Chemistry of the Fe2O3/BiFeO3 Interface in BiFeO3 Thin Film Heterostructures
Abstract
:1. Introduction
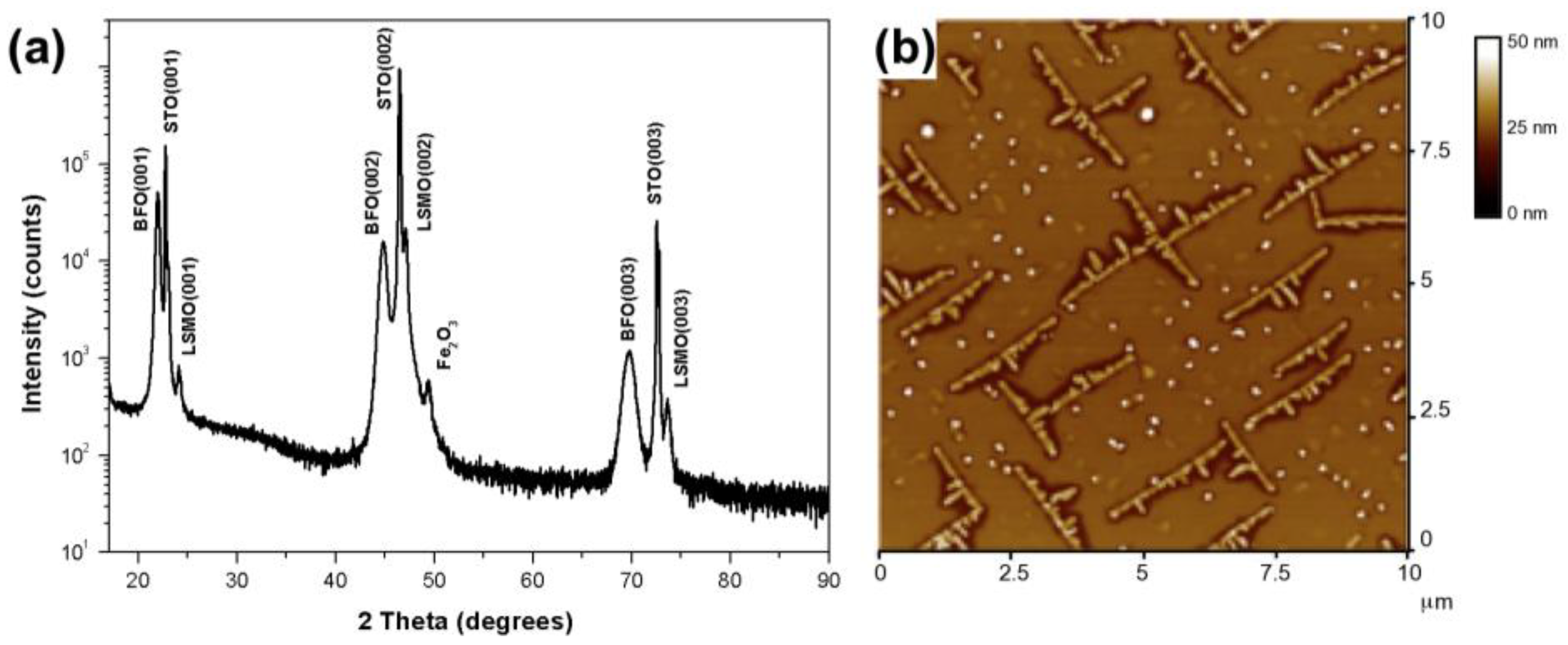
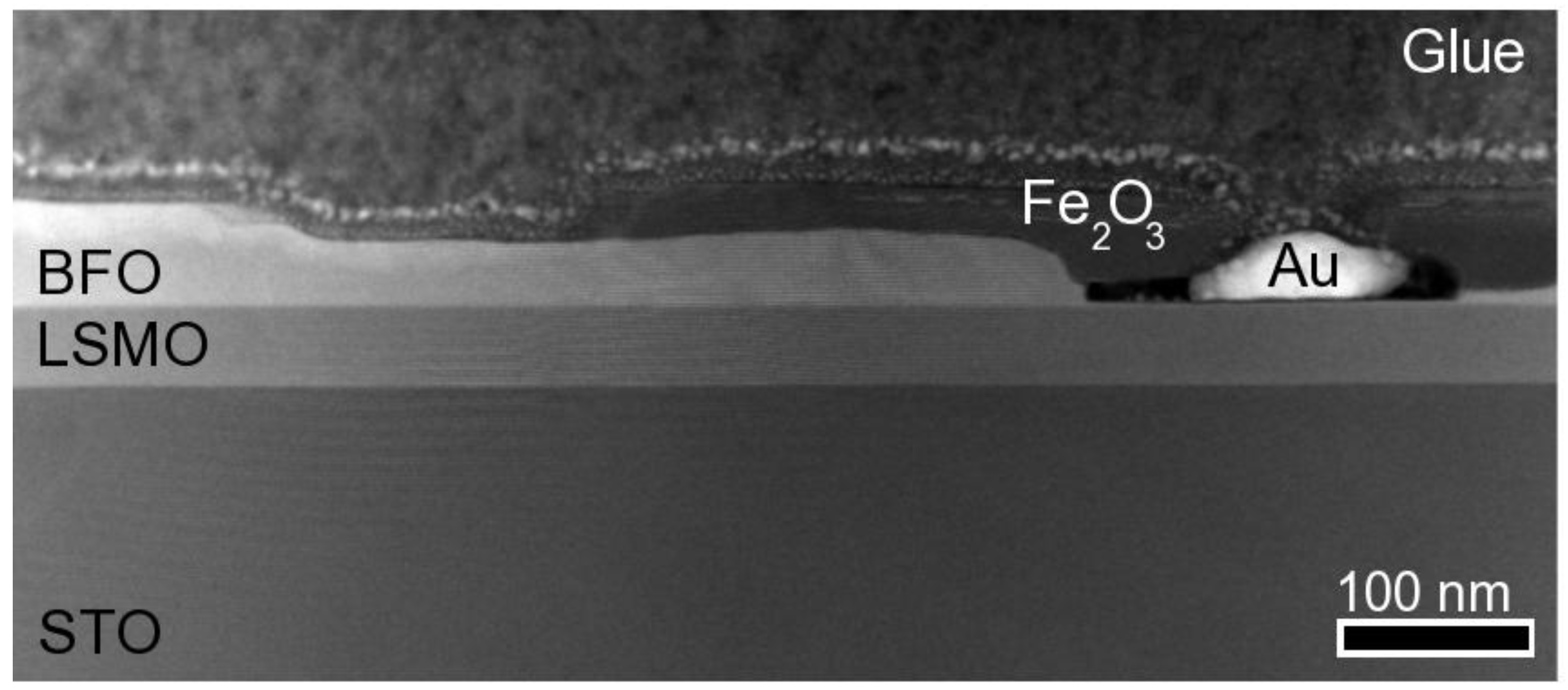
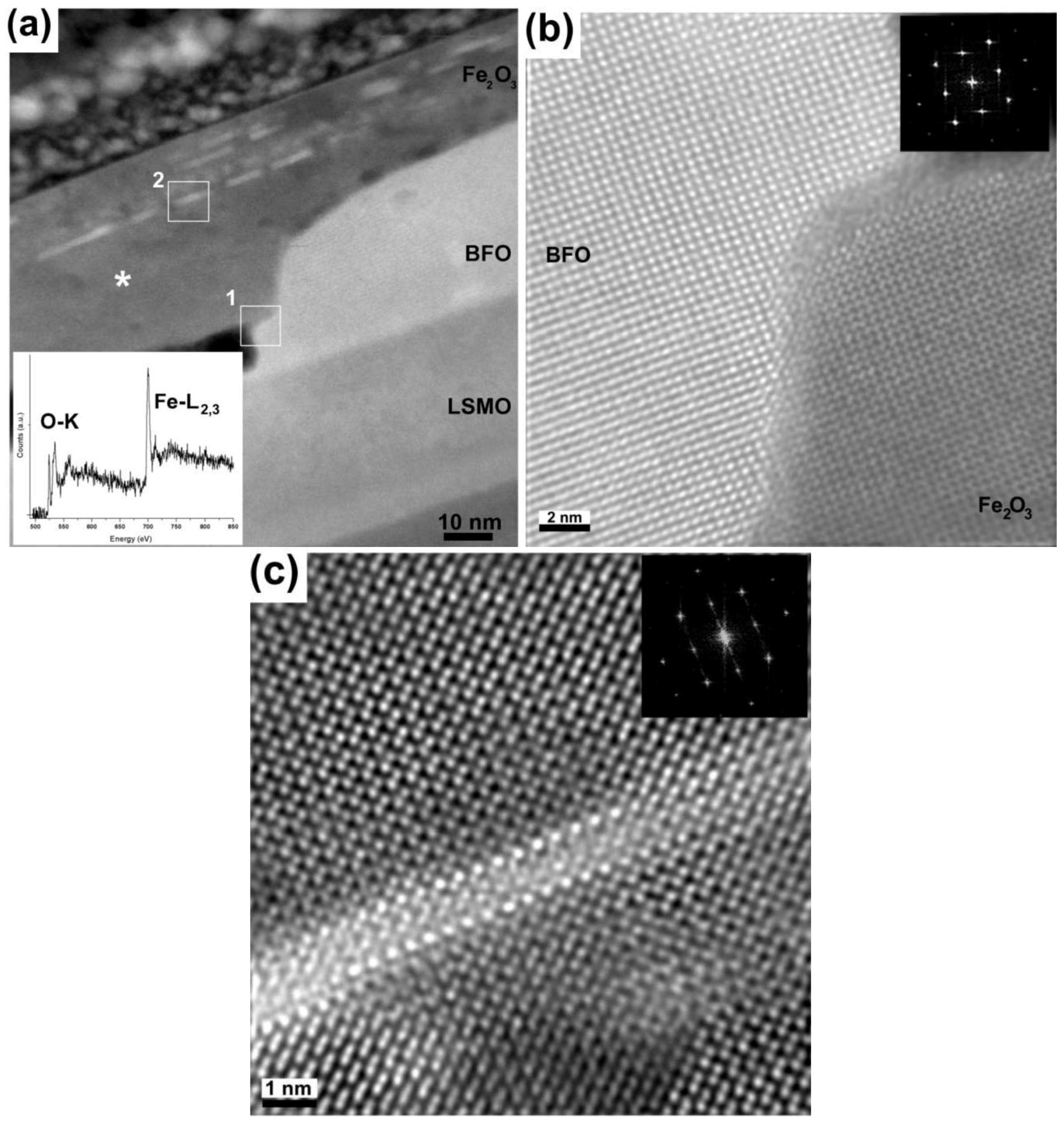
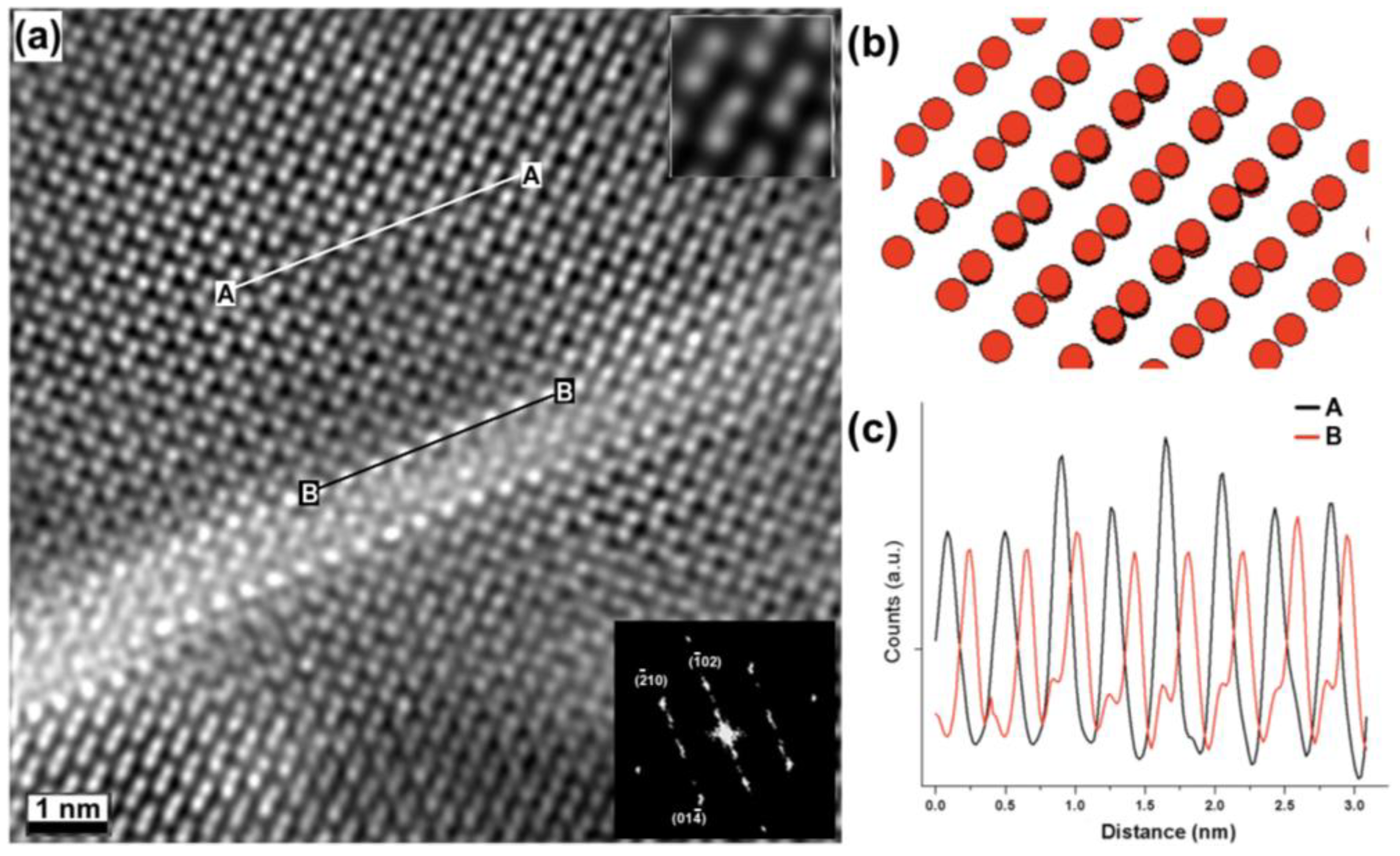
2. Results and Discussion

3. Experimental Section
4. Conclusions
Acknowledgements
References
- Ramesh, R.; Spaldin, N.A. Multiferroics: Progress and prospects in thin films. Nat. Mater. 2007, 6, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Bea, H.; Bibes, M.; Zhu, X.H.; Fusil, S.; Bouzehouane, K.; Petit, S.; Kreisel, J.; Barthelemy, A. Crystallographic, magnetic, and ferroelectric structures of bulklike BiFeO3 thin films. Appl. Phys. Lett. 2008, 93, 072901–072903. [Google Scholar] [CrossRef]
- Kan, D.; Palova, L.; Anbusathaiah, V.; Cheng, C.J.; Fujino, S.; Nagarajan, V.; Rabe, K.M.; Takeuchi, I. Universal behavior and electric-field-induced structural transition in rare-earth-Substituted BiFeO3. Adv. Funct. Mater. 2010, 20, 1108–1115. [Google Scholar] [CrossRef]
- Zeches, R.J.; Rossell, M.D.; Zhang, J.X.; Hatt, A.J.; He, Q.; Yang, C.H.; Kumar, A.; Wang, C.H.; Melville, A.; Adamo, C.; Sheng, G.; Chu, Y.H.; Ihlefeld, J.F.; Erni, R.; Ederer, C.; Gopalan, V.; Chen, L.Q.; Schlom, D.G.; Spaldin, N.A.; Martin, L.W.; Ramesh, R. A strain-driven morphotropic phase boundary in BiFeO3. Science 2009, 326, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Anbusathaiah, V.; Cheng, C.J.; Lim, S.H.; Murakami, M.; Salamanca-Riba, L.G.; Takeuchi, I.; Nagarajan, V. Role of oxygen partial pressure and seed layer chemistry in flux mediated epitaxy of single phase multiferroic BiFeO3 thin films. Appli. Phys. Lett. 2008, 93, 192906. [Google Scholar] [CrossRef]
- Bea, H.; Bibes, M.; Barthelemy, A.; Bouzehouane, K.; Jacquet, E.; Khodan, A.; Contour, J.P.; Fusil, S.; Wyczisk, F.; Forget, A.; Lebeugle, D.; Colson, D.; Viret, M. Influence of parasitic phases on the properties of BiFeO3 epitaxial thin films. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- Murakami, M.; Fujino, S.; Lim, S.H.; Salamanca-Riba, L.G.; Wuttig, M.; Takeuchi, I.; Varughese, B.; Sugaya, H.; Hasegawa, T.; Lofland, S.E. Microstructure and phase control in Bi-Fe-O multiferroic nanocomposite thin films. Appl. Phys. Lett. 2006, 88, 112505. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Arredondo, M.; Saunders, M.; Ramasse, Q.M.; Valanoor, N.; Munroe, P. Microstructural analysis of ferromagnetic-multiferroic epitaxial heterostructure interfaces. J. Appl. Phys. 2010, in press. [Google Scholar]
- Lim, S.H.; Murakami, M.; Yang, J.H.; Young, S.Y.; Hattrick-Simpers, J.; Wuttig, M.; Salamanca-Riba, L.G.; Takeuchi, I. Enhanced dielectric properties in single crystal-like BiFeO3 thin films grown by flux-mediated epitaxy. Appl. Phys. Lett. 2008, 92, 012918. [Google Scholar] [CrossRef]
- Qi, X.D.; Dho, J.; Tomov, R.; Blamire, M.G.; MacManus-Driscoll, J.L. Greatly reduced leakage current and conduction mechanism in aliovalent-ion-doped BiFeO3. Appl. Phys. Lett. 2005, 86, 062903. [Google Scholar] [CrossRef]
- Eerenstein, W.; Morrison, F.D.; Dho, J.; Blamire, M.G.; Scott, J.F.; Mathur, N.D. Comment on epitaxial BiFeO3 multiferroic thin film heterostructures. Science 2005, 307, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borisevich, A.Y.; Chang, H.J.; Huijben, M.; Oxley, M.P.; Okamoto, S.; Niranjan, M.K.; Burton, J.D.; Tsymbal, E.Y.; Chu, Y.H.; Yu, P.; Ramesh, R.; Kalinin, S.V.; Pennycook, S.J. Suppression of octahedral tilts and associated changes in electronic properties at epitaxial oxide heterostructure interfaces. Phys. Rev. Lett. 2010, 105, 087204. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Lee, J.S.; Okamoto, S.; Rossell, M.D.; Huijben, M.; Yang, C.H.; He, Q.; Zhang, J.X.; Yang, S.Y.; Lee, M.J.; Ramasse, Q.M.; Erni, R.; Chu, Y.H.; Arena, D.A.; Kao, C.C.; Martin, L.W.; Ramesh, R. Interface ferromagnetism and orbital reconstruction in BiFeO3-La0.7Sr0.3MnO3 heterostructures. Phys. Rev. Lett. 2010, 105, 027201:1–027201:5. [Google Scholar]
- Borisevich, A.Y.; Ovchinnikov, O.S.; Chang, H.J.; Oxley, M.P.; Yu, P.; Eliseev, E.A.; Morozovska, A.N.; Ramesh, R.; Pennycook, S.J.; Kalinin, S.V. Mapping octahedral tilts and polarization across a domain wall in BiFeO3 from Z-contrast scanning transmission electron microscopy image atomic column shape analysis. ACS Nano 2010, in press. [Google Scholar]
- Hambe, M.; Petraru, A.; Pertsev, N.A.; Munroe, P.; Nagarajan, V.; Kohlstedt, H. Crossing an interface: Ferroelectric control of tunnel currents in magnetic complex oxide heterostructures. Adv. Funct. Mater. 2010, 20, 2436–2441. [Google Scholar] [CrossRef]
- Colliex, C.; Manoubi, T.; Ortiz, C. Electron-energy-loss-spectroscopy near-edge fine-structures in the iron-oxygen system. Phys. Rev. B 1991, 44, 11402–11411. [Google Scholar] [CrossRef]
- Kurata, H.; Lefevre, E.; Colliex, C.; Brydson, R. Electron-energy-loss near-edge structures in the oxygen K-edge spectra of transition-metal oxides. Phys. Rev. B 1993, 47, 13763–13768. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chueh, Y.L.; Hsieh, C.H.; Chang, M.T.; Chou, L.J.; Wang, Z.L.; Lan, Y.W.; Chen, C.D.; Kurata, H.; Isoda, S. p-Type alpha-Fe2O3 nanowires and their n-Type transition in a reductive ambient. Small 2007, 3, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.W.; Ortiz, D.; Baek, S.H.; Folkman, C.M.; Das, R.R.; Shafer, P.; Chen, Y.; Nelson, C.T.; Pan, X.; Ramesh, R.; Eom, C.B. Domain engineering for enhanced ferroelectric properties of epitaxial (001) BiFeO thin films. Advan. Mater. 2009, 21, 817–823. [Google Scholar] [CrossRef]
- Qi, X.D.; Wei, M.; Lin, Y.; Jia, Q.X.; Zhi, D.; Dho, J.; Blamire, M.G.; MacManus-Driscoll, J.L. High-resolution X-ray diffraction and transmission electron microscopy of multiferroic BiFeO3 films. Appl. Phys. Lett. 2005, 86, 071913. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy; Plenum Press: New York, NY, USA, 1996; p. 729. [Google Scholar]
- Ishizuka, K.; Abe, E. Improvement of spatial resolution of STEM-HAADF image by maximum-entropy and Richardson-Lucy Deconvolution. In Proceedings of the 13th European Microscopy Congress (13th EMC) Instrumentation and Methol, Antwerp, Belgium, 22–27 August 2004; volume 1, p. 117.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arredondo, M.; Ramasse, Q.M.; Bogle, K.; Nagarajan, V. Chemistry of the Fe2O3/BiFeO3 Interface in BiFeO3 Thin Film Heterostructures. Materials 2010, 3, 5274-5282. https://doi.org/10.3390/ma3125274
Arredondo M, Ramasse QM, Bogle K, Nagarajan V. Chemistry of the Fe2O3/BiFeO3 Interface in BiFeO3 Thin Film Heterostructures. Materials. 2010; 3(12):5274-5282. https://doi.org/10.3390/ma3125274
Chicago/Turabian StyleArredondo, Miryam, Quentin M. Ramasse, Kashinath Bogle, and Valanoor Nagarajan. 2010. "Chemistry of the Fe2O3/BiFeO3 Interface in BiFeO3 Thin Film Heterostructures" Materials 3, no. 12: 5274-5282. https://doi.org/10.3390/ma3125274



