High-Pressure Study of Anatase TiO2
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
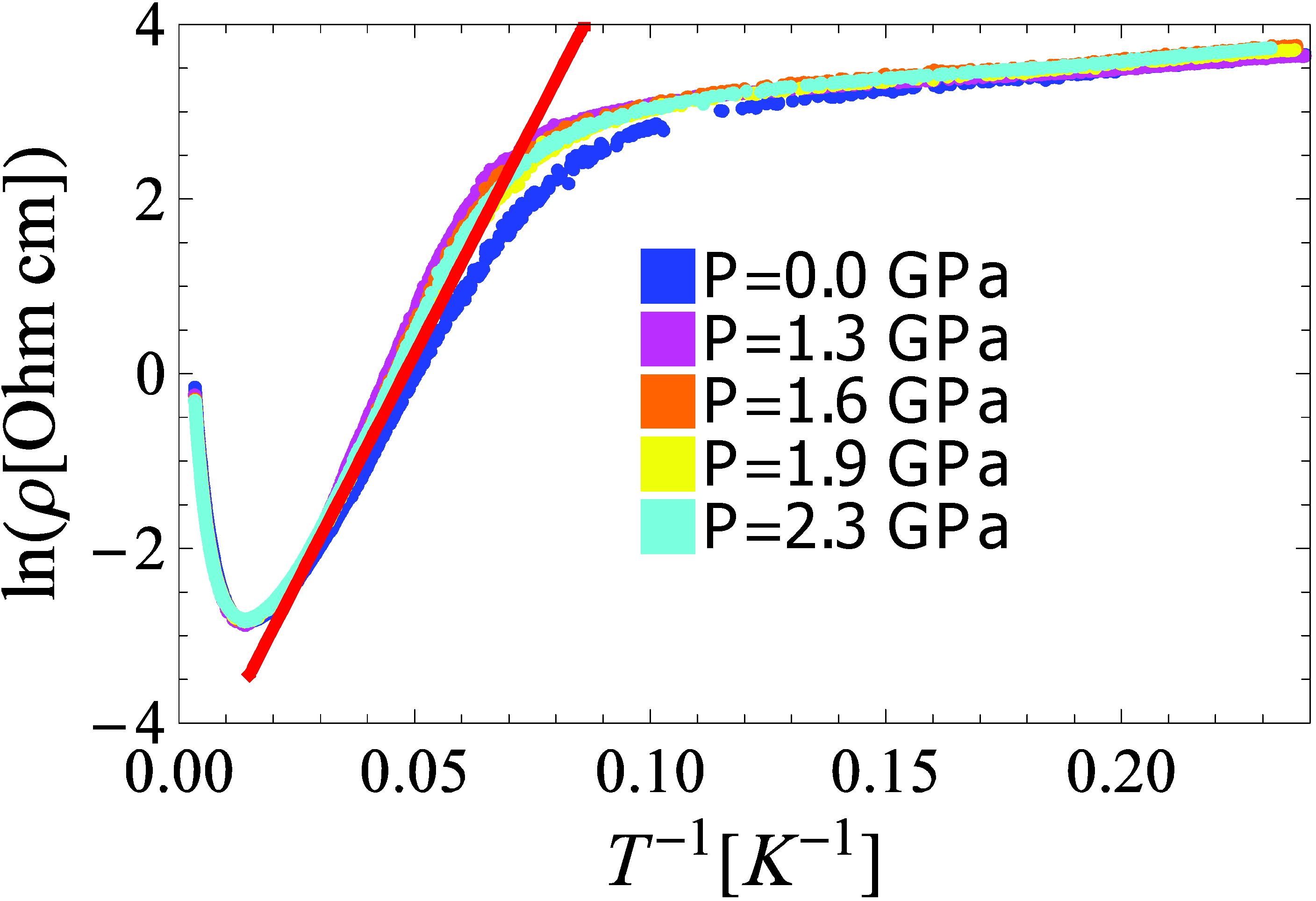
3.1. Resistivity properties

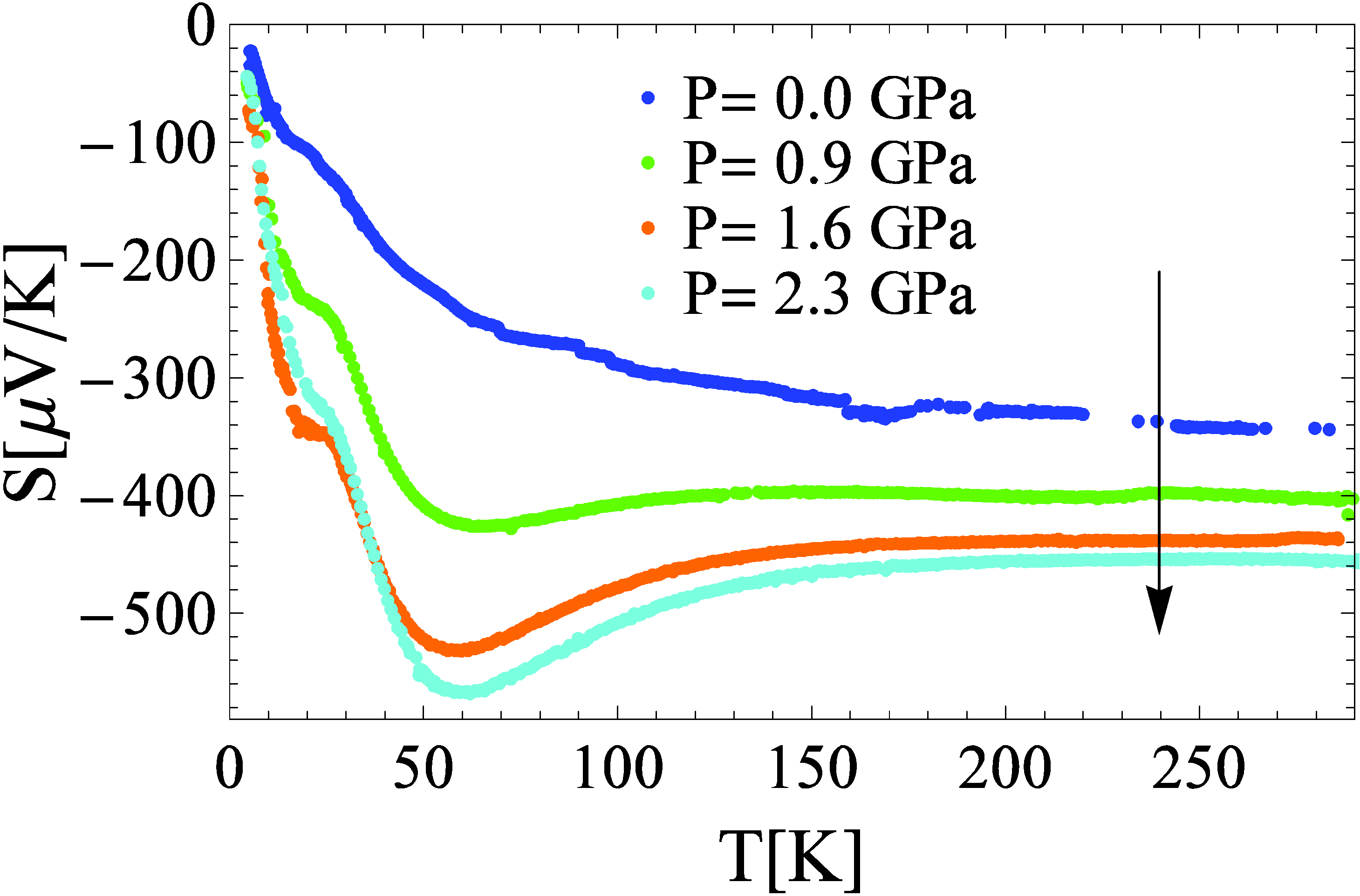
3.2. Thermo-electric power
4. Conclusions
Acknowledgements
References and Notes
- Grätzel, M. The Artificial Leaf Molecular Photovoltaics Achieve Efficient Generation of Electricity from Sunlight. Comments Inorg. Chem. 1991, 12, 93–111. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical Cells. Nature 2001, 414, 338–344. [Google Scholar]
- Burdett, J.K.; Hughbanks, T.; Miller, G.J.; Richardson, J.W.; Smith, J.V. Structural-Electronic Relationships in Inorganic Solids: Powder Neutron Diffraction Studies of the Rutile and Anatase Polymorphs of Titanium Dioxide at 15 and 295 K. J. Am. Chem. Soc. 1987, 109, 3639–3646. [Google Scholar] [CrossRef]
- Tang, H.; Berger, H.; Schmid, P.E.; Lévy, F.J.; Burri, G. Photoluminescence in TiO2 Anatase Single Crystals. Solid State Commun. 1993, 87, 847–850. [Google Scholar]
- Slater, J.C. Introduction to Chemical Physics, 1st ed.; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1939; p. 346. [Google Scholar]
- Forró, L.; Chauvet, O.; Emin, D.; Zuppiroli, L.; Berger, H.; Lévy, F. High mobility n-type charge carriers in large single crystals of anatase TiO2. J. Appl. Phys. 1994, 75, 633–635. [Google Scholar] [CrossRef]
- Berger, H.; Tang, H.; Lévy, F.J. Growth and Raman Spectroscopic Characterization of TiO2 Anatase Single Crystals. J. Cryst. Growth 1993, 130, 108–112. [Google Scholar] [CrossRef]
- De Boer, J.H.; Verwey, E.J.W. Semi-Conductors with Partially and with Completely Filled 3d- Lattice Bands. Proceed. Phys. Soc. 1937, 49, 59–71. [Google Scholar] [CrossRef]
- Sekiya, T.; Kamiya, N.; Ohya, S.; Kurita, S.; Kodaira, T. Electron Paramagnetic Resonance and Optical Absorption of Yellow Anatase TiO2 Single Crystal. J. Phys. Soc. Jpn. 2009, 78, 114701. [Google Scholar] [CrossRef]
- Aselage, T.L.; Emin, D.; McCready, S.S.; Duncan, R.V. Large Enhancement of Boron Carbides' Seebeck Coefficients through Vibrational Softening. Phys. Rev. Lett. 1998, 81, 2316–2319. [Google Scholar]
- Emin, D. Enhanced Seebeck Coefficient from Carrier-Induced Vibrational Softening. Phys. Rev. B 1999, 59, 6205–6210. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jaćimović, J.; Vâju, C.; Gaál, R.; Magrez, A.; Berger, H.; Forró, L. High-Pressure Study of Anatase TiO2. Materials 2010, 3, 1509-1514. https://doi.org/10.3390/ma3031509
Jaćimović J, Vâju C, Gaál R, Magrez A, Berger H, Forró L. High-Pressure Study of Anatase TiO2. Materials. 2010; 3(3):1509-1514. https://doi.org/10.3390/ma3031509
Chicago/Turabian StyleJaćimović, Jaćim, Cristian Vâju, Richard Gaál, Arnaud Magrez, Helmuth Berger, and László Forró. 2010. "High-Pressure Study of Anatase TiO2" Materials 3, no. 3: 1509-1514. https://doi.org/10.3390/ma3031509



