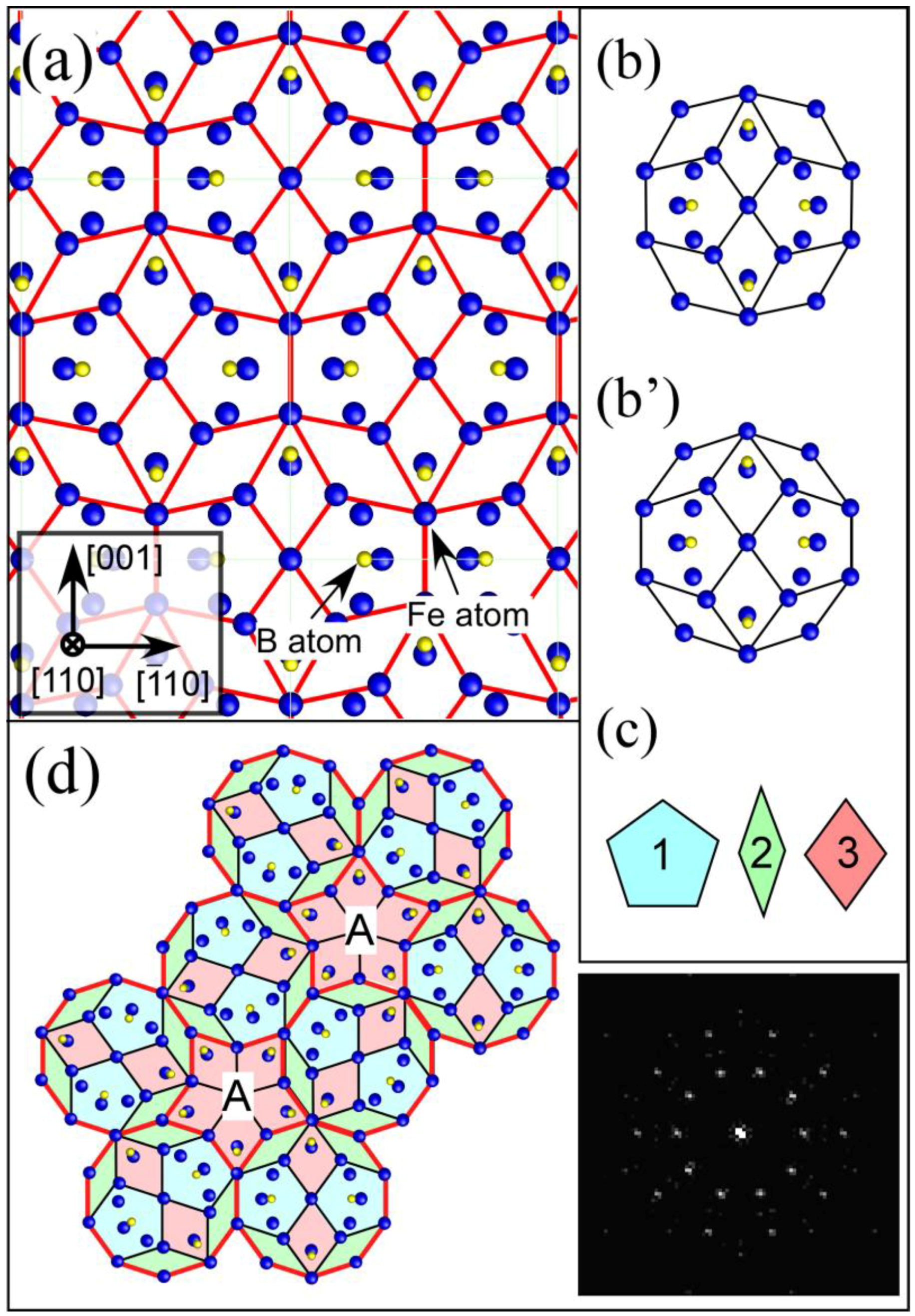
Interestingly, the [110] projection of the Fe
23B
6 structure (
Figure 6 (a)) can be divided into three types of characteristic tiles shown in
Figure 6 (c). These tiles are able to form a quasicrystal-like structure exhibiting a pseudo-tenfold symmetry as shown in
Figure 6 (d). During the formation process of the Fe
23B
6 structure, quasicrystal-like clusters, which have strong correlation with the stable glass structure, are probably stabilized as an intermediate state. We need to focus on the fact that the formation of the intermediate quasicrystal structures in Fe-based systems is very similar to that in the Zr-based systems.
Figure 6.
(
a) [110] projection of an Fe
23B
6 structure; (
b) deformed decagonal structural unit found in (a); (
c) decagonal structural unit obtained by transforming the unit of (b), three types of structure tiles as components of the decagonal unit of (c); and (
e) structure model reproducing the tenfold diffraction pattern shown in
Figure 5 (
d). For the structure model of (e), we first arrange several decagonal structure units (c), and then rotated them by 0, 72, 144, 216, 288 degrees independently. These tiles were subsequently arranged to fill the space by sharing their edges. Though the decagonal structure unit cannot fill the space denoted by A, the space can be filled by using the tile denoted by "3". As a result, three types of tiles shown in (c) can form the structure which reproduces the tenfold diffraction pattern. In the inset, Fourier transform pattern obtained from the structure model is shown. The pattern is well consistent with the experimental diffraction pattern.
Figure 6.
(
a) [110] projection of an Fe
23B
6 structure; (
b) deformed decagonal structural unit found in (a); (
c) decagonal structural unit obtained by transforming the unit of (b), three types of structure tiles as components of the decagonal unit of (c); and (
e) structure model reproducing the tenfold diffraction pattern shown in
Figure 5 (
d). For the structure model of (e), we first arrange several decagonal structure units (c), and then rotated them by 0, 72, 144, 216, 288 degrees independently. These tiles were subsequently arranged to fill the space by sharing their edges. Though the decagonal structure unit cannot fill the space denoted by A, the space can be filled by using the tile denoted by "3". As a result, three types of tiles shown in (c) can form the structure which reproduces the tenfold diffraction pattern. In the inset, Fourier transform pattern obtained from the structure model is shown. The pattern is well consistent with the experimental diffraction pattern.