Toward High-Performance Coatings for Biomedical Devices: Study on Plasma-Deposited Fluorocarbon Films and Ageing in PBS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ageing of coated samples
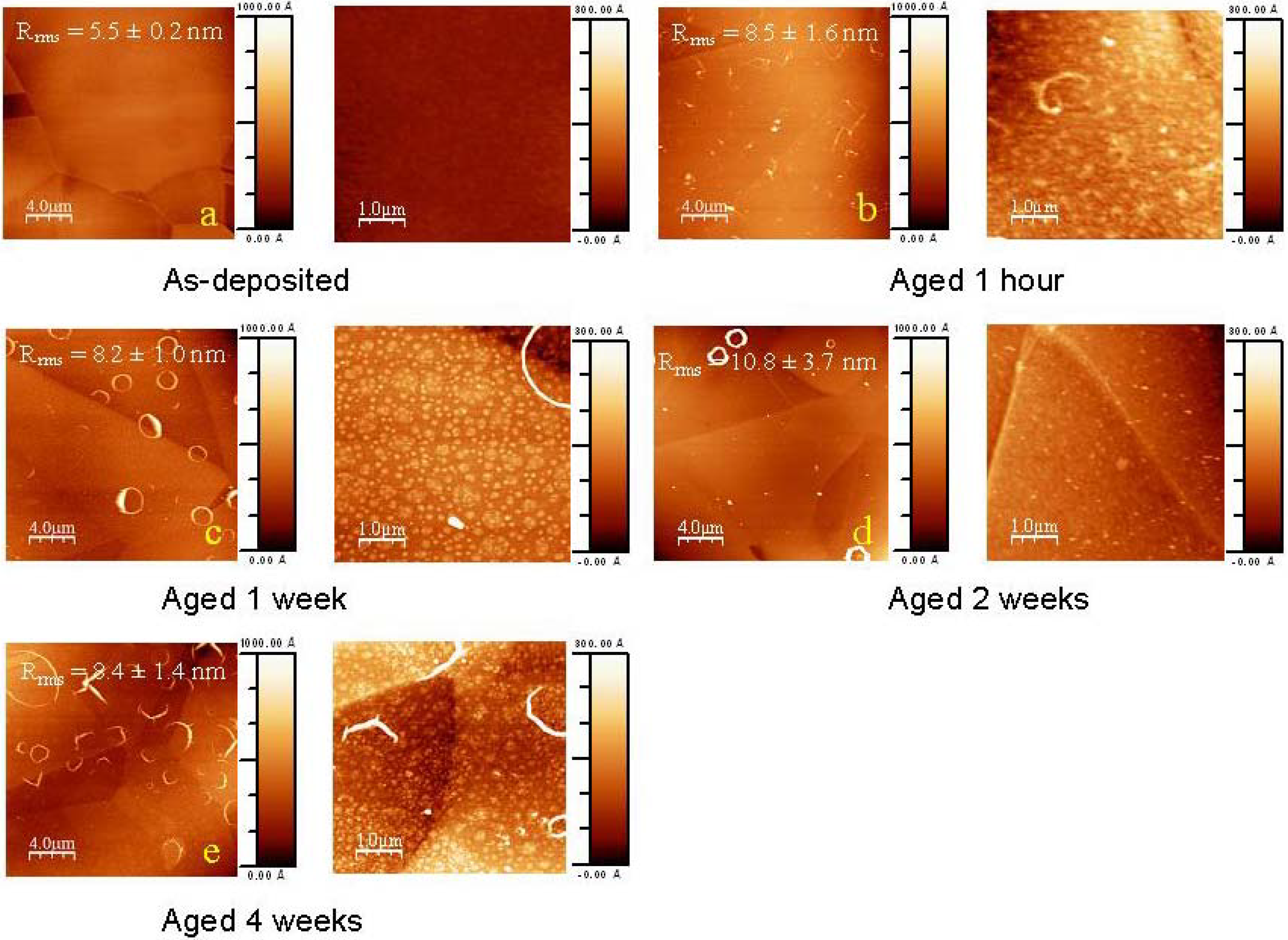
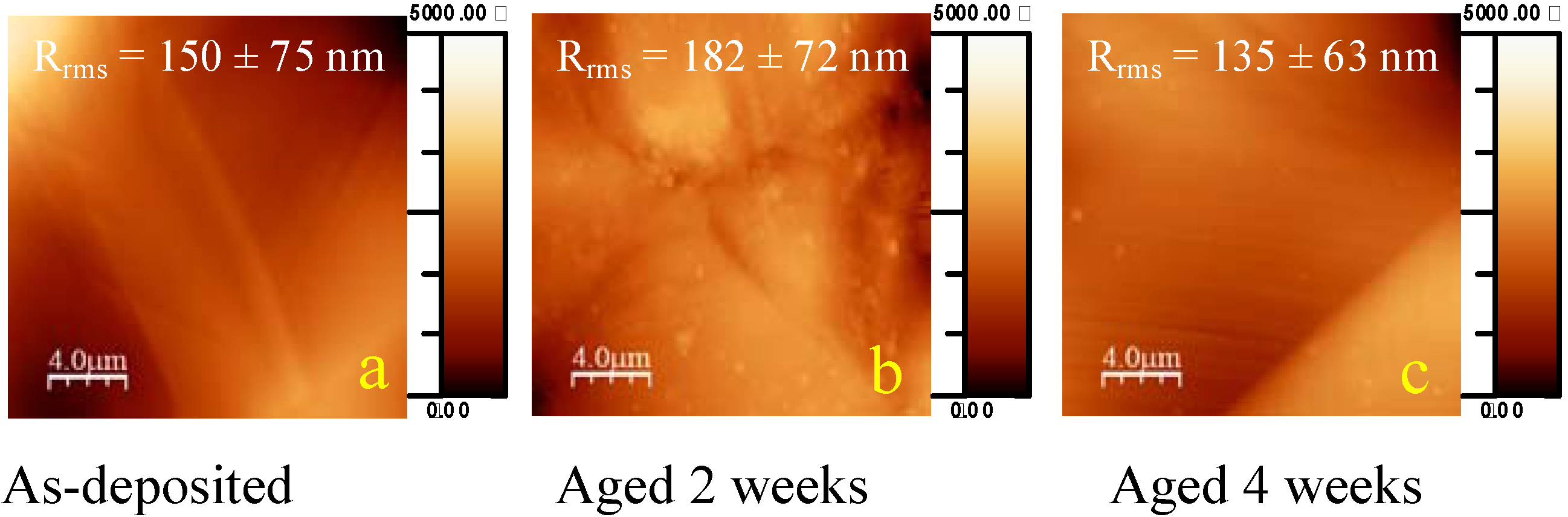


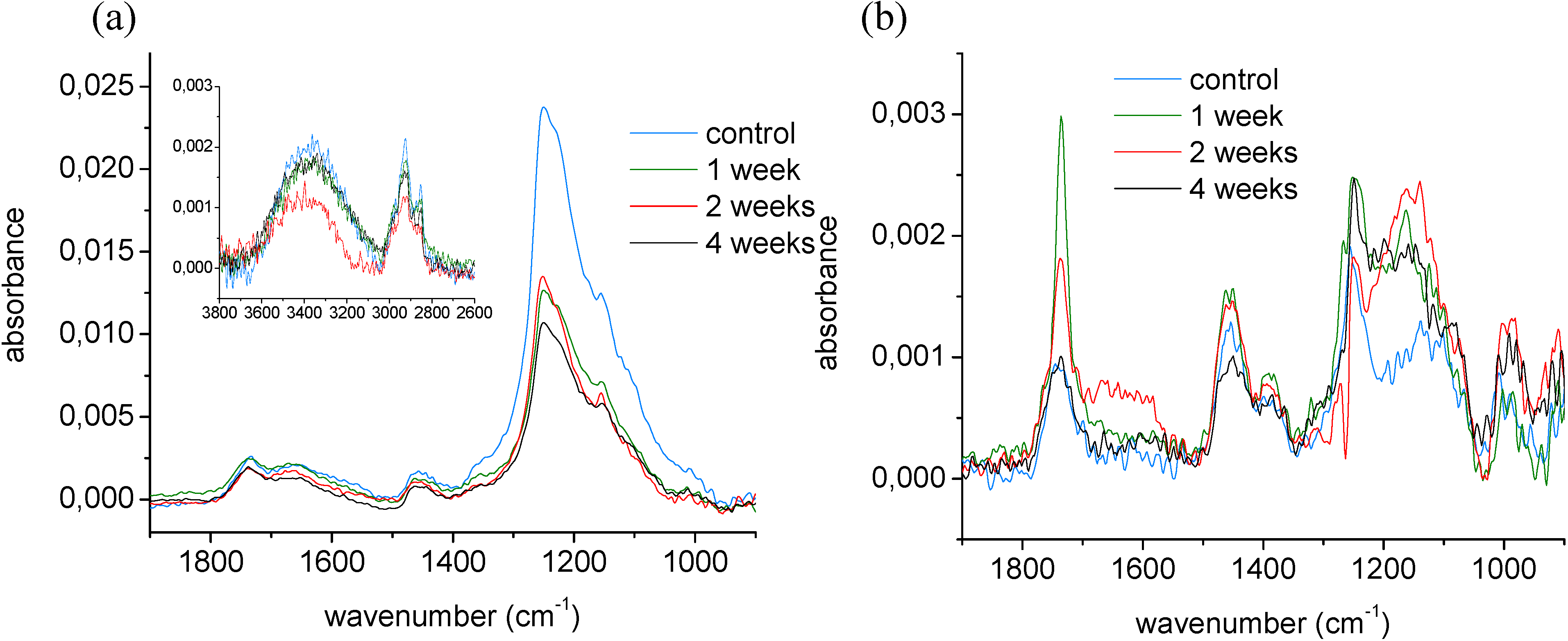
2.2. Mechanisms of the ageing process of fluorocarbon films
2.2.1. Ageing of non-deformed samples
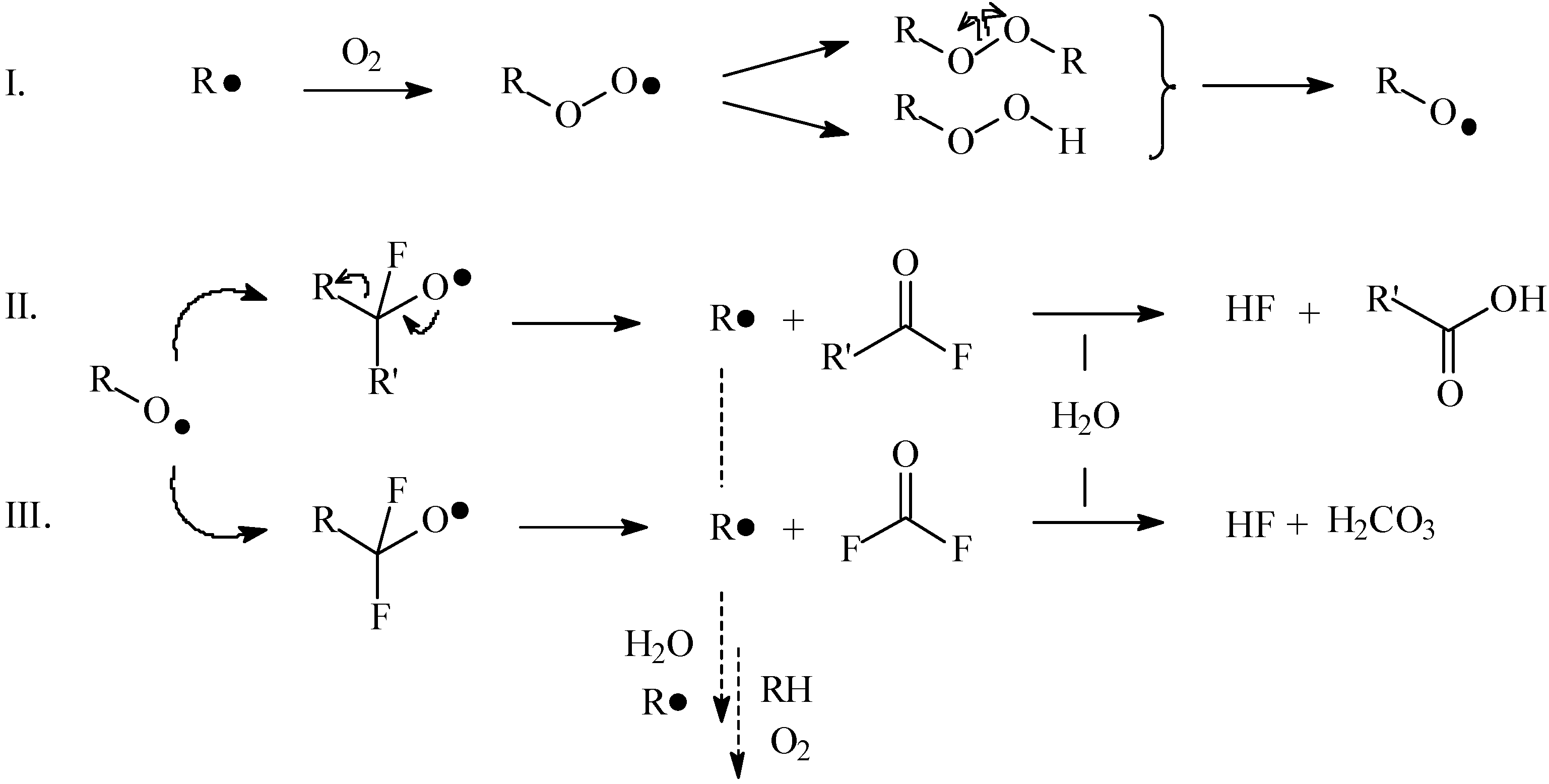
2.2.2. Ageing of plastically deformed samples
3. Experimental Section
3.1. Materials
3.2. Deformation method
3.3. Surface ageing
3.4. Characterization of the ultra-thin coating
4. Conclusions
Acknowledgement
References
- Chevallier, P.; Mantovani, D.; Laroche, G. Functionalization of Vascular Biomaterials Luminal Surfaces. In New Technologies in Vascular Biomaterials Luminal Surfaces: Polytetrafluoroethylene Prostheses, Impregnated Vascular Prostheses and Surface Treatment of Vascular Prostheses; Chakfé, N., Durand, B., Kretz, J.G., Eds.; Europrot: Strasbourg, France, 2003; pp. 87–101. [Google Scholar]
- Dahm, K.L.; Dearnley, P.A. Abrasion response and abrasion-corrosion interactions for a coated biomedical stainless steel. Wear 2005, 259, 933–942. [Google Scholar] [CrossRef]
- Boland, J.L.; Corbeij, H.A.; Van Der Giessen, W.; Seabra-Gomes, R.; Suryapranata, H.; Wijns, W.; Hanet, C.; Suttorp, M.J.; Buller, C.; Bonnier, J.J.; Colombo, A.; Van Birgelen, C.; Pieper, M.; Mangioni, J.A.; Londero, H.; Carere, R.G.; Hamm, C.W.; Bonan, R.; Bartorelli, A.; Kyriakides, Z.S.; Chauhan, A.; Rothman, M.; Grinfeld, L.; Oosterwijk, C.; Serruys, P.W.; Cumberland, D.C. Multicenter evaluation of the phosphorylcholine-coated biodivYsio stent in short de novo coronary lesions: The SOPHOS study. Int. J. Cardiovasc. Intervent. 2000, 3, 215–225. [Google Scholar] [PubMed]
- Lewis, A.L.; Furze, J.D.; Small, S.; Robertson, J.D.; Higgins, B.J.; Taylor, S.; Ricci, D.R. Long-term stability of a coronary stent coating post-implantation. J. Biomed. Mater. Res. 2002, 63, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Stratford, P.W. Phosphorylcholine-coated stents. J. Long. Term. Eff. Med. Implants 2002, 12, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Tolhurst, L.A.; Stratford, P.W. Analysis of a phosphorylcholine-based polymer coating on a coronary stent pre- and post-implantation. Biomaterials 2002, 23, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Bickel, C.; Rupprecht, H.J.; Darius, H.; Binz, C.; Hauroder, B.; Krummenauer, F.; Meyer, J. Substantial reduction of platelet adhesion by heparin-coated stents. J. Interv. Cardiol. 2001, 14, 407–413. [Google Scholar] [CrossRef] [PubMed]
- De Scheerder, I.; Wang, K.; Wilczek, K.; Meuleman, D.; Van Amsterdam, R.; Vogel, G.; Piessens, J.; Van de Werf, F. Experimental study of thrombogenicity and foreign body reaction induced by heparin-coated coronary stents. Circulation 1997, 95, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S.C.; Yoon, H.C.; Wong, G.C.; Bonilla, S.M.; Vedantham, S.; Arora, L.C. Percutaneous delivery of a heparin-impregnated collagen stent-graft in a porcine model of atherosclerotic disease. Invest. Radiol. 2000, 35, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Ritter, E.F.; Kim, Y.B.; Reischl, H.P.; Serafin, D.; Rudner, A.M.; Klitzman, B. Heparin coating of vascular prostheses reduces thromboemboli. Surgery 1997, 122, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Sefton, M.V.; Sawyer, A.; Gorbet, M.; Black, J.P.; Cheng, E.; Gemmell, C.; Pottinger-Cooper, E. Does surface chemistry affect thrombogenicity of surface modified polymers? J. Biomed. Mater. Res. 2001, 55, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Babapulle, M.P.; Eisenberg, M.J. Coated stents for the prevention of restenosis: Part II. Circulation 2002, 106, 2859–2866. [Google Scholar] [CrossRef] [PubMed]
- Babapulle, M.N.; Eisenberg, M.J. Coated stents for the prevention of restenosis: Part I. Circulation 2002, 106, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- Deible, C.R.; Petrosko, P.; Johnson, P.C.; Beckman, E.J.; Russel, A.J.; Wagner, W.R. Molecular barrier to biomaterial thrombosis by modification of surface proteins with polyethylene glycol. Biomaterials 1998, 19, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Deible, C.R.; Petrosko, P.; Johnson, P.C.; Beckman, E.J.; Russel, A.J.; Wagner, W.R. Molecular barrier to biomaterial thrombosis by modification of surface proteins with polyethylene glycol. Biomaterials 1999, 20, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Manolache, S.; Somers, E.B.; Lee Wong, A.C.; Denes, F.S. Generation of antifouling layers on stainless steel surfaces by plasma-enhanced crosslinking of polyethylene glycol. J. Appl. Polym. Sci. 2005, 97, 485–497. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, J.K.; Goo, H.C.; Lee, W.K.; Park, K.D.; Kim, S.H.; Yoo, Y.M.; Kim, Y.H. Improved blood compatibility and decreased VSMC proliferation of surface-modified metal grafted with sulfonated PEG or heparin. J. Biomater. Sci. Polym. Ed. 2002, 13, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Stuhlmeier, K.M.; Lin, Y. Camoufling endothelial cells; Does it prolong graft survival? Biochim. Biophys. Acta 1999, 1428, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.K.; Mintz, G.S.; Lee, C.W.; Song, J.M.; Han, K.H.; Kang, D.H.; Song, J.K.; Kim, J.J.; Weissman, N.J.; Fearnot, N.E.; Park, S.W.; Park, S.J. Paclitaxel coating reduces in-stent intimal hyperplasia in human coronary arteries: a serial volumetric intravascular ultrasound analysis from the Asian Paclitaxel-Eluting Stent Clinical Trial (ASPECT). Circulation 2003, 107, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Schwertz, D.W.; Vaitkus, P. Drug-eluting stents to prevent reblockage of coronary arteries. J. Cardiovasc. Nurs. 2003, 18, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Serruys, P.W.; Grube, E.; Smits, P.C.; Selbach, G.; van der Giessen, W.J.; Staberock, M.; de Feyter, P.; Muller, R.; Regar, E.; Degertekin, M.; Ligthart, J.M.; Disco, C.; Backx, B.; Russell, M.E. TAXUS III Trial: In-stent restenosis treated with stent-based delivery of paclitaxel incorporated in a slow-release polymer formulation. Circulation 2003, 107, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Wieneke, H.; Sawitowski, T.; Wnendt, S.; Fischer, A.; Dirsch, O.; Karoussos, I.A.; Erbel, R. Stent coating: A new approach in interventional cardiology. Herz 2002, 27, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, M.M.; De, S.; Trigwell, S.; Ali, N.; Mazumder, M.K.; Mehta, J.L. Corrosion resistance of polyurethane-coated nitinol cardiovascular stents. J. Biomater. Sci. Polym. Ed. 2003, 14, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Grundmeier, G.; Stratmann, M. Plasma polymerization—A new and promising way for the corrosion protection of steel. Mater. Corros. 1998, 49, 150–160. [Google Scholar] [CrossRef]
- Grundmeier, G.; Thiemann, P.; Carpentier, J.; Barranco, V. Tailored thin plasma polymers for the corrosion protection of metals. Surf. Coat. Technol. 2003, 174, 996–1001. [Google Scholar] [CrossRef]
- Joshi, P.P.; Pulikollu, R.; Higgins, S.R.; Hu, X.; Mukhopadhyay, S.M. Investigation of growth, coverage and effectiveness of plasma assisted nano-films of fluorocarbon. Appl. Surf. Sci. 2006, 252, 5676–5686. [Google Scholar] [CrossRef]
- Moffitt, C.E.; Reddy, C.M.; Yu, Q.S.; Wieliczka, D.M.; Yasuda, H.K. Selective adsorption of fluorocarbons and its effects on the adhesion of plasma polymer protective coatings. Appl. Surf. Sci. 2000, 161, 481–496. [Google Scholar] [CrossRef]
- Caicedo, M.; Jacobs, J.J.; Redyy, A.; Hallab, N.J. Analysis of metal ion-induced DNA damage, apoptosis, and necrosis in human (Jurkat) T-cells demonstrates Ni2+ and V3+ are more toxic than other metals: Al3+, Be2+, Co2+, Cr3+, Cu2+, Fe3+, Mo5+, Nb5+, Zr2+. J. Biomed. Mater. Res. A 2008, 86A, 905–913. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). IARC Monographs Programme on the Evaluation of Carcinogenic Risks to Humans; IARC Scientific Publications: Lyon, France, 1998; Volume 74, pp. 9–31. [Google Scholar]
- Food and Drug Administration (FDA). Guidance for Industry and FDA Staff; Non-clinical Tests and Recommended Labelling for Intravascular Stents and Associated Delivery Systems; US Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health: Rockville, MD, USA, 2005; pp. 1–43.
- Barranco, V.; Carpentier, J.; Grundmeier, G. Correlation of morphology and barrier properties of thin microwave plasma polymer films on metal substrate. Electrochim. Acta 2004, 49, 1999–2013. [Google Scholar] [CrossRef]
- Lin, Y.; Yasuda, H. Effect of plasma polymer deposition methods on copper corrosion protection. J. Appl. Polym. Sci. 1996, 60, 543–555. [Google Scholar] [CrossRef]
- Otsuka, Y.; Chronos, N.A.F.; Aptakarian, R.P.; Robinson, K.A. Scanning electron microscopic analysis of defects in polymer coatings of three commercially available stents: Comparison of biod. J. Invasive Cardiol. 2007, 19, 71–76. [Google Scholar] [PubMed]
- Lewis, F.; Horny, P.; Hale, P.; Turgeon, S.; Tatoulian, M.; Mantovani, D. Study of the adhesion of thin plasma fluorocarbon coatings resisting plastic deformation for stent applications. J. Phys. D: Appl. Phys. 2008, 41, 045310:11–045310:17. [Google Scholar] [CrossRef]
- Marek, M. Oxygen and pH Control in Corrosion Testing of Surgical Implants. In Medical Device Materials–Proceedings of the Materials and Processes for Medical Devices Conference 2003; ASM International: Anaheim, California, CA, USA, 2003; pp. 133–138. [Google Scholar]
- Hale, P.; Turgeon, S.; Horny, P.; Lewis, F.; Brack, N.; Van Riessen, G.; Pigram, P.; Mantovani, D. X-ray photoelectron emission microscopy and time-of-flight secondary ion mass spectrometry analysis of ultrathin fluoropolymer coatings for stent applications. Langmuir 2008, 24, 7897–7905. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.; Chevallier, P.; Turgeon, S.; Cloutier, M.; Pireaux, J.J.; Tatoulian, M.; Mantovani, D. Influence of the 316L stainless steel interface on the adhesion, stability and barrier properties of plasma fluorocarbon films. J. Mater. Sci. Mater. Med. 2010. submitted. [Google Scholar]
- Dai, L.; StJohn, H.A.W.; Bi, J.; Zientek, P.; Chatelier, R.C.; Griesser, H.J. Biomedical coatings by the covalent immobilization of polysaccharides onto gas-plasma-activated polymer surfaces. Surf. Interf. Anal. 2000, 29, 46–55. [Google Scholar] [CrossRef]
- Gengenbach, T.R.; Griesser, H.J. Compositional changes in plasma-deposited fluorocarbon films during ageing. Surf. Interf. Anal. 1998, 26, 498–511. [Google Scholar] [CrossRef]
- Moffitt, C.E.; Yu, Q.S.; Reddy, C.M.; Wieliczka, D.M.; Yasuda, H.K. XPS analysis of the aging of thin, adhesion promoting, fluorocarbon treatments of DC plasma polymers. Plasmas Polym. 2001, 6, 193–209. [Google Scholar] [CrossRef]
- Wijesundara, M.B.J.; Zajac, G.; Fuoco, E.; Hanley, L. Aging of fluorocarbon thin films deposited on polystyrene from hyperthermal C3F5+ and CF3+ ion beams. J. Adhes. Sci. Technol. 2001, 15, 599. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers. In The Scienta ESCA300 Database, 1st ed.; Wiley: Chichester, UK, 1992; p. 1. [Google Scholar]
- Rastogi, A.C.; Desu, S.B. Thermal chemical vapor deposition of fluorocarbon polymer thin films in a hot filament reactor. Polymer 2005, 46, 3440–3451. [Google Scholar] [CrossRef]
- Milella, A.; Palumbo, F.; Favia, P.; Cicala, G.; d'Agostino, R. Continuous and modulated deposition of fluorocarbon films from c-C4F8 plasmas. Plasma Process. Polym. 2004, 1, 164–170. [Google Scholar] [CrossRef]
- Horie, M. Plasma-structure dependence of the growth mechanism of plasma-polymerized fluorocarbon films with residual radicals. J. Vac. Sci. Technol. A−Vac. Surf. Films 1995, 13, 2490–2497. [Google Scholar] [CrossRef]
- Gengenbach, T.R.; Vasic, Z.R.; Chatelier, R.C.; Griesser, H.J. A multi-technique study of the spontaneous oxidation of N-hexane plasma polymers. J. Polym. Sci. A− Polym. Chem. 1994, 32, 1399–1414. [Google Scholar] [CrossRef]
- Tanikella, R.V.; Agraharam, S.; Bidstrup Allen, S.A.; Hess, D.W.; Kohl, P.A. Moisture absorption studies of fluorocarbon films deposited from pentafluoroethane and octafluorocyclobutane plasmas. J. Electron. Mater. 2001, 31, 1096–1103. [Google Scholar] [CrossRef]
- Haidopoulos, M.; Turgeon, S.; Laroche, G.; Mantovani, D. Chemical and morphological characterization of ultra-thin fluorocarbon plasma-polymer deposition on 316 stainless steel substrates: A first step toward the improvement of the long-term safety of coated-stents. Plasma Processes Polym. 2005, 2, 424–440. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Favia, P. Plasma Deposition of Fluoropolymer Films in Different Glow Discharge Regimes. In Plasma Polymer Films; Biederman, H., Ed.; Imperial College Press: London, UK, 2004; p. 25. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Holvoet, S.; Chevallier, P.; Turgeon, S.; Mantovani, D. Toward High-Performance Coatings for Biomedical Devices: Study on Plasma-Deposited Fluorocarbon Films and Ageing in PBS. Materials 2010, 3, 1515-1532. https://doi.org/10.3390/ma3031515
Holvoet S, Chevallier P, Turgeon S, Mantovani D. Toward High-Performance Coatings for Biomedical Devices: Study on Plasma-Deposited Fluorocarbon Films and Ageing in PBS. Materials. 2010; 3(3):1515-1532. https://doi.org/10.3390/ma3031515
Chicago/Turabian StyleHolvoet, Servaas, Pascale Chevallier, Stéphane Turgeon, and Diego Mantovani. 2010. "Toward High-Performance Coatings for Biomedical Devices: Study on Plasma-Deposited Fluorocarbon Films and Ageing in PBS" Materials 3, no. 3: 1515-1532. https://doi.org/10.3390/ma3031515



