Probing the Texture of the Calamitic Liquid Crystalline Dimer of 4-(4-Pentenyloxy)benzoic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and thermal behavior of of 4-(4-pentenyloxy)benzoic acid (2)

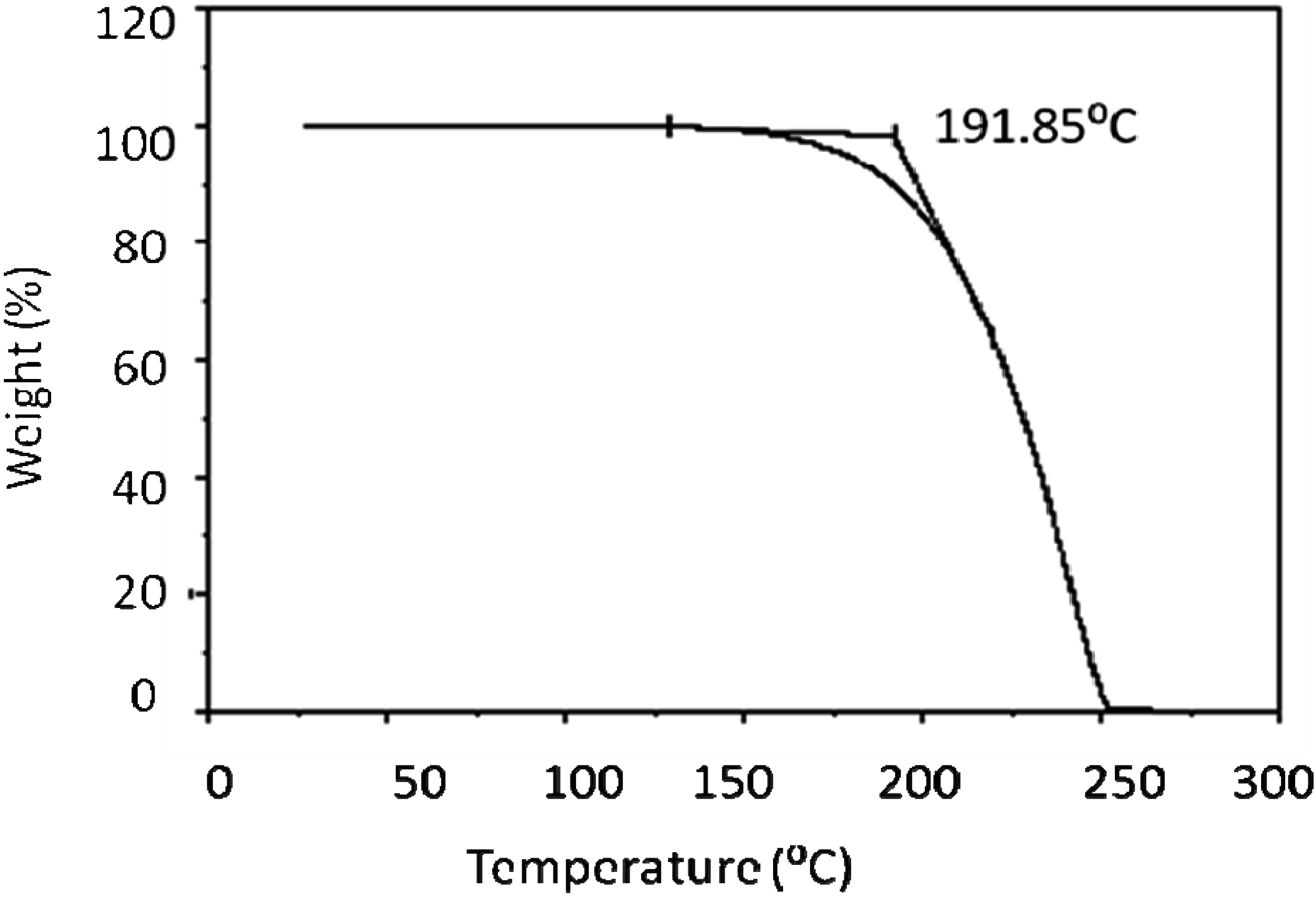
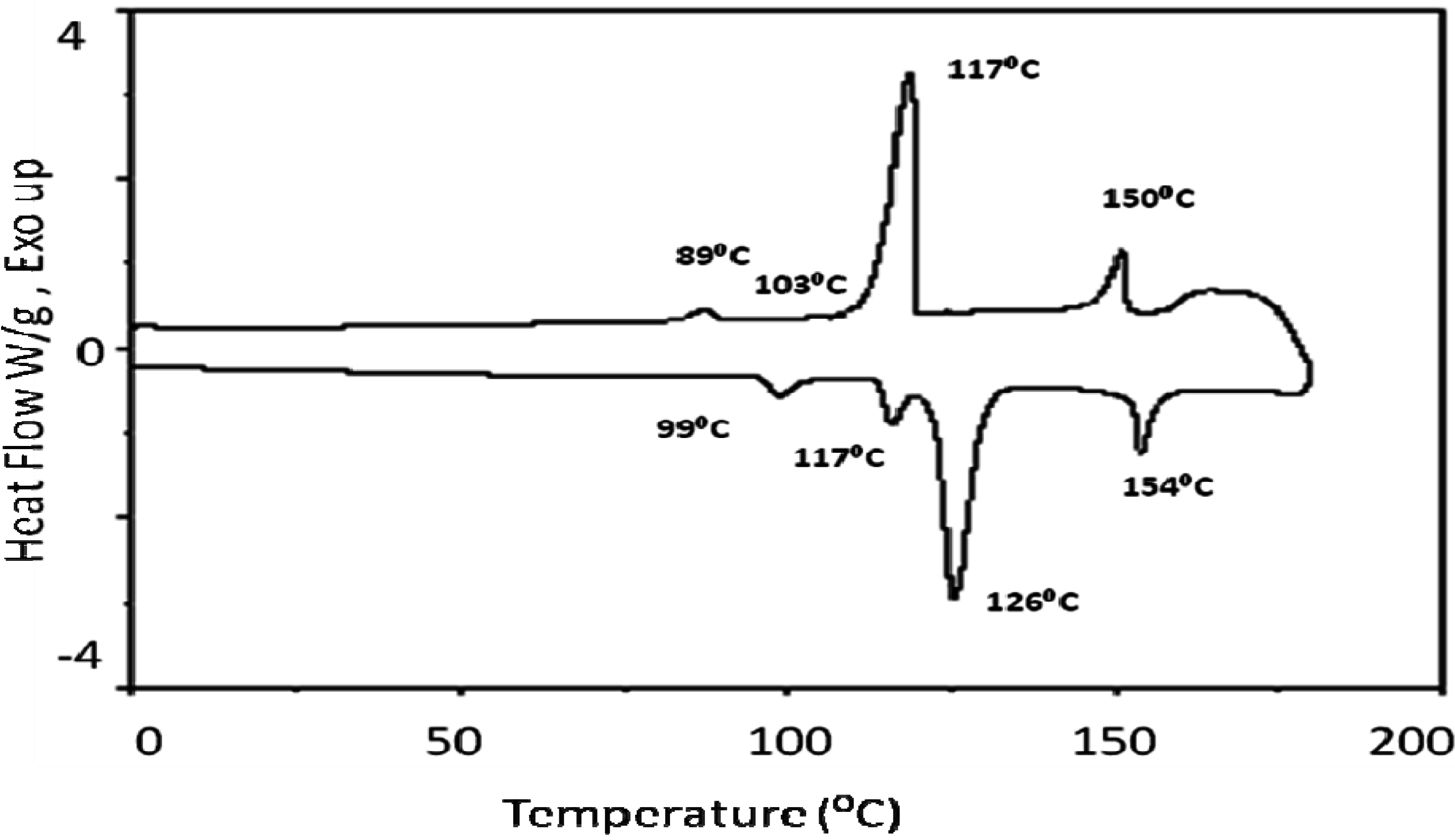
| Heating | ||
| Phase Transition | Temperature ○C | Δ H kJmol-1 |
| Cr-SmX1 | 99 | 1.14 |
| SmX1-SmX2 | 117 | 1.43 |
| SmX2-N | 126 | 14.2 |
| N-I | 154 | 2.9 |
| Cooling | ||
| Phase Transition | Temperature ○C | Δ H kJmol-1 |
| I-N | 150 | -2.80 |
| N-SmX1 | 117 | -15.0 |
| SmX1-SmX2 | 103 | Very small |
| SmX2-Cr | 89.0 | -0.40 |
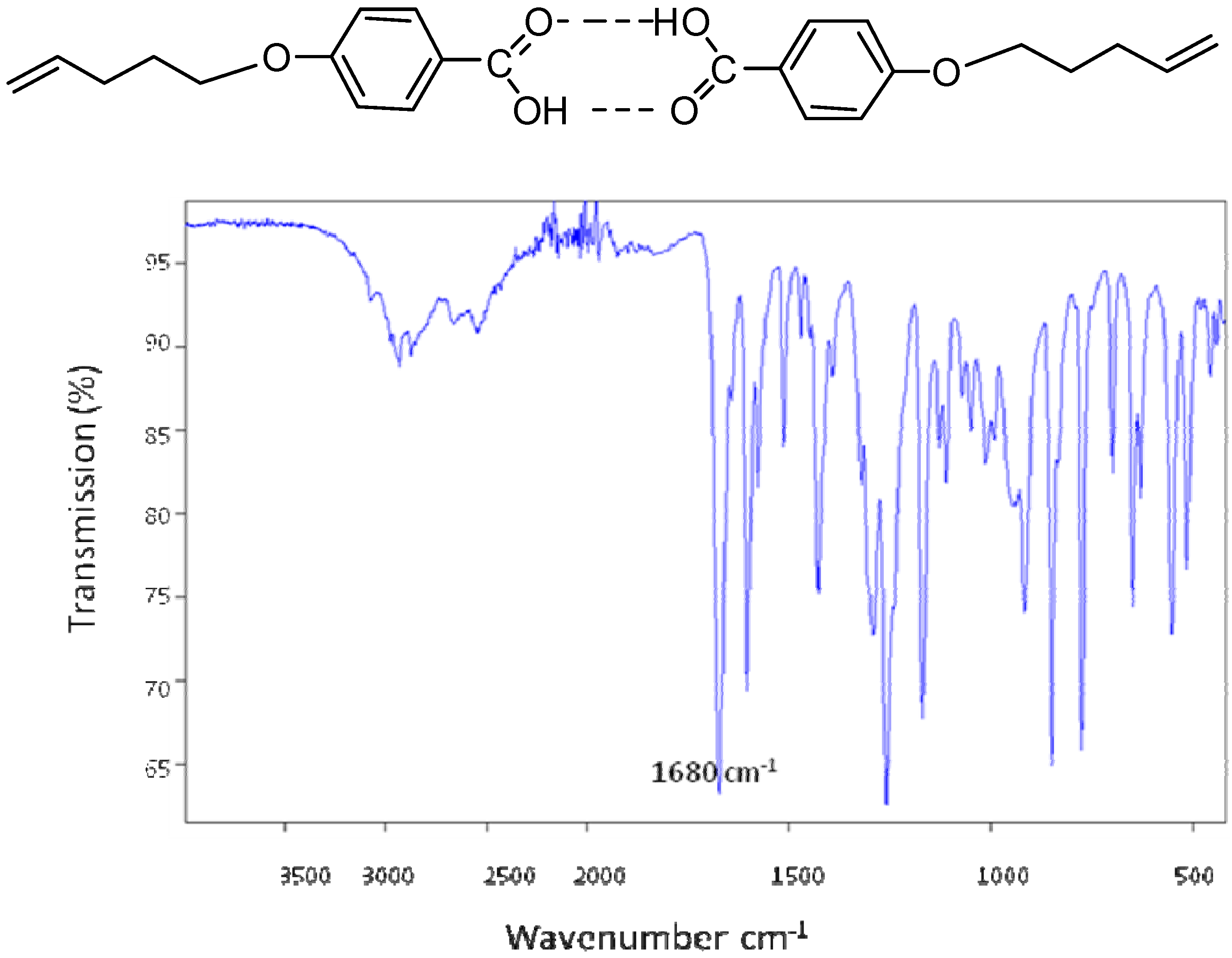
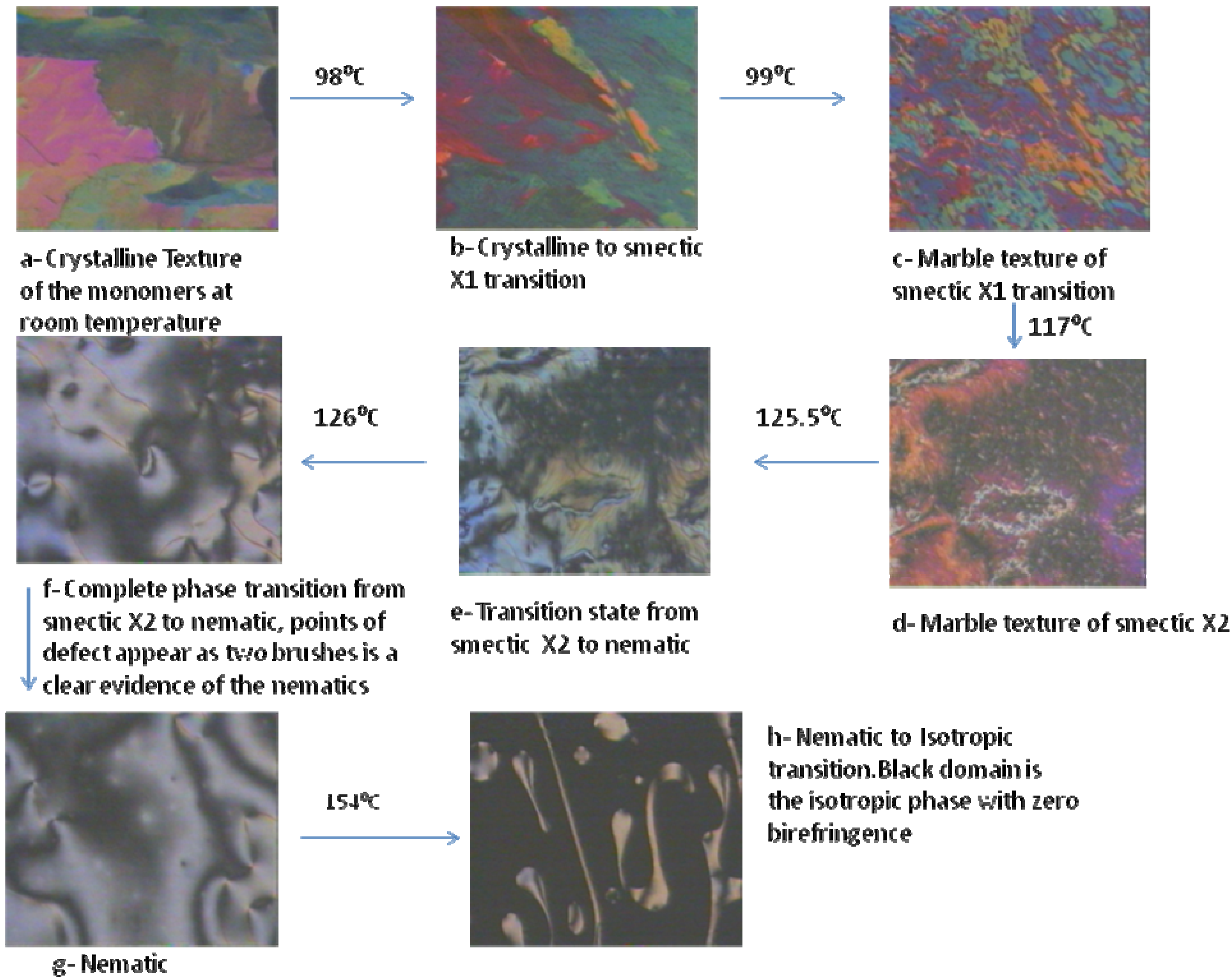
2.2. Texture study
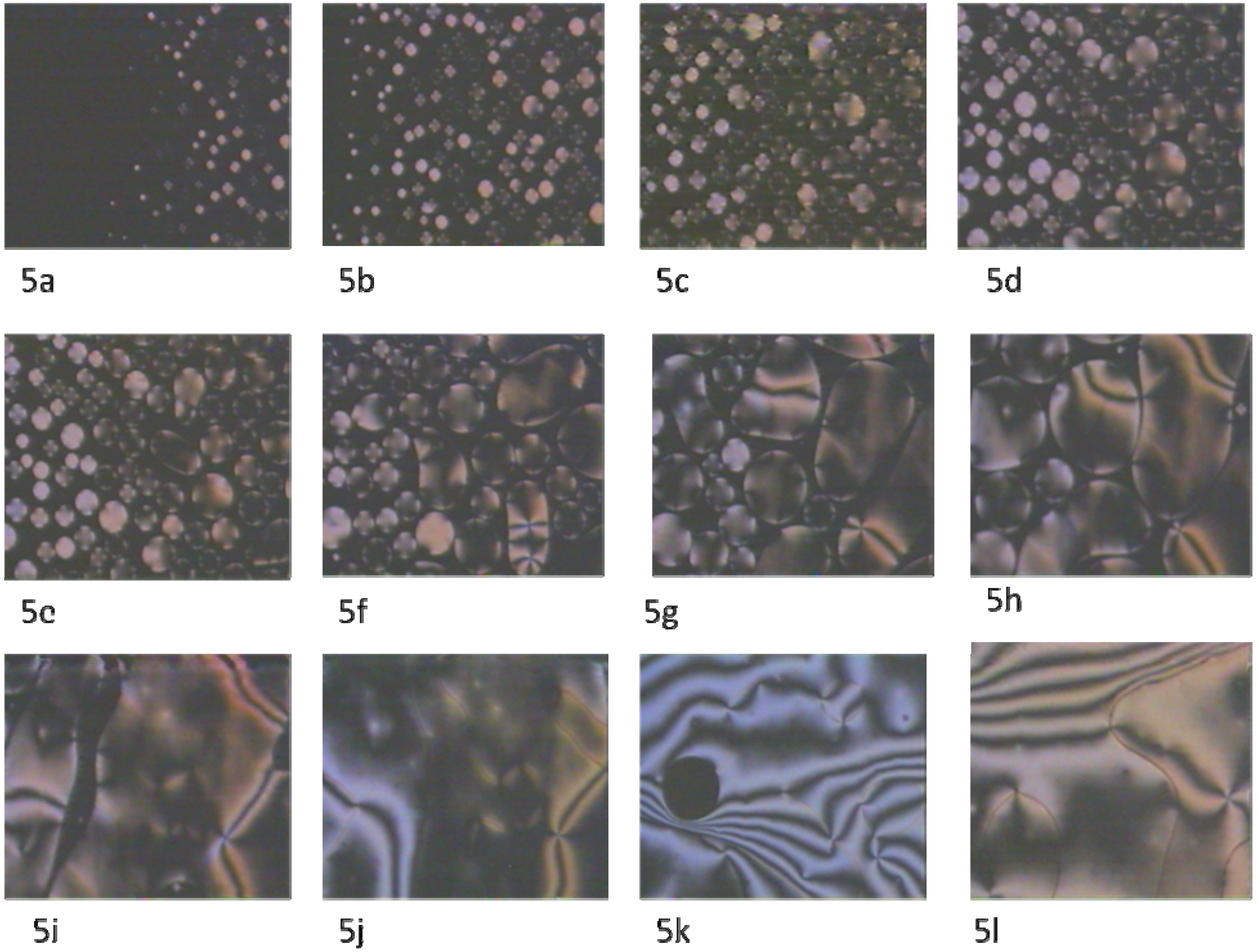

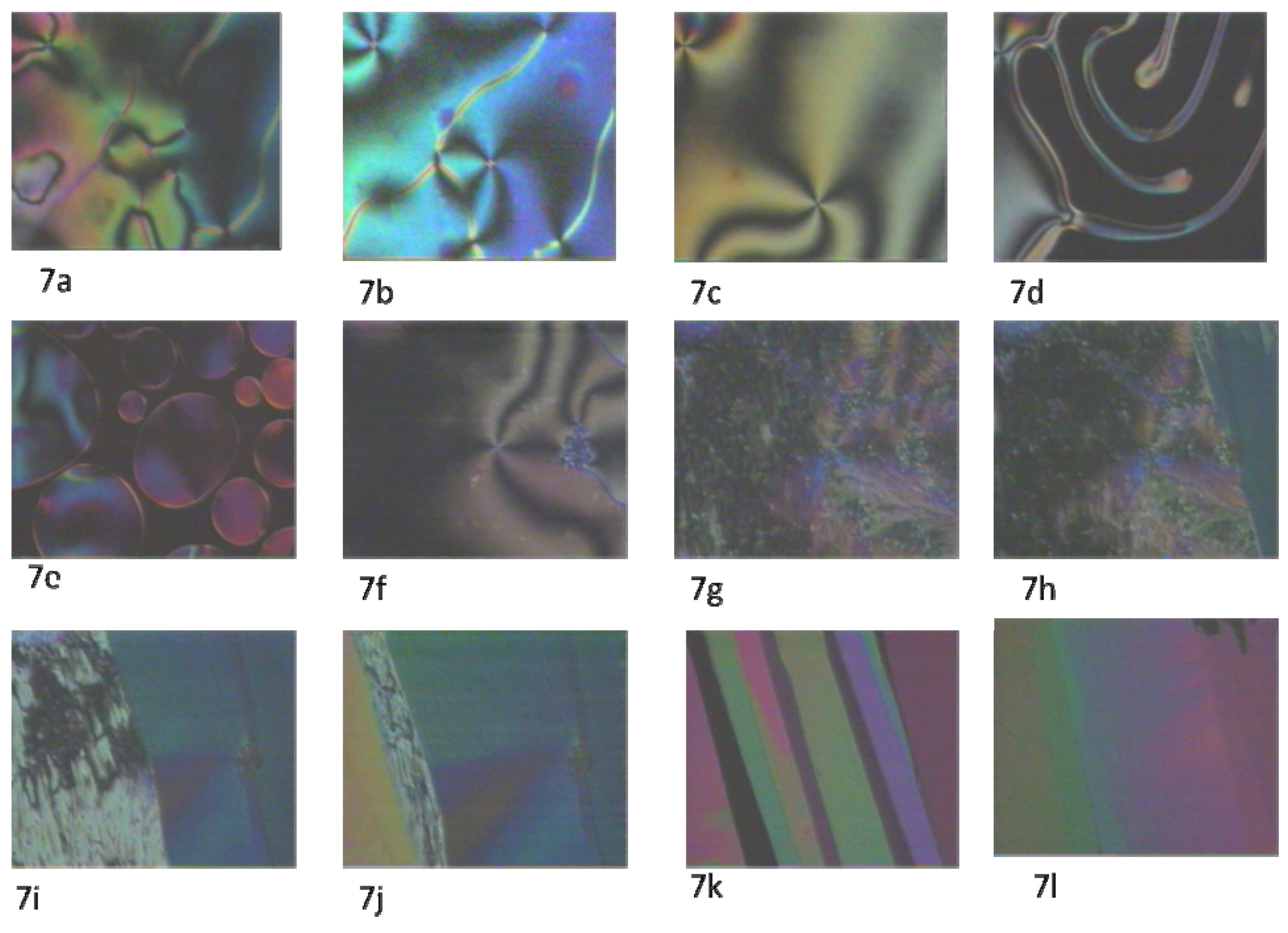

3. Experimental Section
3.1. Materials
3.2. Instrumentation
3.3. Synthesis
3.3.1. Preparation of the K2CO3·Al2O3 Catalyst
3.3.2. Ethyl 4-(4-Pentenyloxy)benzoate (1)
3.3.3. 4-(4-Pentenyloxy)benzoic Acid (2)
4. Conclusions
Acknowledgments
References
- Lemieux, P. Chirality Transfer in Ferroelectric Liquid Crystals. Acc. Chem. Res. 2001, 34, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, P.; Dinescu, L.; Maly, E. Optical switching of a ferroelectric liquid crystal spatial light modulator using chiral thioindigo dopants. ACS Symp. Ser. 2001, 798, 227–238. [Google Scholar]
- Jones, B. Apparent cases of liquid-crystal formation in p-alkoxybenzoic acids. J. Chem. Soc. 1935, 1874. [Google Scholar]
- Gray, G.W.; Jones, B. Mesomorphism and chemical constitution. III. The effect of halogen substitution on the mesomorphism of the 4-alkoxybenzoic acids. J. Chem. Soc. 1954, 2556–2562. [Google Scholar] [CrossRef]
- Ganicz, T.; Stanczyk, W.l.A.; Gladkova, N.K.; Sledzinska, I. Vinylsilanes as monomers for side chain polymer liquid crystals. Macromolecules 2000, 33, 289–293. [Google Scholar] [CrossRef]
- Yao, N.; Jamieson, A.M. Transient shear flow behavior of dilute solutions of side-chain liquid-crystalline polysiloxanes in 4,4'-(N-pentyloxy)cyanobiphenyl. Macromolecules 1998, 31, 5399–5406. [Google Scholar] [CrossRef]
- Hsu, C.-S.; Chu, P.-H.; Chang, H.-L.; Hsieh, T.-H. Effect of lateral substituents on the mesomorphic properties of side-chain liquid crystalline polysiloxanes containing 4-[(S)-2-methyl-1-butoxy]phenyl 4-(alkenyloxy)benzoate side groups. J. Polym. Sci. A: Polym. Chem. 1997, 35, 2793–2800. [Google Scholar] [CrossRef]
- Inoue, A.; Maniwa, S.; Ide, V. Relation between molecular structure and electrorheological effects in liquid crystalline polymers. J. Appl. Polym. Sci. 1997, 64, 303–310. [Google Scholar] [CrossRef]
- Jeng, G.P.C.; Kuo, J.F.; Chen, C.Y. An X-ray investigation of terminally carboxyl oligo(ethylene oxide) monomethyl ethers-substituted side-chain liquid-crystalline polysiloxanes. J. Appl. Polym. Sci. 1993, 47, 781–788. [Google Scholar] [CrossRef]
- Kaeding-Koppers, A.; Zugenmaier, P.; Roman, M. Mixed-dimer formation in binary systems of 4-substituted benzoic acids and structure considerations. Can. J. Chem. 2008, 86, 525–532. [Google Scholar] [CrossRef]
- Torgova, S.I.; Petrov, M.P.; Strigazzi, A. Textures of homologous 4-n-alkyloxybenzoic acids: Spontaneous chirality and surface memory. Liq. Cryst. 2001, 28, 1439–1449. [Google Scholar] [CrossRef]
- Kumar, U.; Kato, T.; Frechet, J.M.J. Use of intermolecular hydrogen bonding for the induction of liquid crystallinity in the side chain of polysiloxanes. J. Am. Chem. Soc. 1992, 114, 6630–6639. [Google Scholar] [CrossRef]
- Plass, M. Hydrogen bonding in liquid crystalline phases of p-n-alkoxybenzoic acids. Z. Phys. Chem. (Munich) 1996, 194, 223–230. [Google Scholar] [CrossRef]
- Kirov, N.; Fontana, M.P.; Cavatorta, F.; Ratajczak, H. IR, FIR, Raman and thermodynamic investigations of p-n-alkoxybenzoic acids in their solid, mesomorphic and isotropic phases. Mol. Cryst. Liq. Cryst. 1981, 75, 303–320. [Google Scholar] [CrossRef]
- Takase, A.; Sakagami, S.; Nakamizo, M. Temperature dependence of the Raman spectra of the homologous series of alkoxybenzoic acids. Jpn. J. Appl. Phys. 1977, 16, 1497–1498. [Google Scholar] [CrossRef]
- Konstantinov, I.I.; Khodzhaeva, V.L.; Shishkina, M.V.; Amerik, Y.B. Determination of the degree of order of mesomorphic p-n-alkoxybenzoic acids by IR dichroism method. J. Phys. (Paris), Colloq. 1975, 55–57. [Google Scholar]
- Kresse, H.; Luecke, K.H.; Schmidt, P.; Demus, D. Dielectric anisotropy of a liquid crystal, 4-n-alkyloxybenzoic acid in the nematic phase. Z. Phys. Chem. (Leipzig) 1977, 258, 785–787. [Google Scholar]
- Tian, Y.; Xu, X.; Zhao, Y.; Tang, X.; Li, T.; Sun, J.; Li, C.; Pan, A. Temperature-dependent FTIR study on self-assembly chiral liquid crystals through intermolecular hydrogen bonding. Thin Solid Films 1996, 284–285, 603–605. [Google Scholar] [CrossRef]
- Pugh, C.; Arehart, S.; Liu, H.; Narayanan, R. New architectures for side chain liquid crystalline polymers with chiral smectic C mesophases. J. Macromol. Sci., Pure Appl. Chem. 1994, A31, 1591–1607. [Google Scholar] [CrossRef]
- Bristol, D.W.; Schroeder, J.P. Liquid crystals. V. Molecular structural effects on the mesomorphism of phenylene esters. J. Org. Chem. 1974, 39, 3138–3141. [Google Scholar] [CrossRef]
- Schroeder, J.P.; Bristol, D.W. Liquid crystals. IV. Effects of terminal substituents on the nematic mesomorphism of p-phenylene dibenzoates. J. Org. Chem. 1973, 38, 3160–3164. [Google Scholar] [CrossRef]
- Jahromi, S. Liquid crystalline epoxide thermosets: A deuterium nuclear magnetic resonance study. Macromolecules 1994, 27, 2804–2813. [Google Scholar] [CrossRef]
- Krasnogolovets, V.V.; Puchkovskaya, G.A.; Yakubov, A.A. Mechanism of the formation of closed hydrogen associates in alkyl- and alkoxybenzoic acids during the phase transitions from the crystal to the liquid-crystal state. Khim. Fiz. 1992, 11, 806–813. [Google Scholar]
- Krasnogholovets, V.V.; Puchkovskaya, G.A.; Yakubov, A.A. Thermoinduced rearrangement of the hydrogen bonded systems in liquid crystalline carboxylic acids. Mol. Cryst. Liq. Cryst. Sci. Technol. A 1995, 265, 2709–2716. [Google Scholar]
- Sideratou, Z.; Tsiourvas, D.; Paleos, C.M.; Skoulios, A. Liquid crystalline behavior of hydrogen bonded complexes of a nonmesogenic anil with p-n-alkoxybenzoic acids. Liq. Cryst. 1997, 22, 51–60. [Google Scholar] [CrossRef]
- Katranchev, B.; Naradikian, H.; Petrov, M. The role of hydrogen bonding for initiation of chirality, dendrites and physical gel in nematics with short range smectic C order. J. Optoelectron. Adv. Mater. 2005, 7, 273–276. [Google Scholar]
- Suriyakala, R.; Pisipati, V.G.K.M.; Swamy, G.N.; Potukuchi, D.M. Alternating intermolecular hydrogen bonding in linear liquid-crystalline complexes. Mol. Cryst. Liq. Cryst. 2006, 457, 181–189. [Google Scholar] [CrossRef]
- Sui, D.; Hou, Q.; Chai, J.; Ye, L.; Zhao, L.; Li, M.; Jiang, S. Symmetric bi-pyridyl banana-shaped molecule and its intermolecular hydrogen bonding liquid-crystalline complexes. J. Mol. Struct. 2008, 891, 312–316. [Google Scholar] [CrossRef]
- Pisupati, S.; Kumar, P.A.; Pisipati, V.G.K.M. Induced crystal G phase through intermolecular hydrogen bonding II. Influence of alkyl chain length of n-alkyl p-hydroxybenzoates on thermal and phase behaviour. Liq. Cryst. 2000, 27, 665–669. [Google Scholar] [CrossRef]
- Demus, D.; Richter, L. Textures of Liquid Crystals; Wiley-VCH: Weinheim, Germany, 1978; p. 228. [Google Scholar]
- Demus, D.; Richter, L. Textures of Liquid Crystals, 2nd ed; Wiley-VCH: Weinheim, Germany, 1980; p. 228. [Google Scholar]
- Bobadova-Parvanova, P.; Parvanov, V.; Petrov, M.; Tsonev, L. Molecular structure of the nematic liquid crystals made by hydrogen bonded in dimers molecules. Cryst. Res. Technol. 2000, 35, 1321–1330. [Google Scholar] [CrossRef]
- Sage, I. Liquid Crystals. In Ulmann’s Encyclopedia of Industrial Chemistry; Elvers, B., Hawkins, S., Shulz, G., Eds.; VCH: Weiheim, Germany, 1990; Volume A15, p. 359. [Google Scholar]
- Engelen, B.; Heppke, G.; Hopf, R.; Schneider, F. Induced smectic phases. Ann. Phys. (Paris) 1978, 3, 403–407. [Google Scholar]
- Petrov, M.; Braslau, A.; Levelut, A.M.; Durand, G. Surface induced transitions in the nematic phase of 4-octyloxybenzoic acid. J. Phys. II 1992, 2, 1159–1193. [Google Scholar]
- Petrov, M.; Alexe-Ionescu, A.L.; Barbero, G.; Kirov, N. Landau theory for the surface induced order transitions in the nematic phase with hydrogen bonded molecules. Mol. Cryst. Liq. Cryst. Sci. Technol. C 1995, 5, 41–49. [Google Scholar]
- Petrove, M.; Durand, G. Polar surface bifurcation in dimerized nematic phase of 4-n alkyloxybenzoic acids. J. Phys. II 1996, 6, 1259–1272. [Google Scholar]
- Qaddoura, A.M.; Belfield, K. Synthesis, Characterization and Texture Observations of Calamitic Liquid Crystalline Compounds. Int. J. Mol. Sci. 2009, 10, 4772–4788. [Google Scholar] [CrossRef] [PubMed]
- Swathi, P.; Sastry, S.S.; Kumar, P.A.; Pisipati, V.G.K.M. Induced smectic-G phase through intermolecular H-bonding. Part III. Influence of alkyl chain length of p-n-alkoxybenzoic acids on thermal and phase behaviour. Mol. Cryst. Liq. Cryst. Sci. Technol. A 2001, 365, 523–533. [Google Scholar] [CrossRef]
- Ingo, D. Texture of Liquid Crystals, 1st ed.; Wiley-VCH: Weinheim, Germany, 2003; p. 199. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qaddoura, M.A.; Belfield, K.D. Probing the Texture of the Calamitic Liquid Crystalline Dimer of 4-(4-Pentenyloxy)benzoic Acid. Materials 2010, 3, 827-840. https://doi.org/10.3390/ma3020827
Qaddoura MA, Belfield KD. Probing the Texture of the Calamitic Liquid Crystalline Dimer of 4-(4-Pentenyloxy)benzoic Acid. Materials. 2010; 3(2):827-840. https://doi.org/10.3390/ma3020827
Chicago/Turabian StyleQaddoura, Maher A., and Kevin D. Belfield. 2010. "Probing the Texture of the Calamitic Liquid Crystalline Dimer of 4-(4-Pentenyloxy)benzoic Acid" Materials 3, no. 2: 827-840. https://doi.org/10.3390/ma3020827




