Azine- and Azole-Functionalized Oligo´ and Polythiophene Semiconductors for Organic Thin-Film Transistors
Abstract
:1. Introduction
1.1. General Overview
1.2. Organic Field-Effect Transistor Structure and Operation
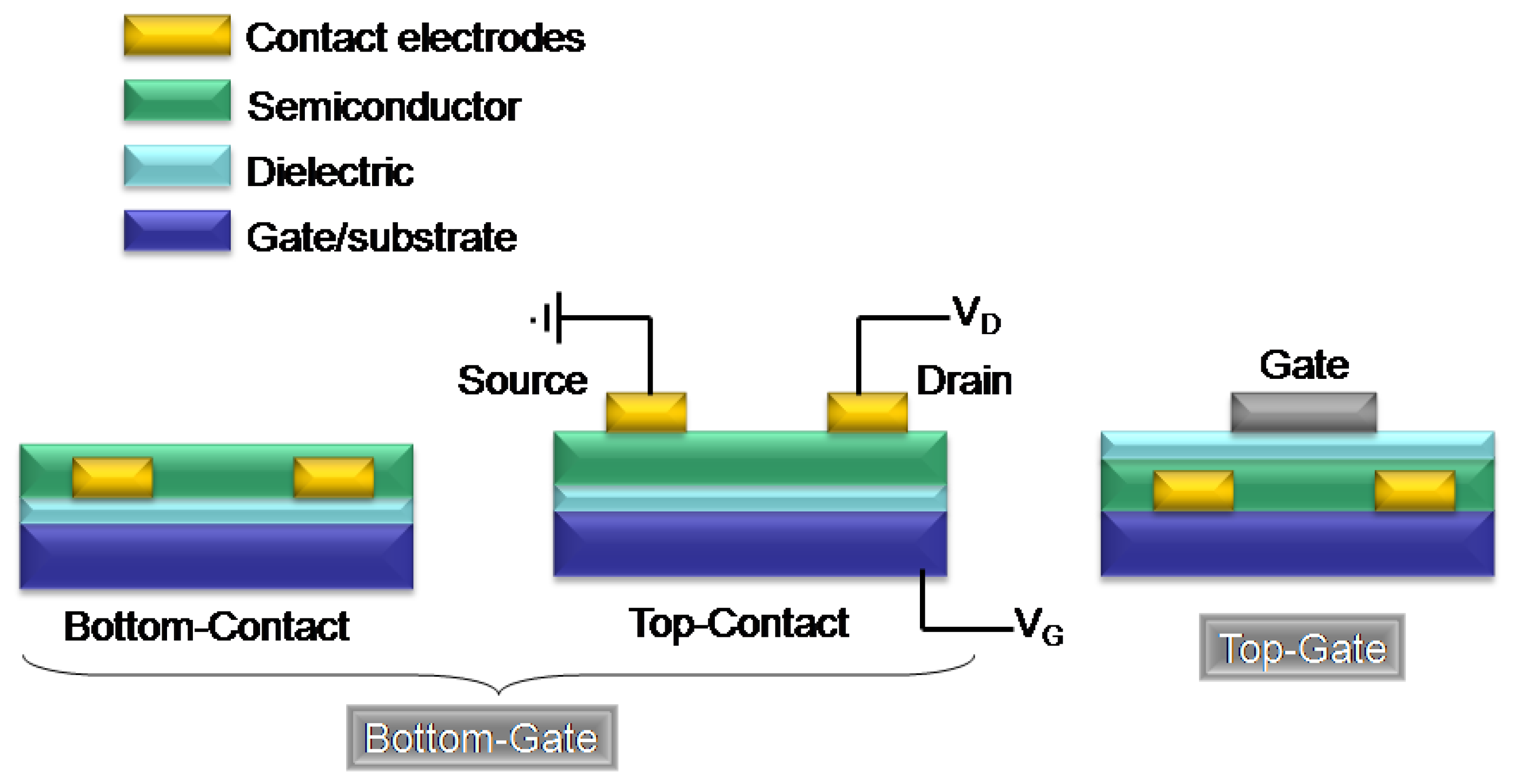
2. Azine/Azole-Thiophene Oligomers
2.1. P-Channel Semiconductors
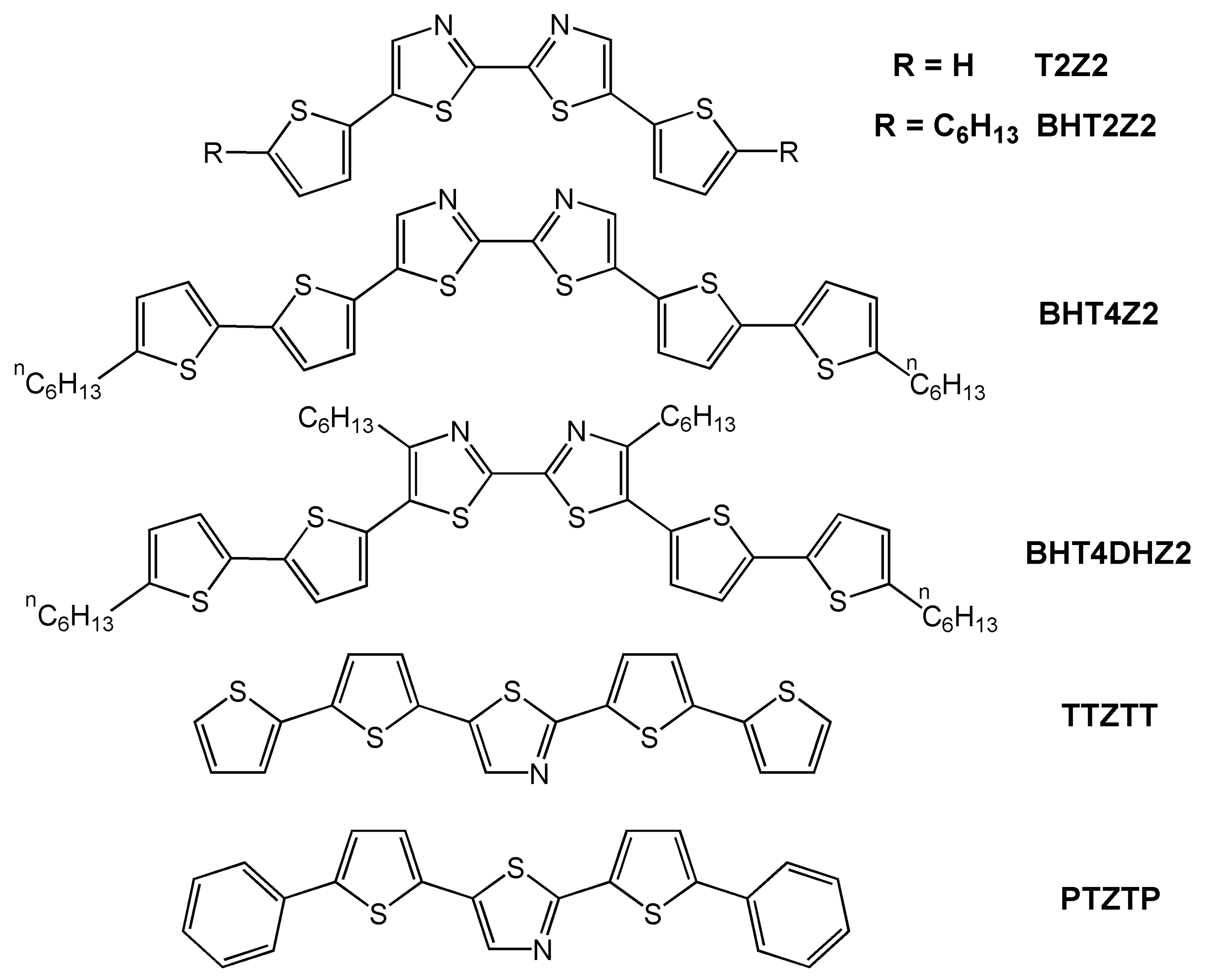
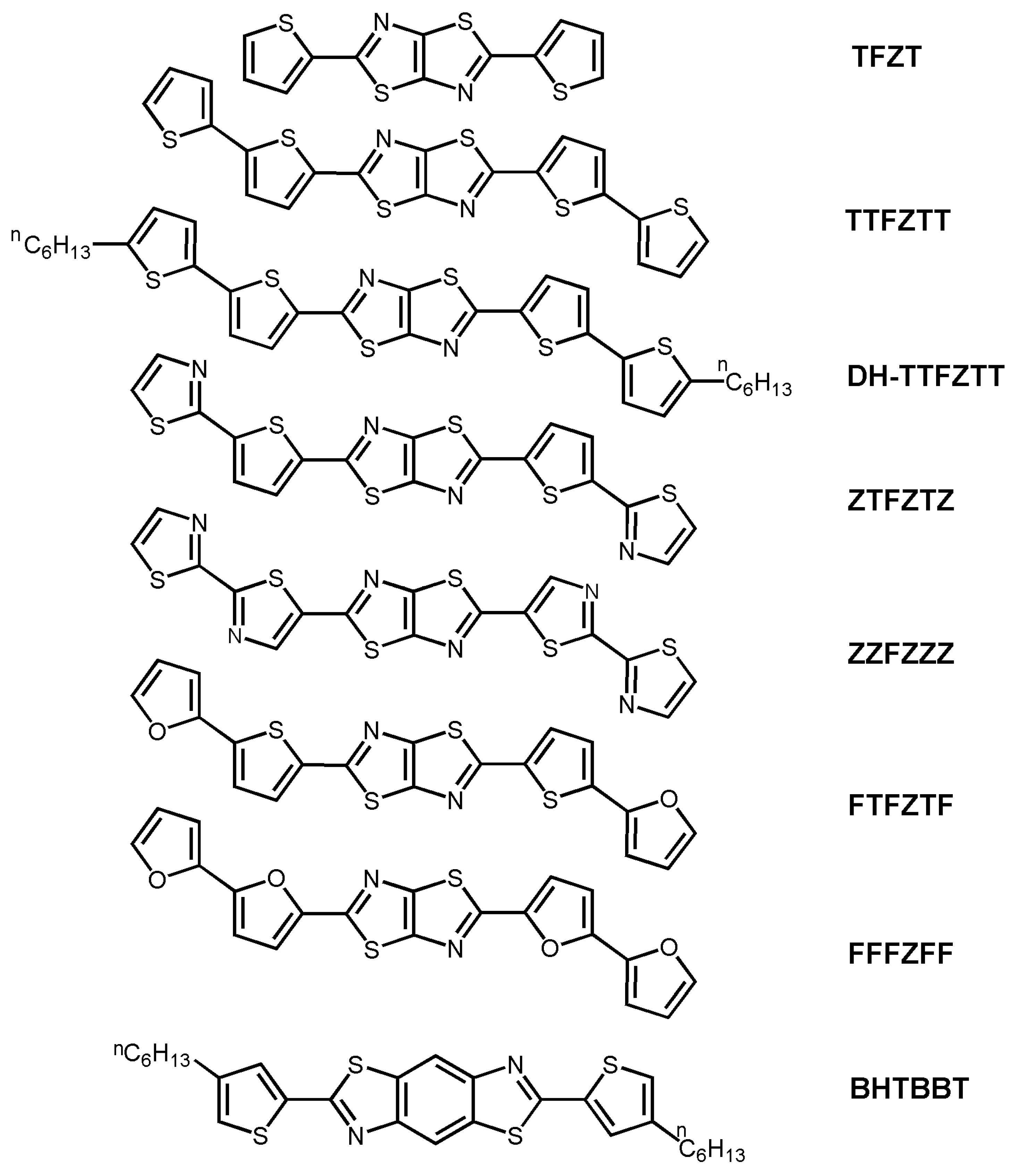
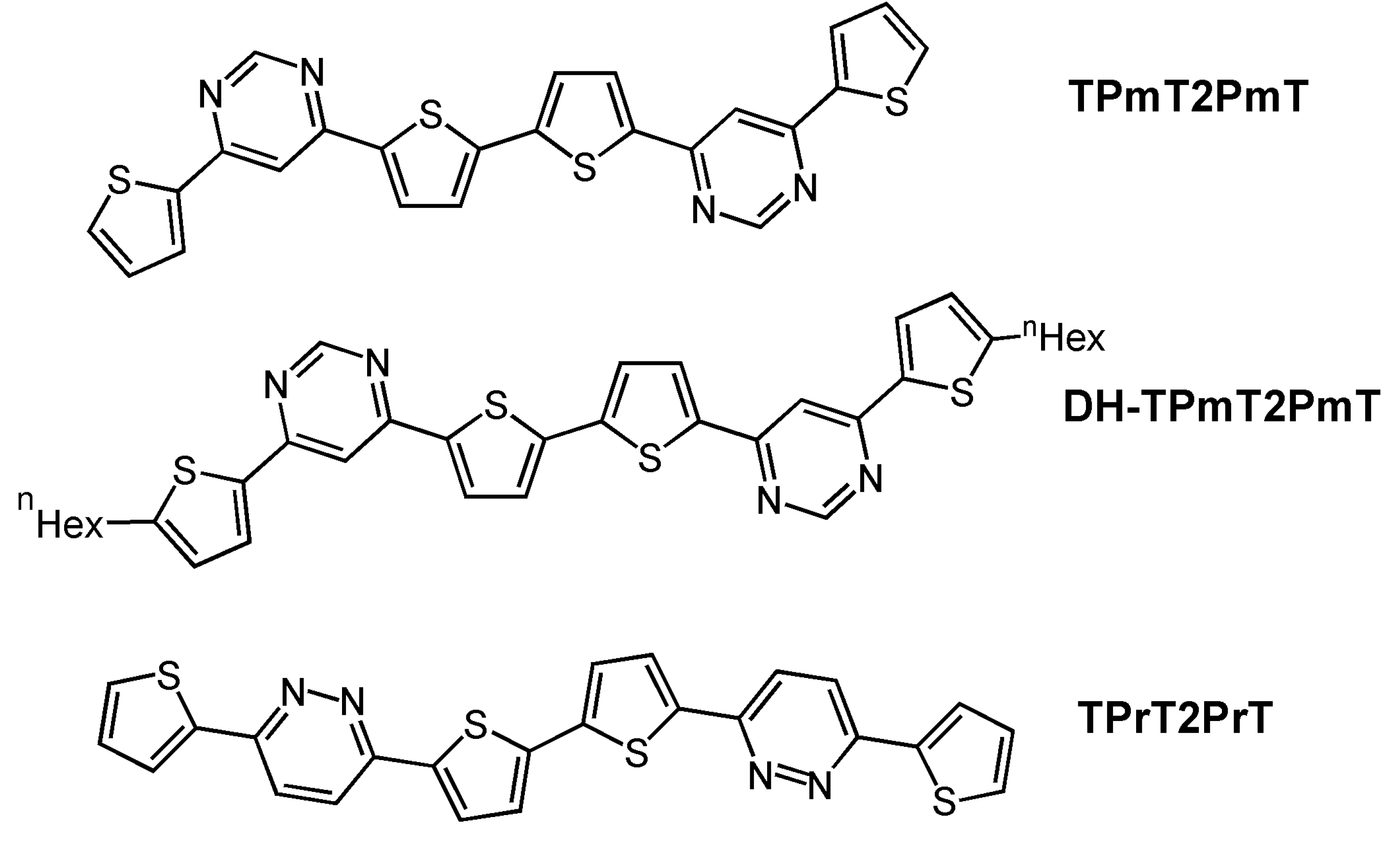
| Oligomer | Dielectrica | TD (°C) | μh (cm2V-1s-1) | VT (V) | Ion/Ioff | Ref. |
|---|---|---|---|---|---|---|
| T2Z2b | S | 25 | 1 × 10-5 | NR | NR | 20 |
| BHT2Z2b | S | 25 | 2 × 10-5 | NR | NR | 20 |
| BHT4Z2 | S | 55 | 0.011 | −11 | >104 | 20 |
| BHT4DHZ2 | S | 25 | 3.5 × 10-4 | NR | NR | 20 |
| TTZTT | S | 60–80 | 7 × 10-3 | −7 | 104 | 47 |
| PTZTP | S | 25 | 1 × 10-4 | −23 | 80 | 47 |
| TTFZTT | S | 50 | 2 × 10-2 | −7 | 104 | 48 |
| DH-TTFZTT | S | 20 | 3 × 10-3 | −2 | 103 | 48 |
| ZTFZTZb | S | 20 | 1 × 10-7 | NR | 103 | 49 |
| FTFZTF | S | 70 | 1 × 10-3 | NR | 102 | 49 |
| FFFZFF | S | 50 | 4 × 10-4 | NR | 103 | 49 |
| BHTBBTb | H | 25 | 0.01 | −20 | 105 | 50 |
| TPmT2PmT | H | 70 | 1 × 10-4 | −88 | 2 × 105 | 40 |
| DH-TPmT2PmT | H | 90 | 3 × 10-3 | −55 | 2 × 107 | 40 |
| TPrT2PrT | H | 110 | 4 × 10-3 | −68 | 107 | 40 |
2.2. N-Channel Semiconductors

| Oligomer | Dielectrica | TD(°C) | μe (cm2V-1s-1) | VT (V) | Ion/Ioff | Ref. |
|---|---|---|---|---|---|---|
| 1 | O | 25 | 1.2 | 67 | 107 | 53 |
| 2 | S | 25 | 0.085 | 63 | 104 | 54 |
| 3 | S | 25 | 0.018 | 61 | 104 | 54 |
| 4b | S | 50 | 8.3 × 10-4 | 105 | 2.8 × 106 | 55 |
| 5 | H | 80 | 0.19 | 3 | 106 | 56 |
| 8 | O | 50 | 0.24 | 24 | 3 × 106 | 57 |
3. Azine- and Azole-Containing Polymers
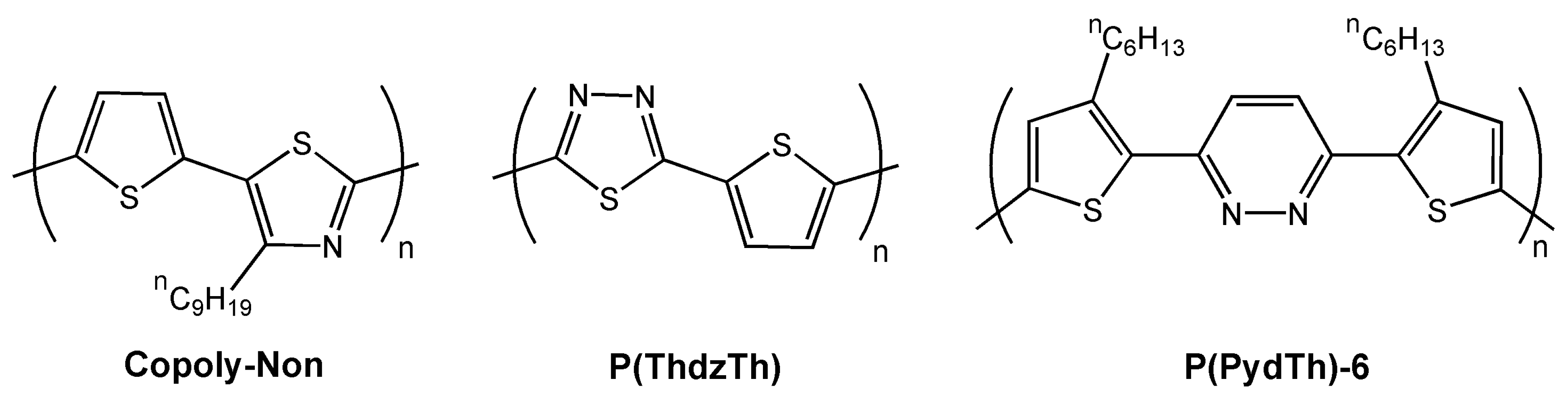



| Polymer | Dielectrica | μ (cm2V-1s-1)b | VT (V) | Ion/Ioff | Ref. |
|---|---|---|---|---|---|
| Copoly-Nonc | H | 2.5 × 10-3 | 16 | 200 | 58 |
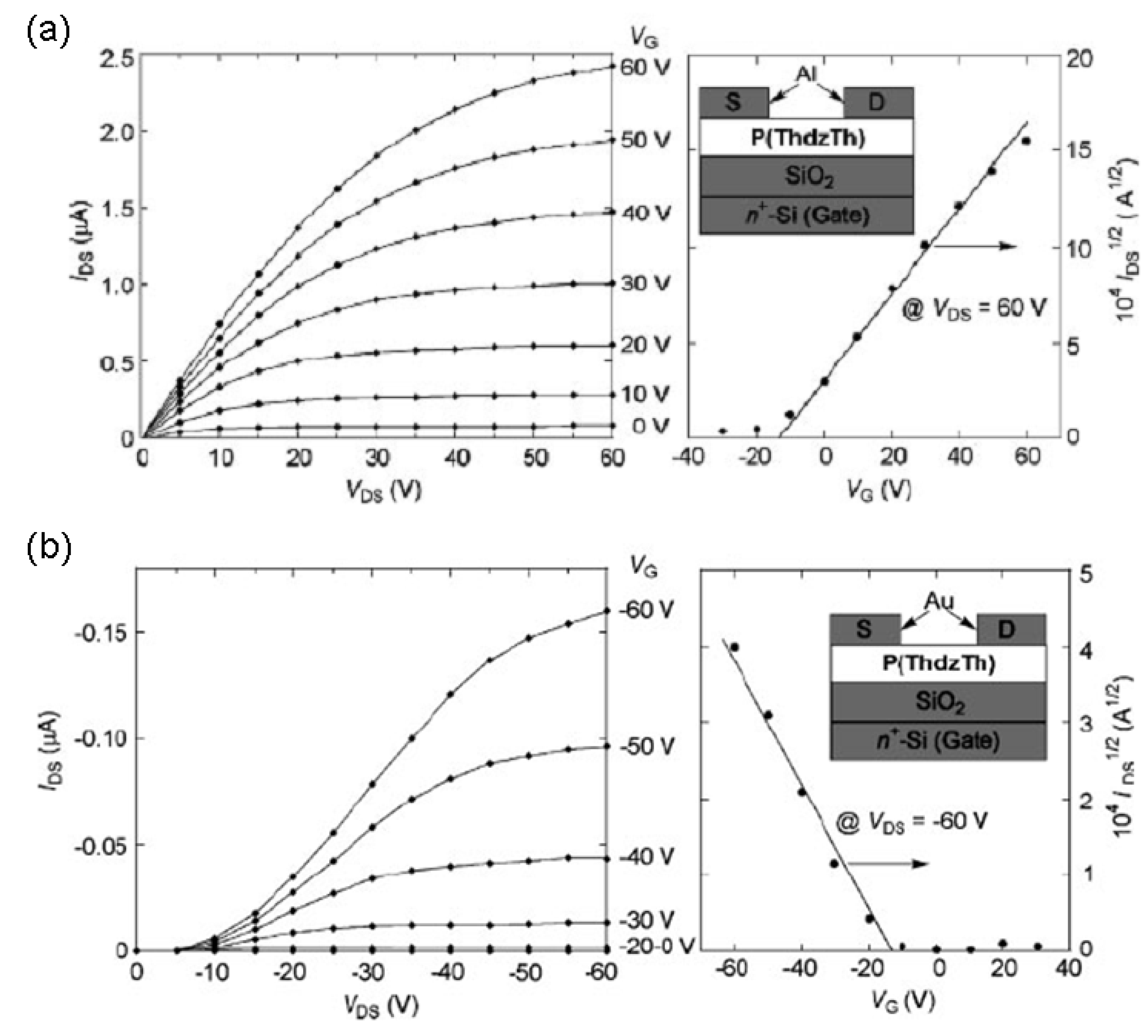
| P(ThdzTh)c | H | 5.4 × 10-3 (e.) | −13 | 3 × 104 | 38 |
| H | 3.4 × 10-4 | NR | 1 × 104 | 38 | |
| P(PydTh)-6c | S | 3 × 10-3 | NR | 4 × 103 | 36 |
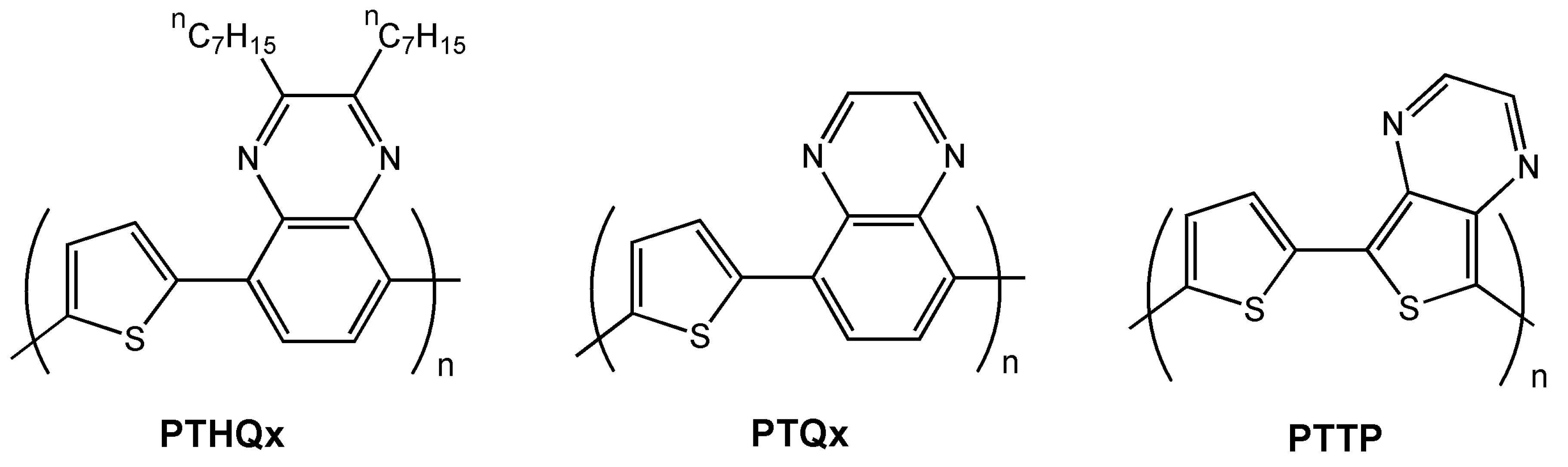
| PTHQx | O-8 | 3.6 × 10-3 | −2 | 6 × 105 | 64 |
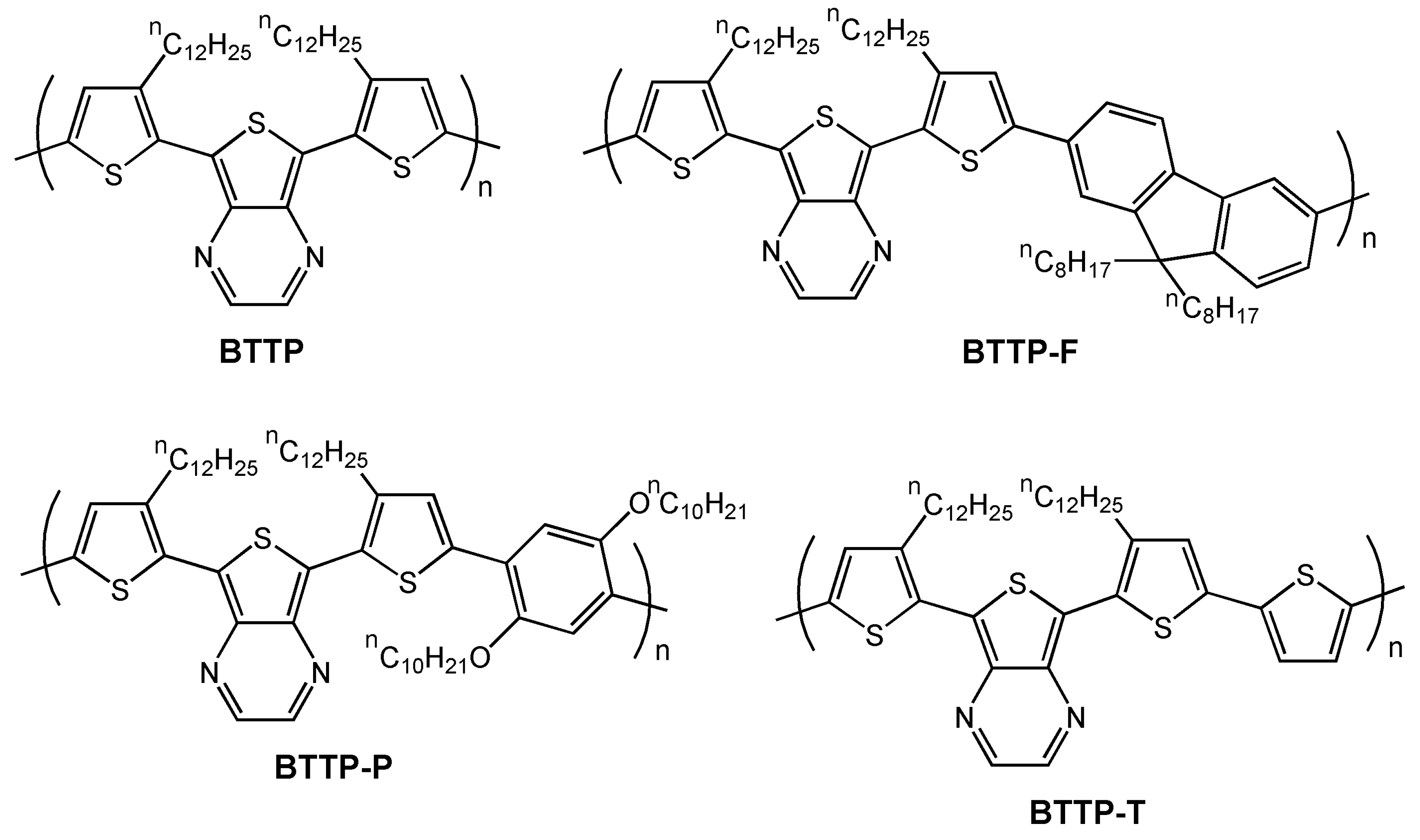
| BTTP | O | 7.1 × 10-4 | >80 | NR | 66 |
| BTTP-F | O | 1.6 × 10-3 | −3 | 2 × 104 | 66 |
| BTTP-P | O | 1.1 × 10-3 | NR | 100 | 66 |
| BTTP-T | O | 4.2 × 10-4 | >80 | NR | 66 |
| PTHTP-C7 | H | 3.6 × 10-5 | NR | 3 | 68 |
| PBTHTP-C7 | H | 1.2 × 10-4 | NR | 300 | 68 |
| PTHTP-C12 | O-8 | 0.011 | 21 | 227 | 68 |
| AFPO-Green1 | S | 0.03 | ~0 | 5 × 104 | 69 |
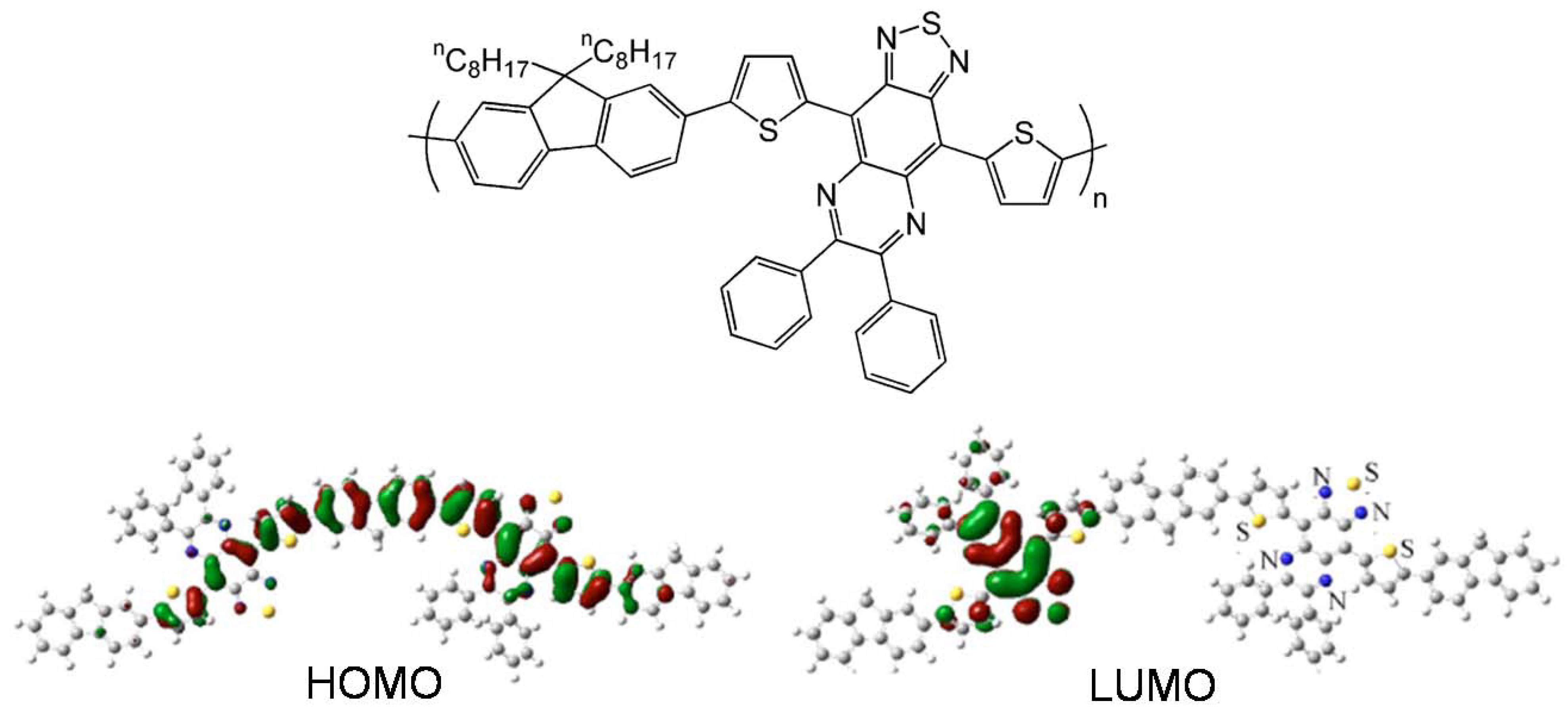
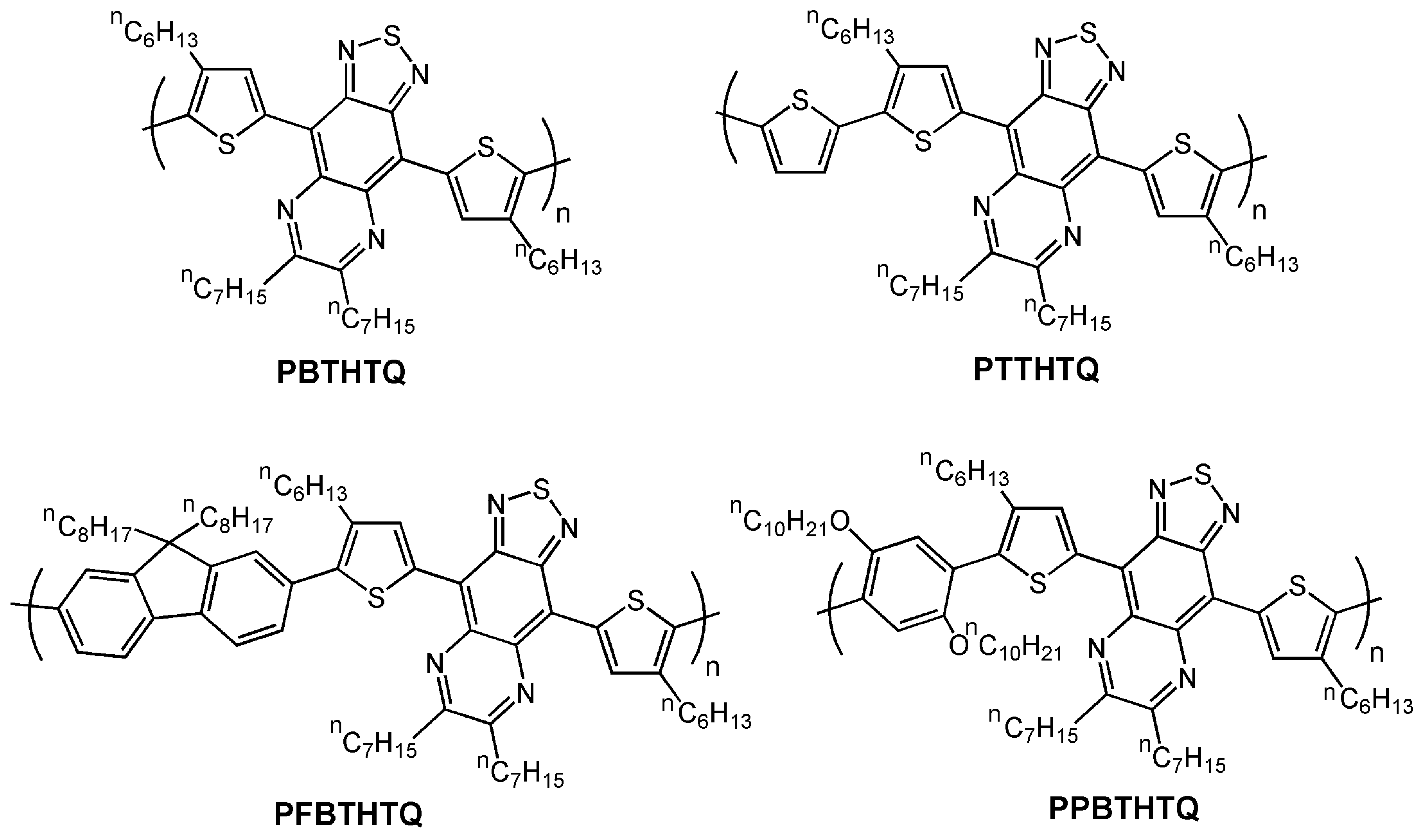
| PBTHTQ | O-8 | 1.6 × 10-4 | NR | 215 | 71 |
| PTTHTQ | O-8 | 3.8 × 10-3 | NR | 505 | 71 |
| PFBTHTQ | O-8 | 1.5 × 10-4 | NR | 324 | 71 |
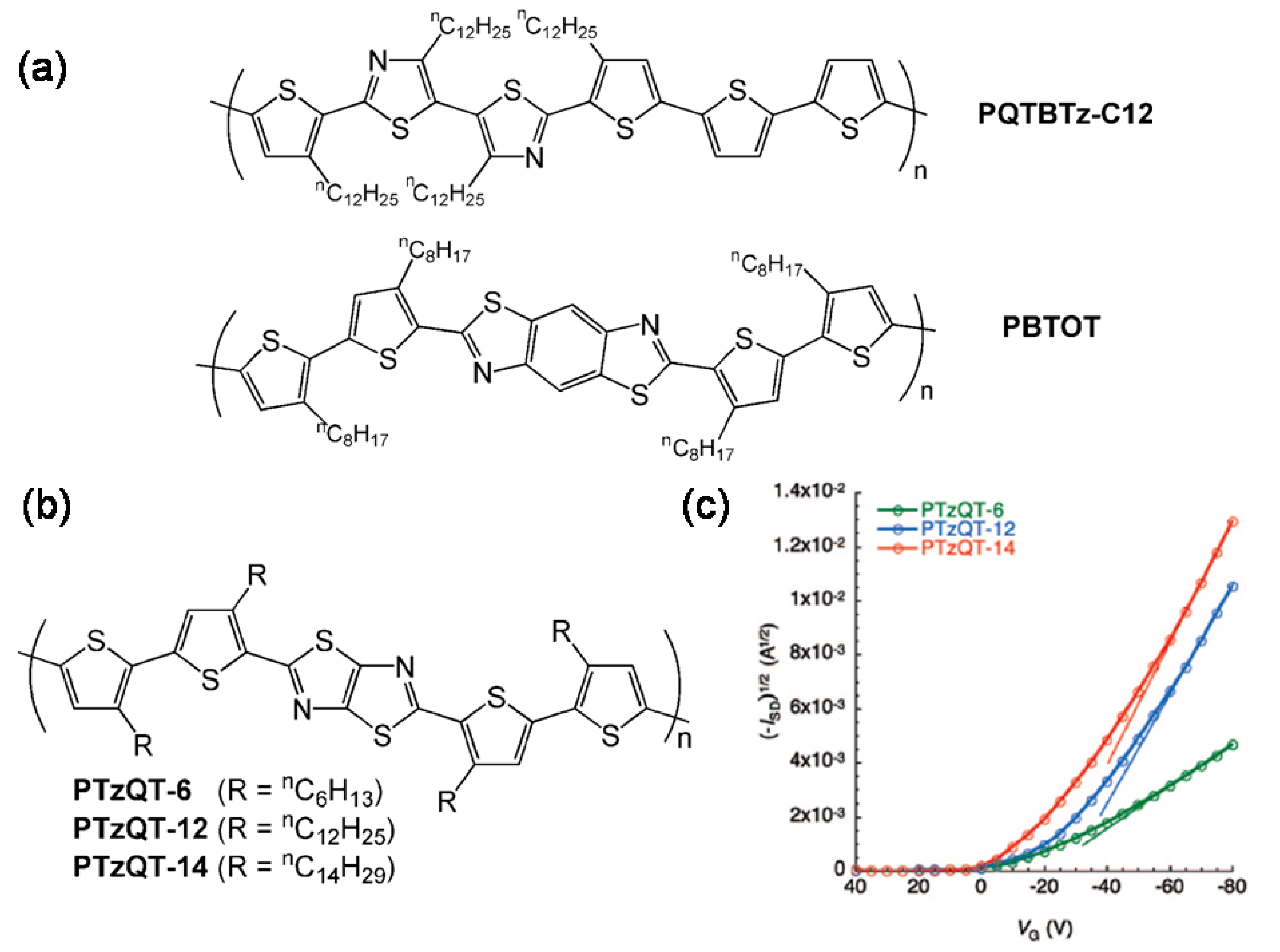
| PBTOT | S | 0.01 | −5.2 | >106 | 73 |
| PQTBTz-C12 | O-m | 0.33 | NR | 107 | 74 |
| PTzQT-14 | O-8 | 0.20–0.30 | −23 to −30 | 105–107 | 76 |
| P(DTB-BThBBT)nc | O | 1.2 × 10-3 | NR | NR | 72 |
| O | 5.8 × 10-4 (e.) | NR | NR | 72 |
4. Conclusions
Acknowledgements
References and Notes
- Handbook of Oligo- and Polythiophenes; Fichou, D. (Ed.) Wiley-VCH: Weinheim, Germany, 1999.
- Meng, H.; Zheng, J.; Lovinger, A.J.; Wang, B.-C.; Van Patten, P.G.; Bao, Z. Oligofluorene-thiophene derivatives as high-performance semiconductors for organic thin film transistors. Chem. Mater. 2003, 15, 1778–1787. [Google Scholar] [CrossRef]
- Mushrush, M.; Facchetti, A.; Lefenfeld, M.; Katz, H.E.; Marks, T.J. Easily processable phenylene-thiophene-based organic field-effect transistors and solution-fabricated nonvolatile transistor memory elements. J. Am. Chem. Soc. 2003, 125, 9414–9423. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, A.; Mushrush, M.; Katz, H.E; Marks, T.J. n-Type building blocks for organic electronics: A homologous family of fluorocarbon-substituted thiophene oligomers with high carrier mobility. Adv. Mater. 2003, 15, 33–38. [Google Scholar] [CrossRef]
- Delongchamp, D.M.; Sambasivan, S.; Fischer, E.K.L.; Chang, P.; Murphy, A.R.; Fréchet, J.M.J.; Subramanian, V. Direct correlation of organic semiconductor film structure to field-effect mobility. Adv. Mater. 2005, 17, 2340–2344. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Z.; Zheng, Y.; Newman, C.; Quinn, J.R.; Dötz, F.; Kastler, M.; Facchetti, A. A high-mobility electron-transporting polymer for printed transistors. Nature 2009, 457, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Pasini, M.; Destri, S.; Porzio, W.; Botta, C.; Giovanella, U. Electroluminescent poly(fluorene-co-thiophene-S,S-dioxide): Synthesis, characterisation and structure-property relationships. J. Mater. Chem. 2003, 13, 807–813. [Google Scholar] [CrossRef]
- Suzuki, M.; Fukuyama, M.; Hori, Y.; Hotta, S. Electroluminescence features of oligothiophenes dispersed as a dopant in host matrices. J. Appl. Phys. 2002, 91, 5706. [Google Scholar] [CrossRef]
- Pisignano, D.; Anni, M.; Gigli, G.; Cingolani, R.; Zavelani-Rossi, M.; Lanzani, G.; Barbarella, G.; Favaretto, L. Amplified spontaneous emission and efficient tunable laser emission from a substituted thiophene-based oligomer. Appl. Phys. Lett. 2002, 81, 3534–3536. [Google Scholar] [CrossRef]
- Roncali, J. Electrogenerated functional conjugated polymers as advanced electrode materials. J. Mater. Chem. 1999, 9, 1875–1893. [Google Scholar] [CrossRef]
- Torsi, L.; Lovinger, A.J.; Crone, B.; Someya, T.; Dodabalapur, A.; Katz, H.E.; Gelperin, A. Correlation between oligothiophene thin film transistor morphology and vapor responses. J. Phys. Chem. B 2002, 106, 12563–12568. [Google Scholar] [CrossRef]
- Hara, K.; Kurashige, M.; Dan-Oh, Y.; Kasada, C.; Shinpo, A.; Suga, S.; Sayama, K.; Arakawa, H. Design of new coumarin dyes having thiophene moieties for highly efficient organic-dye-sensitized solar cells. New J. Chem. 2003, 27, 783. [Google Scholar] [CrossRef]
- Azfali, A.; Breen, T.L.; Kagan, C.R. An efficient synthesis of symmetrical oligothiophenes: Synthesis and transport properties of a soluble sexithiophene derivative. Chem. Mater. 2002, 14, 1742–1746. [Google Scholar] [CrossRef]
- Turbiez, M.; Frère, P.; Roncali, R. Stable and soluble oligo(3,4-ethylenedioxythiophene)s end-capped with alkyl chains. J. Org. Chem. 2003, 68, 5357–5360. [Google Scholar] [CrossRef] [PubMed]
- Garnier, F.; Hajlaoui, R.; El Kassmi, A.; Horowitz, G.; Laigre, L.; Porzio, W.; Armanini, M.; Provasoli, F. Dihexylquaterthiophene, a two-dimensional liquid crystal-like organic semiconductor with high transport properties. Chem. Mater. 1998, 10, 3334–3339. [Google Scholar] [CrossRef]
- Ackermann, J.; Videlot, C.; Dumas, P.; El Kassmi, A.; Guglielmetti, R.; Safarov, V. Control of growth and charge transport properties of quaterthiophene thin films via hexyl chain substitutions. Org. Electr. 2004, 5, 213–222. [Google Scholar] [CrossRef]
- Chwang, A.B.; Frisbie, C.D. Field effect transport measurements on single grains of sexithiophene: Role of the contacts. J. Phys. Chem. B 2000, 104, 12202–12209. [Google Scholar] [CrossRef]
- Horowitz, G.; Hajlaoui, R.; Bouchriha, H.; Bourguiga, R.; Hajlaoui, M. The concept of “threshold voltage” in organic field-effect transistors. Adv. Mater. 1998, 10, 923–927. [Google Scholar] [CrossRef]
- Hajlaoui, R.; Horowitz, G.; Garnier, F.; ArceBrouchet, A.; Laigre, L.; Elkassmi, A.; Demanze, F.; Kouki, F. Improved field-effect mobility in short oligothiophenes: Quaterthiophene and quinquethiophene. Adv. Mater. 1997, 9, 389–391. [Google Scholar] [CrossRef]
- Li, W.; Katz, H.E.; Lovinger, A.J.; Laquindanum, J. Field-effect transistors based on thiophene hexamer analogues with diminished electron donor strength. Chem. Mater. 1999, 11, 458–465. [Google Scholar] [CrossRef]
- Tsumara, A.; Koezuka, H.; Ando, T. Macromolecular electronic device: Field-effect transistor with a polythiophene thin film. Appl. Phys. Lett. 1986, 49, 1210–1212. [Google Scholar] [CrossRef]
- Assadi, A.; Svensson, C.; Willander, M.; Inganäs, O. Field effect mobility of poly(3-hexylthiophene). Appl. Phys. Lett. 1988, 53, 195–197. [Google Scholar] [CrossRef]
- Akimichi, H.; Waragai, K.; Hotta, S.; Kano, H.; Sakaki, H. Field-effect transistors using alkyl substituted oligothiophenes. Appl. Phys. Lett. 1991, 58, 1500–1503. [Google Scholar] [CrossRef]
- Garnier, F.; Yassar, A.; Hajlaoui, R.; Horowitz, G.; Deloffre, F.; Servet, B.; Ries, S.; Alnot, P. Molecular engineering of organic semiconductors: Design of self-assembly properties in conjugated thiophene oligomers. J. Am. Chem. Soc. 1993, 115, 8716–8721. [Google Scholar] [CrossRef]
- Mushrush, M.; Facchetti, A.; Lefenfeld, M.; Katz, H.E.; Marks, T.J. Easily processable phenylene-thiophene based organic field-effect transistors and solution-fabricated nonvolatile transistor memory elements. J. Am. Chem. Soc. 2003, 125, 9414–9423. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, A.; Mushrush, M.; Katz, H.E.; Marks, T.J. n-Type building blocks for organic electronics: A homologous family of fluorocarbon-substituted thiophene oligomers with high carrier mobility. Adv. Mater. 2003, 15, 33–38. [Google Scholar] [CrossRef]
- Pappenfus, T.M.; Newman, C.R.; Pappenfus, T.M.; Ewbank, P.C.; Haukaas, M.H.; Mann, K.R.; Miller, L.L.; Frisbie, C.D. High electron mobility and ambipolar transport in organic thin-film transistors based on a π-stacking quinoidal terthiophene. Adv. Mater. 2003, 15, 1278–1282. [Google Scholar] [CrossRef]
- Handa, S.; Miyazaki, E.; Takimiya, K.; Kunugi, Y. Solution-processible n-channel organic field-effect transistors based on dicyanomethylene-substituted terthienoquinoid derivative. J. Am. Chem. Soc. 2007, 129, 11684–11685. [Google Scholar] [CrossRef] [PubMed]
- Ponce Ortiz, R.; Facchetti, A.; Marks, T.J.; Casado, J.; Zgierski, M.; Kozaki, M.; Hernández, V.; López Navarrete, J.T. Ambipolar organic field-effect transistors from cross-conjugated aromatic quaterthiophenes; Comparisons with quinoidal parent materials. Adv. Funct. Mater. 2009, 19, 386–394. [Google Scholar] [CrossRef]
- Facchetti, A.; Yoon, M.-H.; Marks, T.J. Gate dielectrics for organic field-effect transistors: New opportunities for organic electronics. Adv. Mater. 2005, 17, 1705–1725. [Google Scholar] [CrossRef]
- Veres, J.; Ogier, S.; Lloyd, G.; de Leeuw, D. Gate insulators in organic field-effect transistors. Chem. Mater. 2004, 16, 4543–4555. [Google Scholar] [CrossRef]
- Delongchamp, D.M.; Vogel, B.M.; Jung, Y.; Gurau, M.C.; Richter, C.A.; Kirillov, O.A.; Obrzut, J.; Fischer, D.A.; Sambasivan, S.; Richter, L.J.; Lin, E.K. Variations in semiconducting polymer microstructure and hole mobility with spin-coating speed. Chem. Mater. 2005, 17, 5610–5612. [Google Scholar] [CrossRef]
- Casado, J.; Ponce Ortiz, R.; Ruiz Delgado, M.C.; Hernández, V.; López Navarrete, J.T.; Raimundo, J.-M.; Blanchard, P.; Allain, M.; Roncali, J. Alternated quinoid/aromatic units in terthiophenes building blocks for electroactive narrow band gap polymers. Extended spectroscopic, solid state, electrochemical, and theoretical study. J. Phys. Chem. B 2005, 109, 16616–16627. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Zhang, F.; Veenstra, S.C.; Verhees, W.J.H.; Hummelen, J.C.; Kroon, J.M.; Inganäs, O.; Andersson, M.R. High-performance polymer solar cell of an alternating polyfluorene copolymer and a fullerene derivative. Adv. Mater. 2003, 15, 988–991. [Google Scholar]
- Campos, L.M.; Tontcheva, A.; Gunes, S.; Sonmez, G.; Neugebauer, H.; Sariciftci, N.S.; Wudl, F. Extended photocurrent spectrum of a low band gap polymer in a bulk heterojunction solar cell. Chem. Mater. 2005, 17, 4031–4033. [Google Scholar] [CrossRef]
- Yasuda, T.; Sakai, Y.; Aramaki, S.; Yamamoto, T. New coplanar (ABA)n-type donor-acceptor π-conjugated copolymers constituted of alkylthiophene (unit A) and pyridazine (unit B): Synthesis using hexametylditin, self-organized solid structure, and optical and electrochemical properties of the copolymers. Chem. Mater. 2005, 17, 6060–6068. [Google Scholar] [CrossRef]
- Sonmez, G.; Shen, C.K.F.; Rubin, Y.; Wudl, F. The unusual effect of bandgap lowering by C60 on a conjugated polymer. Adv. Mater. 2005, 17, 897–900. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yasuda, T.; Sakai, Y.; Aramaki, S. Ambipolar field-effect transistor (FET) and redox characteristics of a π-conjugated thiophene/1,3,4-thiadizole CT-type copolymer. Macromol. Rapid Commun. 2005, 26, 1214–1217. [Google Scholar] [CrossRef]
- Yamashita, Y. Development of high-performance n-type organic field-effect transistors based on nitrogen heterocycles. Chem. Lett. 2009, 38, 870–875. [Google Scholar] [CrossRef]
- Ponce Ortiz, R.; Casado, J.; Hernández, V.; López Navarrete, J.T.; Letizia, J.A.; Ratner, M.A.; Facchetti, A.; Marks, T.J. Thiophene-diazine molecular semiconductors: Synthesis, structural, electrochemical, optical, and electronic structural properties; implementation in organic field-effect transistors. Chem. Eur. J. 2009, 15, 5023–5039. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.R.; Fréchet, T.M.J. Organic semiconducting oligomers for use in thin film transistors. Chem. Rev. 2007, 107, 1066–1096. [Google Scholar] [CrossRef] [PubMed]
- DiBenedetto, S.A.; Facchetti, A.; Ratner, M.A.; Marks, T.J. Molecular self-assembled monolayers and multilayers for organic and unconventional inorganic thin-film transistors applications. Adv. Mater. 2009, 21, 1407–1433. [Google Scholar] [CrossRef]
- Ponce Ortiz, R.; Facchetti, A.; Marks, T.J. High-k organic, inorganic, and hybrid dielectrics for low-voltage organic field-effect transistors. Chem. Rev. 2010, 110, 205–239. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, G. Organic thin film transistors: From theory to real devices. J. Mater. Res. 2004, 19, 1946–1962. [Google Scholar] [CrossRef]
- Katz, H.E.; Laquindanum, J.G.; Lowinger, A.J. Synthesis, solubility, and field-effect mobility of elongated and oxa-substituted α,ω-dialkyl thiophene oligomers. Extension of “polar intermediate” synthetic strategy and solution deposition on transistor substrates. Chem. Mater. 1998, 10, 633–638. [Google Scholar] [CrossRef]
- Katz, H.E.; Lovinger, A.J.; Laquindanum, J.G. α,ω-dihexylquaterthiophene: A second thin film single-crystal organic semiconductor. Chem. Mater. 1998, 10, 457–459. [Google Scholar] [CrossRef]
- Hong, X.M.; Katz, H.E.; Lovinger, A.J.; Wang, B.-C.; Raghavachari, K. Thiophene-phenylene and thiophene-thiazole oligomeric semiconductors with high field-effect transistor on/off ratios. Chem. Mater. 2001, 13, 4686–4691. [Google Scholar] [CrossRef]
- Ando, S.; Nishida, J.-I.; Inoue, Y.; Tokito, S.; Yamashita, Y. Synthesis, physical properties, and field-effect transistors of novel thiophene/thiazolothiazole co-oligomers. J. Mater. Chem. 2004, 14, 1787–1790. [Google Scholar] [CrossRef]
- Ando, S.; Nishida, J.-I.; Fujiwara, E.; Tada, H.; Inoue, Y.; Tokito, S.; Yamashita, Y. Characterization and field-effect transistor performance of heterocyclic oligomers containing a thiazolothiazole unit. Chem. Lett. 2004, 33, 1170–1171. [Google Scholar] [CrossRef]
- Pang, H.; Vilela, F.; Skabara, P.J.; McDouall, J.J.W.; Crouch, D.J.; Anthopoulos, T.D.; Bradley, D.D.C.; de Leeuw, D.M.; Horton, P.N.; Hursthouse, M.B. Advantageous 3D ordering of π-conjugated systems: A new approach towards efficient charge transport in any direction. Adv. Mater. 2007, 19, 4438. [Google Scholar] [CrossRef]
- Abbotto, A.; Bradamante, S.; Facchetti, A.; Pagani, G.A. Metal chelation aptitudes of bis(o-azaheteroaryl)methanes as tuned by heterocycle charge demands. J. Org. Chem. 2002, 67, 5753–5772. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Nishida, J.-I.; Tada, H.; Inoue, Y.; Tokito, S.; Yamashita, Y. High performance n-type organic field-effect transistors based on π-electronic systems with trifluoromethylphenyl groups. J. Am. Chem. Soc. 2005, 127, 5336–5337. [Google Scholar] [CrossRef]
- Kumaki, D.; Ando, S.; Shimono, S.; Yamashita, Y.; Umeda, T.; Tokito, S. Significant improvement of electron mobility in organic thin-film transistors based on thiazolothiazole derivative by employing self-assembled monolayer. Appl. Phys. Lett. 2007, 90, 053506:1–053506:3. [Google Scholar]
- Ando, S.; Murakami, R.; Nishida, J.-I.; Tada, H.; Inoue, Y.; Tokito, S.; Yamashita, Y. n-type organic field-effect transistors with very high electron mobility based on thiazole oligomers with trifluoromethylphenyl groups. J. Am. Chem. Soc. 2005, 127, 14996–14997. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Nishida, J.-I.; Tokito, S.; Tada, H.; Yamashita, Y. Organic field-effect transistors based on heterocyclic co-oligomers containing a pyrazine ring. Chem. Commun. 2007, 1430–1432. [Google Scholar] [CrossRef]
- Kono, T.; Kumaki, D.; Nishida, J.-I.; Sakanoue, T.; Kakita, M.; Tada, H.; Tokito, S.; Yamashita, Y. High-performance and light-emitting n-type organic field-effect transistors based on dithienylbenzothiadiazole and related heterocycles. Chem. Mater. 2007, 19, 1218–1220. [Google Scholar] [CrossRef]
- Mamada, M.; Nishida, J.-I.; Tokito, S.; Yamashita, Y. Preparation, characterization, and field-effect transistor performance of benzo[1,2-d:4,5-d´]bisthiazole derivatives. Chem. Lett. 2008, 37, 766–767. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kokubo, H.; Kobashi, M.; Sakai, Y. Aligment and field-effect transistor behavior of an alternative π-conjugated copolymer of thiophene and 4-alkylthiazole. Chem. Mater. 2004, 16, 4616–4618. [Google Scholar] [CrossRef]
- Yasuda, T.; Imase, T.; Sasaki, S.; Yamamoto, T. Synthesis, solid structure, and optical properties of new thiophene-based alternating π-conjugated copolymers containing 4-Alkyl-1,2,4-triazole or 1,3,4-thiadiazole units as the partner unit. Macromolecules 2005, 38, 1500–1503. [Google Scholar] [CrossRef]
- Ishii, H.; Sugiyama, K.; Ito, E.; Seki, K. Energy level alignment and interfacial electronic structures at organic/metal and organic/organic interfaces. Adv. Mater. 1999, 11, 605–625. [Google Scholar] [CrossRef]
- Ghosh, R.; Simonsen, S.H. Trans-diaquatetrakis(imidazole)manganese(II) dichloride, [Mn(C3H4N2)4(H2O)2]Cl2. Acta Crystallogr., C 1993, C39, 1031–1034. [Google Scholar] [CrossRef]
- Ackers, B.; Blake, A.J.; Hill, S.J.; Hubberstey, P. 3,6-Di(thiophen-2-yl)pyridazine. Acta Crystallogr., C 2002, C58, o640–o641. [Google Scholar]
- Suzuki, T.; Fujii, H.; Yamashita, Y.; Kabuto, C.; Tanaka, S.; Harasawa, M.; Mukai, T.; Miyashi, T. Clathrate formation and molecular recognition by novel chalcogen-cyano interactions in tetracyanoquinodimethanes fused with thiadiazole and selenadiazole rings. J. Am. Chem. Soc. 1992, 114, 3034–3043. [Google Scholar] [CrossRef]
- Champion, R.D.; Cheng, K.-F.; Pai, C.-L.; Chen, W.-C.; Jenekhe, S.A. Electronic properties and field-effect transistors of thiophene-based donor-acceptor conjugated copolymers. Macromol. Rapid Commun. 2005, 26, 1835–1840. [Google Scholar] [CrossRef]
- Knipp, D.; Street, R.A.; Völkel, A.; Ho, J. Pentacene thin film transistors on inorganic dielectrics: Morphology, structural properties, and electronic transport. J. Appl. Phys. 2003, 93, 347–355. [Google Scholar] [CrossRef]
- Zhu, Y.; Champion, R.D.; Jenekhe, S.A. Conjugated donor-acceptor copolymer semiconductors with large intramolecular charge transfer: Synthesis, optical properties, electrochemistry, and field effect carrier mobility of thienopyrazine-based copolymers. Macromolecules 2006, 39, 8712–8719. [Google Scholar] [CrossRef]
- Wu, P.-T.; Kim, F.S.; Champion, R.D.; Jenekhe, S.A. Conjugated donor-acceptor copolymer semiconductors. Synthesis, optical properties, electrochemistry, and field-effect carrier mobility of pyridopyrazine-based copolymers. Macromolecules 2008, 41, 7021–7028. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Cheng, K.-F.; Liu, C.-L.; Lin, S.-T.; Chueh, C.-C.; Tsai, F.-Y.; Chen, W.-C. High hole mobility from thiophene-thienopyrazine copolymer based thin film transistors. J. Polym. Res. 2009, 16, 239–244. [Google Scholar] [CrossRef]
- Chen, M.; Crispin, X.; Perzon, E.; Andersson, M.R.; Pullerits, T.; Andersson, M.; Inganäs, O.; Berggren, M. High carrier mobility in low band gap polymer-based field-effect transistors. Appl. Phys. Lett. 2005, 87, 252105:1–252105:3. [Google Scholar]
- Chen, M.X.; Perzon, E.; Robisson, N.; Jönsson, S.K.M.; Andersson, M.R.; Fahlman, M.; Berggren, M. Low band gap donor-acceptor-donor polymers for infra-red electroluminescence and transistors. Synth. Met. 2004, 146, 233–236. [Google Scholar] [CrossRef]
- Cheng, K.F.; Chueh, C.-C.; Lin, C.-H.; Chen, W.-C. Synthesis, properties and field effect transistor characteristics of new thiophene-[1,2,5]thiadiazolo[3,4-g]quinoxaline-thiophene-based conjugated polymers. J. Polym. Sci. A: Polym. Chem. 2008, 46, 6305–6316. [Google Scholar] [CrossRef]
- Steckler, T.T.; Zhang, X.; Hwang, J.; Honeyager, R.; Ohira, S.; Zhang, X.-H.; Grant, A.; Ellinger, S.; Odom, S.A.; Sweat, D.; Tanner, D.B.; Rinzler, A.G.; Barlow, S.; Brèdas, J.-L.; Kippelen, B.; Marder, S.R.; Reynolds, J.R. A spray-processable, low bandgap, and ambipolar donor-acceptor conjugated polymer. J. Am. Chem. Soc. 2009, 131, 2824–2826. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Kim, F.S.; Xin, H.; Jenekhe, S.A. Benzobisthiazole-thiophene copolymer semiconductors: Synthesis, enhanced stability, field-effect transistors, and efficient solar cells. Macromolecules 2009, 42, 8615–8618. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, B.-L.; Moon, H.; Kang, H.M.; Jeong, E.J.; Park, J.; Han, K.-M.; Lee, S.; Yoo, B.W.; Koo, B.W.; Kim, J.Y.; Lee, W.H.; Cho, K.; Becerril, H.A.; Bao, Z. Liquid-crystalline semiconducting copolymers with intramolecular donor-acceptor building blocks for high-stability polymer transistors. J. Am. Chem. Soc. 2009, 131, 6124–6132. [Google Scholar] [CrossRef] [PubMed]
- Osaka, I.; Sauvé, G.; Zhang, R.; Kowalewski, T.; McCullough, R.D. Novel thiophene-thiazolothiazole copolymers for organic field-effect transistors. Adv. Mater. 2007, 19, 4160–4165. [Google Scholar] [CrossRef]
- Osaka, I.; Zhang, R.; Sauvé, G.; Smilgies, D.-M.; Kowalewski, T.; McCullough, R.D. High-lamellar ordering and amorphous-like π-network in short-chain thiazolothiazole-thiophene copolymers lead to high mobilities. J. Am. Chem. Soc. 2009, 131, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, R.P.; Yan, H.; Facchetti, A.; Marks, T.J. Azine- and Azole-Functionalized Oligo´ and Polythiophene Semiconductors for Organic Thin-Film Transistors. Materials 2010, 3, 1533-1558. https://doi.org/10.3390/ma3031533
Ortiz RP, Yan H, Facchetti A, Marks TJ. Azine- and Azole-Functionalized Oligo´ and Polythiophene Semiconductors for Organic Thin-Film Transistors. Materials. 2010; 3(3):1533-1558. https://doi.org/10.3390/ma3031533
Chicago/Turabian StyleOrtiz, Rocío Ponce, He Yan, Antonio Facchetti, and Tobin J. Marks. 2010. "Azine- and Azole-Functionalized Oligo´ and Polythiophene Semiconductors for Organic Thin-Film Transistors" Materials 3, no. 3: 1533-1558. https://doi.org/10.3390/ma3031533





