Crystal and Electronic Structures, Photoluminescence Properties of Eu2+-Doped Novel Oxynitride Ba4Si6O16-3x/2Nx
Abstract
:1. Introduction
2. Computational Details
3. Experimental Section
3.1. Synthetic approaches
3.2. Characterization
4. Results and Discussion
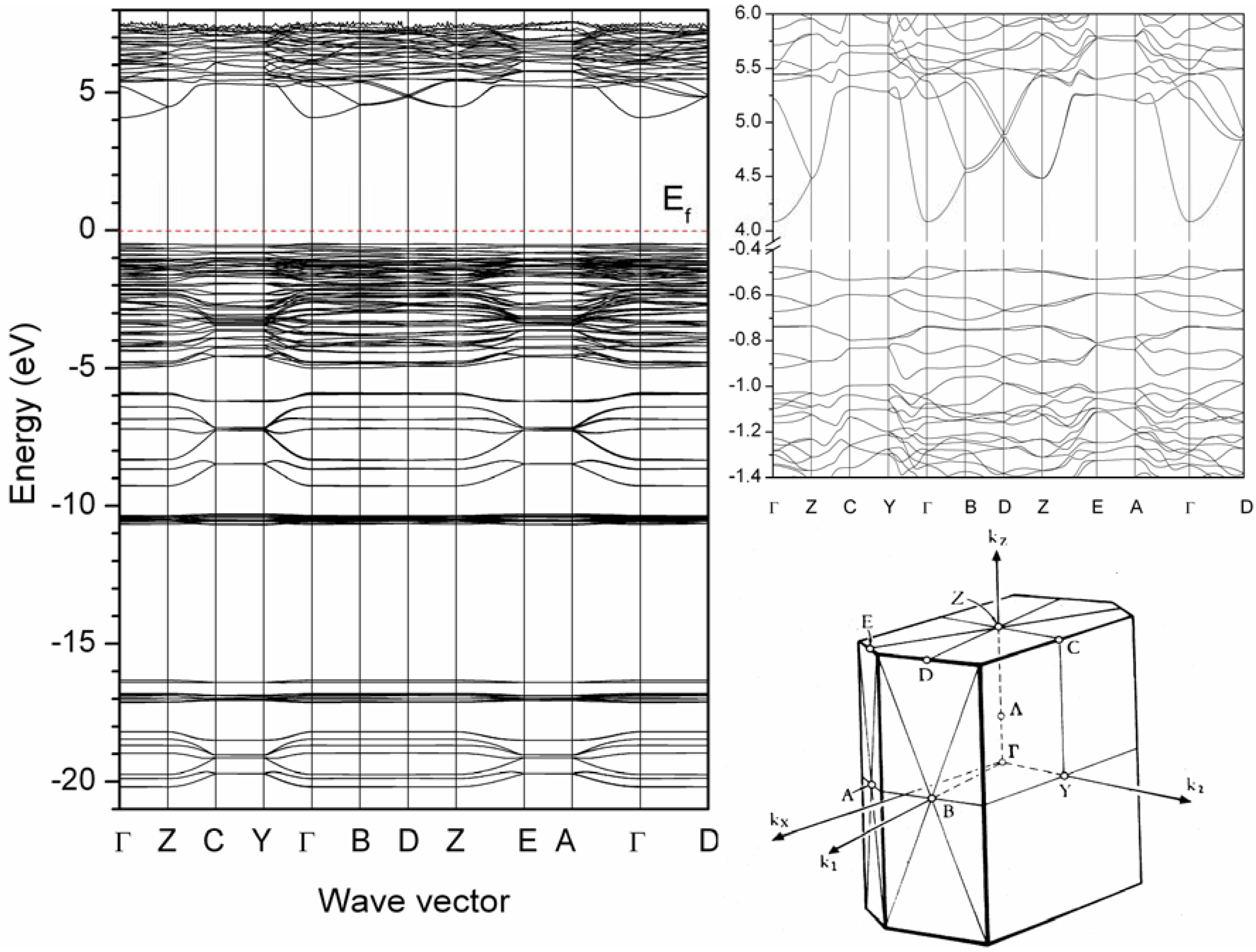
4.1. Electronic structures of the Ba4Si6O16 host

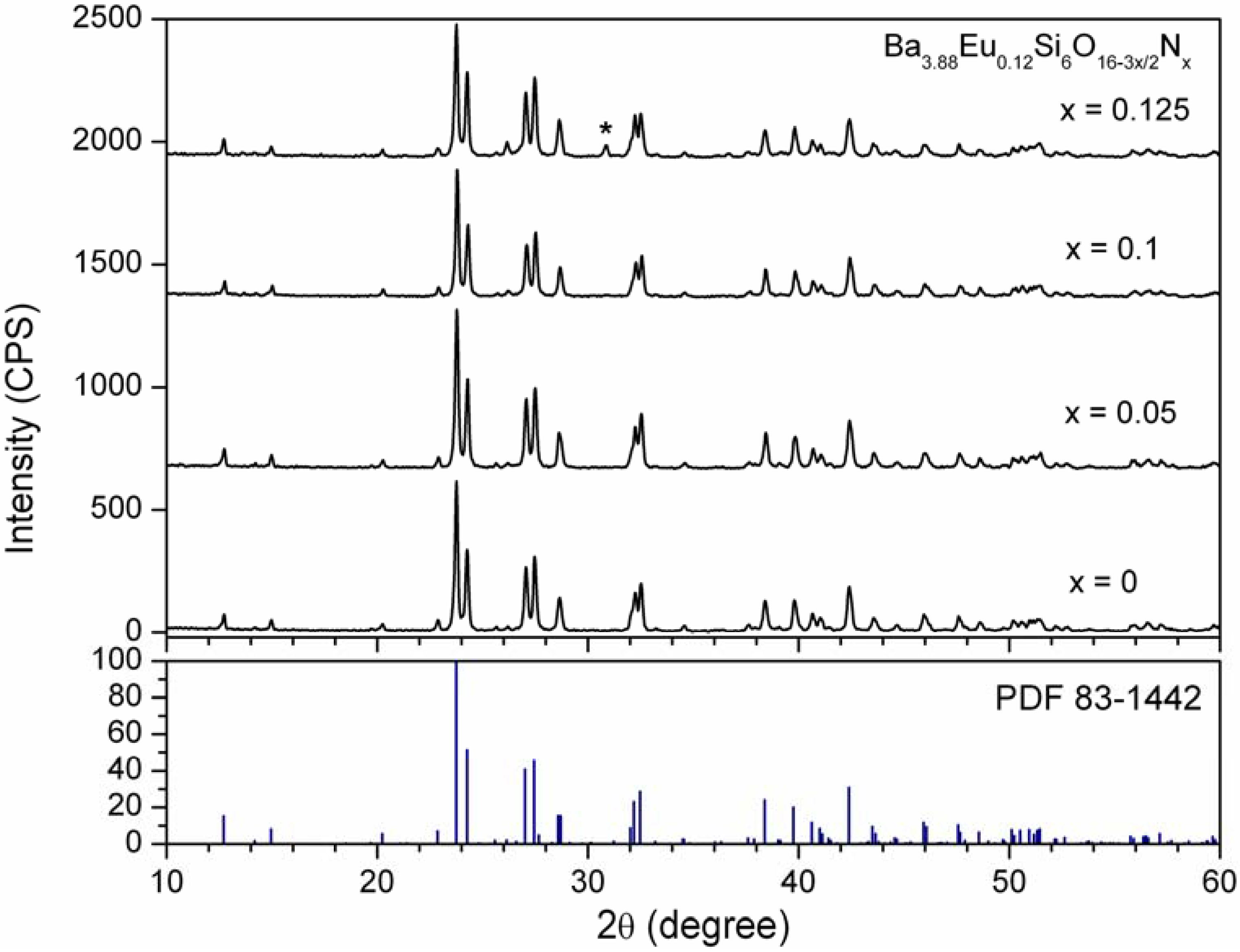
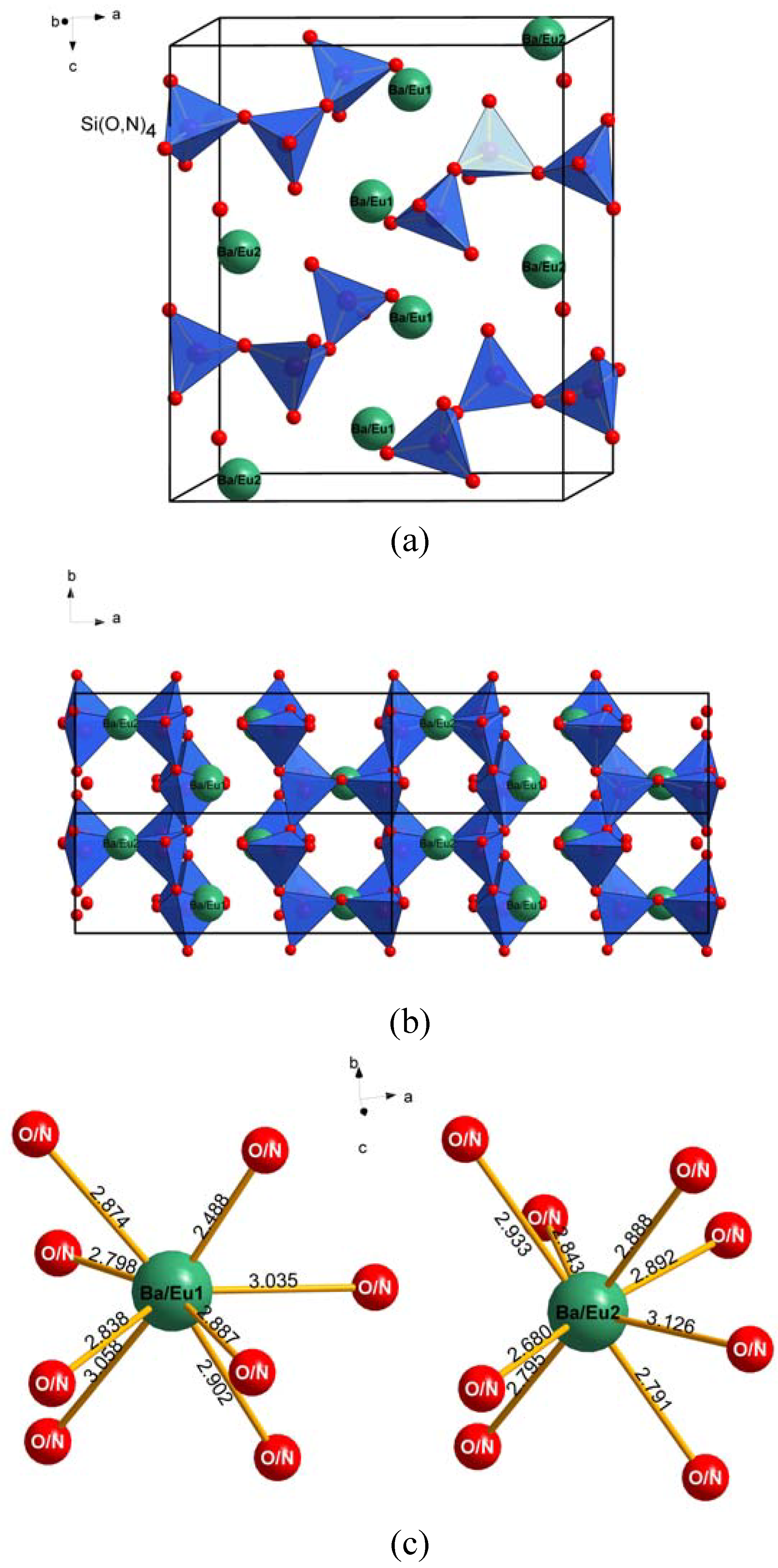
4.2.Formation and crystal structure of Ba4-yEuySi6O16-3x/2Nx

| Formula | Ba3.88Eu0.12Si6O16 | |||||||||
| Formula weight | 975.57 | |||||||||
| Crystal system | Monoclinic | |||||||||
| Space group | P21/c (14) | |||||||||
| Z | 2 | |||||||||
| Lattice parameters | ||||||||||
| a (Å) | 12.4651(4) | |||||||||
| b (Å) | 4.6826(1) | |||||||||
| c (Å) | 13.9242(4) | |||||||||
| β (°) | 93.60(1) | |||||||||
| Unit cell volume (Å3) | 811.13(4) | |||||||||
| Density (g•cm-3) | ||||||||||
| ρcalc. | 3.994 | |||||||||
| Rwp | 9.6% | |||||||||
| Rp | 7.3% | |||||||||
| χ2 | 2.78 | |||||||||
| Atom | Wyck. | x/a | y/b | z/c | S.O.F. | U (100Å2) | ||||
| Ba/Eu1 | 4e | 0.5806(23) | 0.7673(11) | 0.1123(17) | 0.97/0.03 | 1.67 | ||||
| Ba/Eu2 | 4e | 0.8548(23) | 0.2523(12) | 0.0329(18) | 0.97/0.03 | 1.53 | ||||
| Si1 | 4e | 0.6475 | 0.8026 | 0.3892 | 1.00 | 2.23 | ||||
| Si2 | 4e | 0.7240 | 0.3230 | 0.2761 | 1.00 | 1.96 | ||||
| Si3 | 4e | 0.9717(9) | 0.3201(33) | 0.3054(8) | 1.00 | 1.42 | ||||
| O1 | 4e | 0.5282(15) | 0.7620(11) | 0.4091(14) | 1.00 | 1.62 | ||||
| O2 | 4e | 0.7410(14) | 0.7810(8) | 0.4759(13) | 1.00 | 0.88 | ||||
| O3 | 4e | 0.6445 | 0.1494 | 0.3486 | 1.00 | 2.62 | ||||
| O4 | 4e | 0.6805(19) | 0.639(5) | 0.2970(16) | 1.00 | 1.04 | ||||
| O5 | 4e | 0.7151 | 0.2375 | 0.1692 | 1.00 | 1.29 | ||||
| O6 | 4e | 0.8409(16) | 0.2220(8) | 0.3255(13) | 1.00 | 1.64 | ||||
| O7 | 4e | 0.0373(14) | 0.2430(10) | 0.4072(12) | 1.00 | 2.55 | ||||
| O8 | 4e | -0.0053(17) | 0.6510(6) | 0.2826(19) | 1.00 | 1.76 | ||||
| (Ba/Eu)1-O | (Ba/Eu)2-O | |||||||||
| Ba/Eu1-O1 | 2.730(5) | Ba/Eu2-O2 | 2.96(4) | |||||||
| Ba/Eu1-O1 | 2.690(4) | Ba/Eu2-O2 | 2.697(32) | |||||||
| Ba/Eu1-O1 | 2.866(19) | Ba/Eu2-O5 | 2.655(3) | |||||||
| Ba/Eu1-O2 | 2.851(17) | Ba/Eu2-O6 | 2.885(18) | |||||||
| Ba/Eu1-O3 | 2.943(3) | Ba/Eu2-O7 | 2.840(4) | |||||||
| Ba/Eu1-O4 | 2.852(19) | Ba/Eu2-O7 | 2.770(4) | |||||||
| Ba/Eu1-O5 | 3.070(4) | Ba/Eu2-O7 | 2.957(17) | |||||||
| Ba/Eu1-O5 | 2.850(5) | Ba/Eu2-O8 | 3.120(21) | |||||||
| mean | 2.857 ± 0.117 | mean | 2.860 ± 0.154 | |||||||
| polyhedron volume | 97.21 | polyhedron volume | 96.88 | |||||||
| Formula | Ba3.88Eu0.12Si6O15.85N0.1 | |||||||||
| Formula weight | 974.57 | |||||||||
| Crystal system | Monoclinic | |||||||||
| Space group | P21/c (14) | |||||||||
| Z | 4 | |||||||||
| Lattice parameters | ||||||||||
| a (Å) | 12.4663(5) | |||||||||
| b (Å) | 4.6829(1) | |||||||||
| c (Å) | 13.9236(6) | |||||||||
| β (°) | 93.61(1) | |||||||||
| Unit cell volume (Å3) | 811.22(6) | |||||||||
| Density (g•cm-3) | ||||||||||
| ρcalc. | 3.990 | |||||||||
| Rwp | 9.8% | |||||||||
| Rp | 7.3% | |||||||||
| χ2 | 2.48 | |||||||||
| Atom | Wyck. | x/a | y/b | z/c | S.O.F. | U (100Å2) | ||||
| Ba/Eu1 | 4e | 0.5802(2) | 0.7629(13) | 0.1124(2) | 0.97/0.03 | 1.61 | ||||
| Ba/Eu2 | 4e | 0.8556(2) | 0.2515(13) | 0.0319(2) | 0.97/0.03 | 1.41 | ||||
| Si1 | 4e | 0.6494 | 0.7871 | 0.3915 | 1.00 | 2.05 | ||||
| Si2 | 4e | 0.7241 | 0.3220 | 0.2759 | 1.00 | 1.81 | ||||
| Si3 | 4e | 0.9715 | 0.3202 | 0.3052 | 1.00 | 1.33 | ||||
| O/N1 | 4e | 0.5254(15) | 0.7120(8) | 0.4080(14) | 0.9906/0.0063 | 1.68 | ||||
| O/N2 | 4e | 0.7366 | 0.7665 | 0.4732 | 0.9906/0.0063 | 1.80 | ||||
| O/N3 | 4e | 0.6495(17) | 0.1220(5) | 0.3446(14) | 0.9906/0.0063 | 2.12 | ||||
| O/N4 | 4e | 0.6777(20) | 0.6460(5) | 0.2947(15) | 0.9906/0.0063 | 1.53 | ||||
| O/N5 | 4e | 0.7153 | 0.2379 | 0.1700 | 0.9906/0.0063 | 1.23 | ||||
| O/N6 | 4e | 0.8416(16) | 0.2490(11) | 0.3273(11) | 0.9906/0.0063 | 1.27 | ||||
| O/N7 | 4e | 0.0314(14) | 0.2390(11) | 0.4060(11) | 0.9906/0.0063 | 2.48 | ||||
| O/N8 | 4e | -0.0054 | 0.6488 | 0.2827 | 0.9906/0.0063 | 1.56 | ||||
| (Ba/Eu)1-O/N | (Ba/Eu)2-O/N | |||||||||
| Ba/Eu1-O/N1 | 2.900(4) | Ba/Eu2-O/N2 | 2.933(5) | |||||||
| Ba/Eu1-O/N1 | 2.490(34) | Ba/Eu2-O/N2 | 2.795(5) | |||||||
| Ba/Eu1-O/N1 | 2.887(20) | Ba/Eu2-O/N5 | 2.680(3) | |||||||
| Ba/Eu1-O/N2 | 2.837(3) | Ba/Eu2-O/N6 | 2.844(16) | |||||||
| Ba/Eu1-O/N3 | 3.035(21) | Ba/Eu2-O/N7 | 2.890(4) | |||||||
| Ba/Eu1-O/N4 | 2.798(19) | Ba/Eu2-O/N7 | 2.790(4) | |||||||
| Ba/Eu1-O/N5 | 3.058(5) | Ba/Eu2-O/N7 | 2.892(16) | |||||||
| Ba/Eu1-O/N5 | 2.873(5) | Ba/Eu2-O/N8 | 3.126(2) | |||||||
| mean | 2.860 ± 0.175 | mean | 2.868 ± 0.130 | |||||||
| polyhedron volume | 97.22 | polyhedron volume | 98.03 | |||||||
4.3. Photoluminescence properties
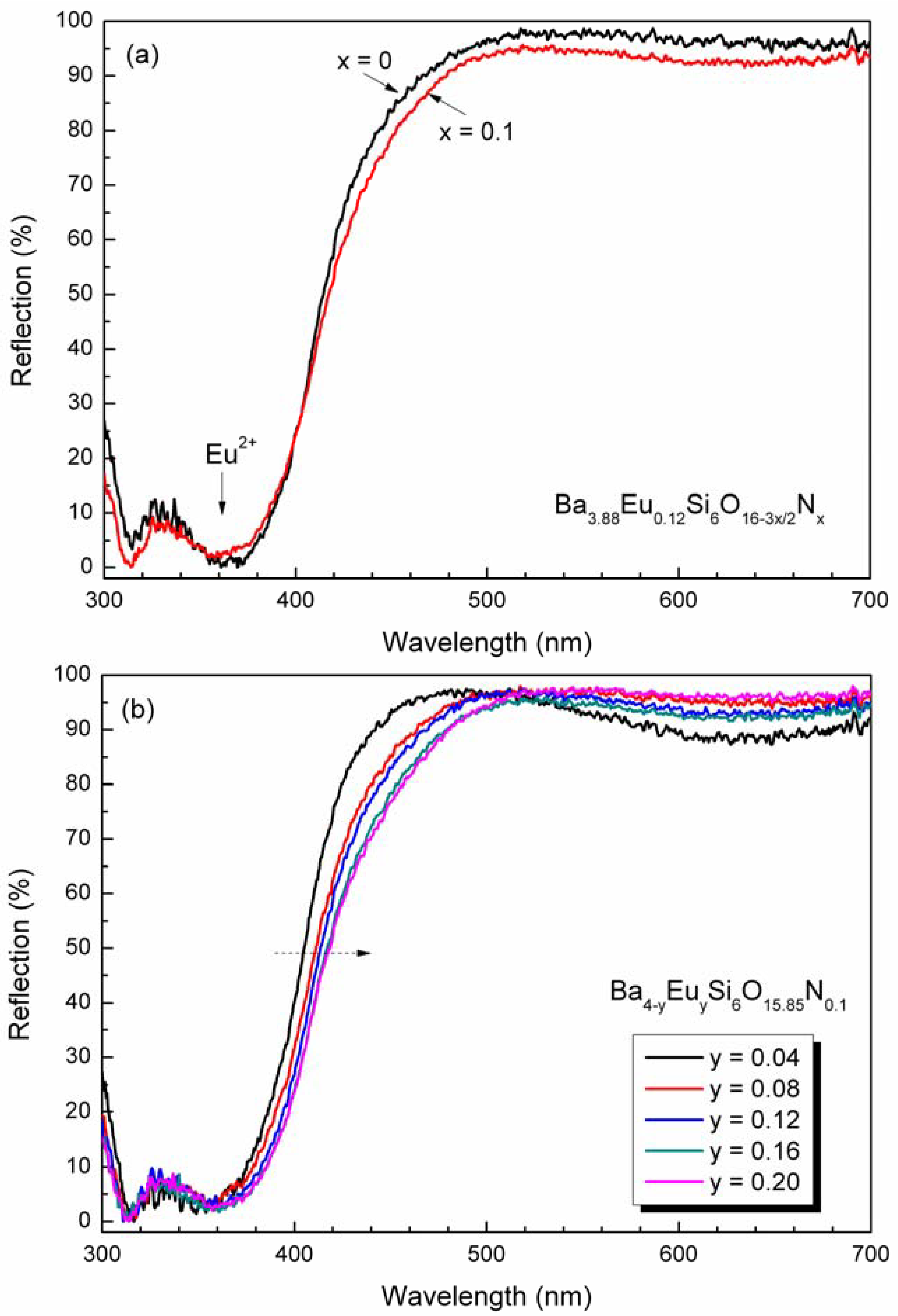
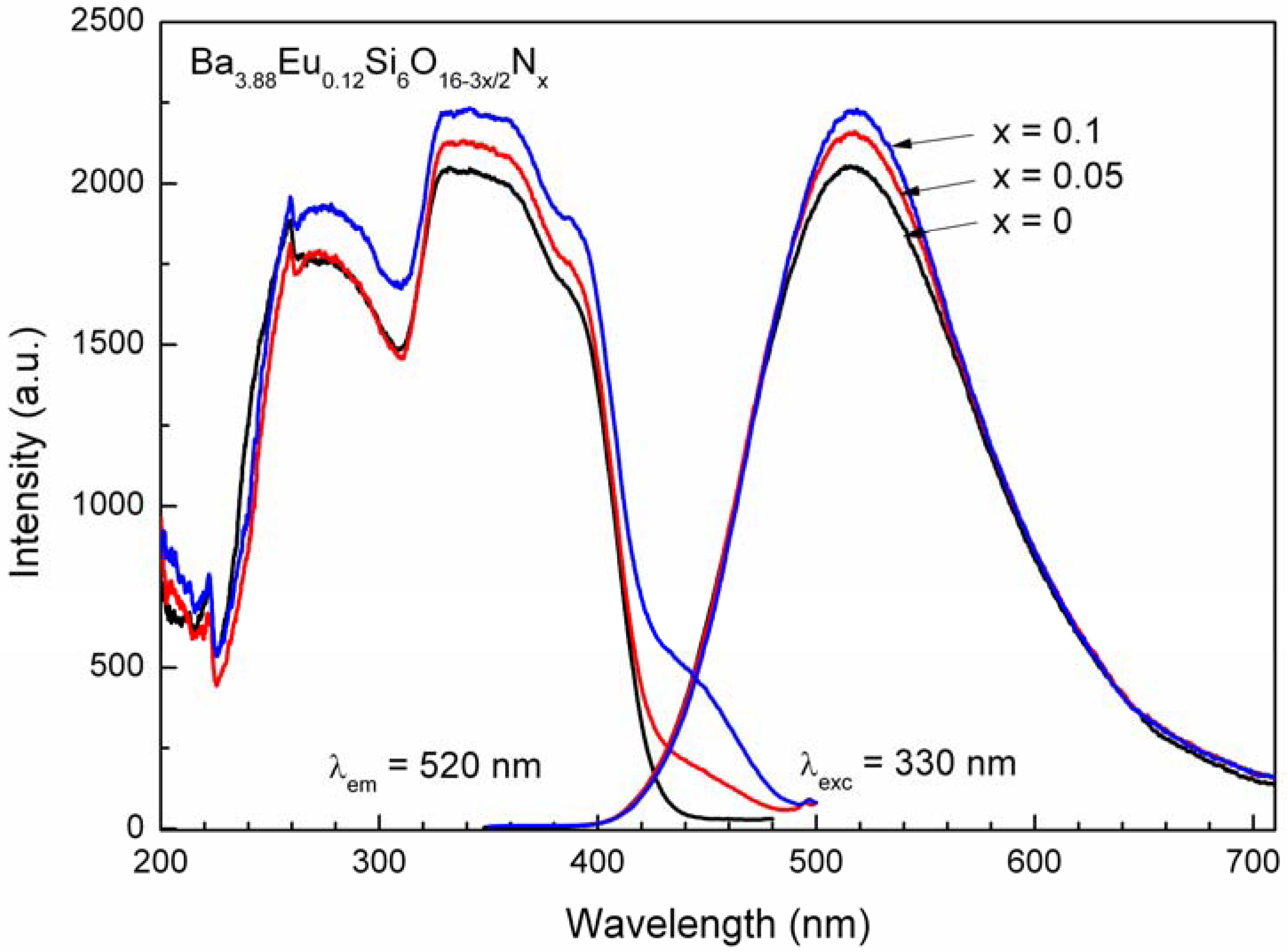
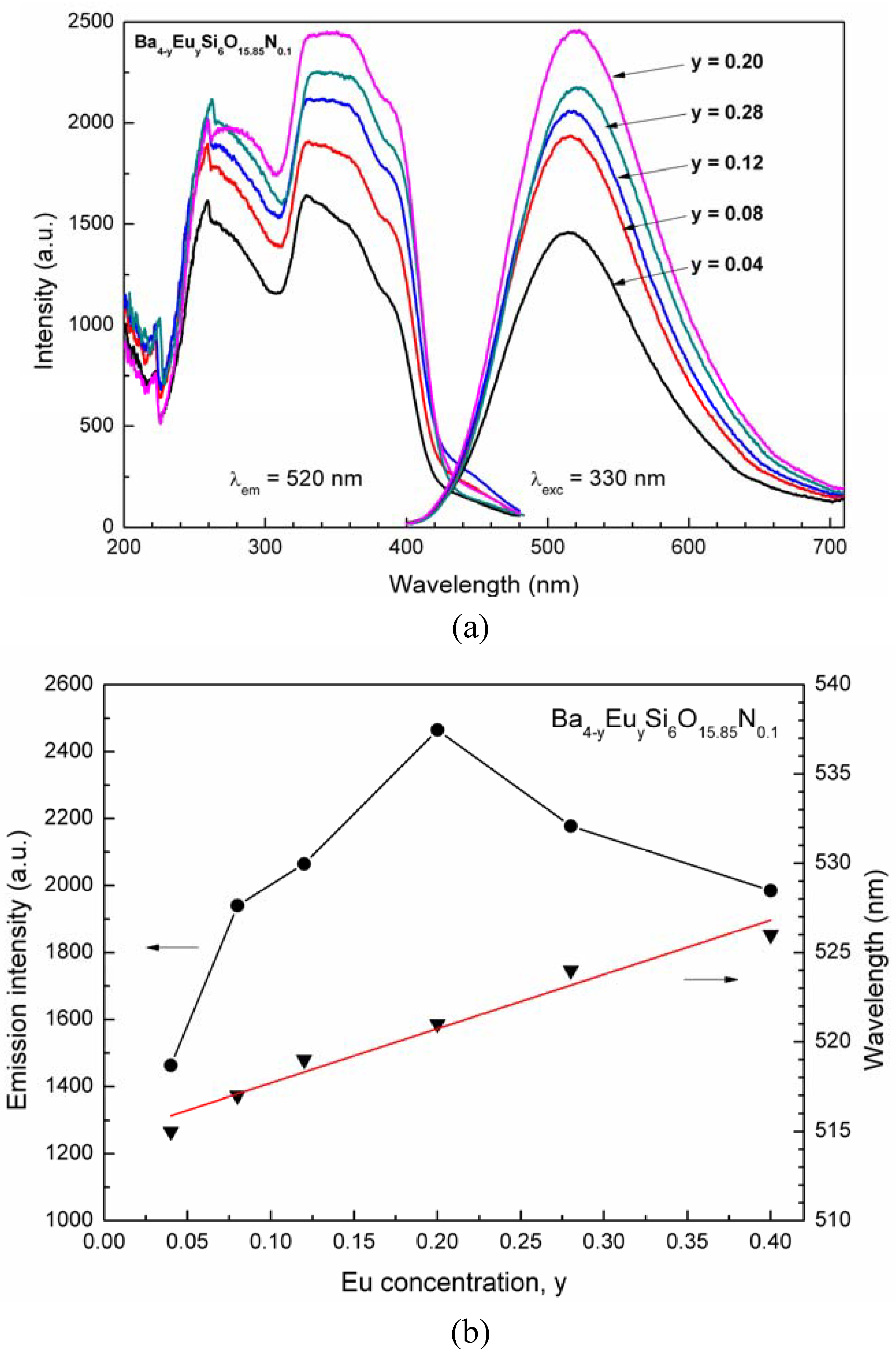
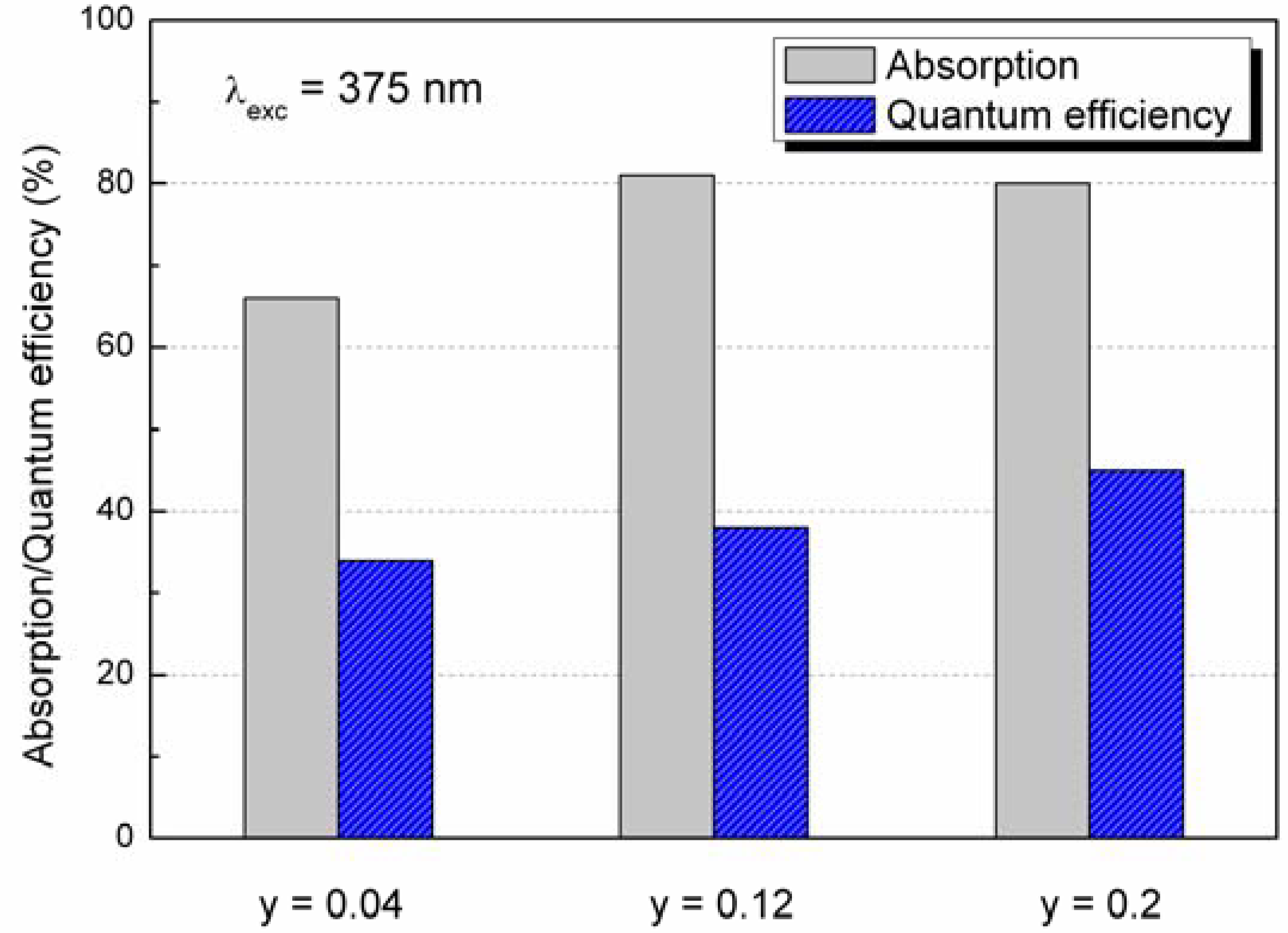
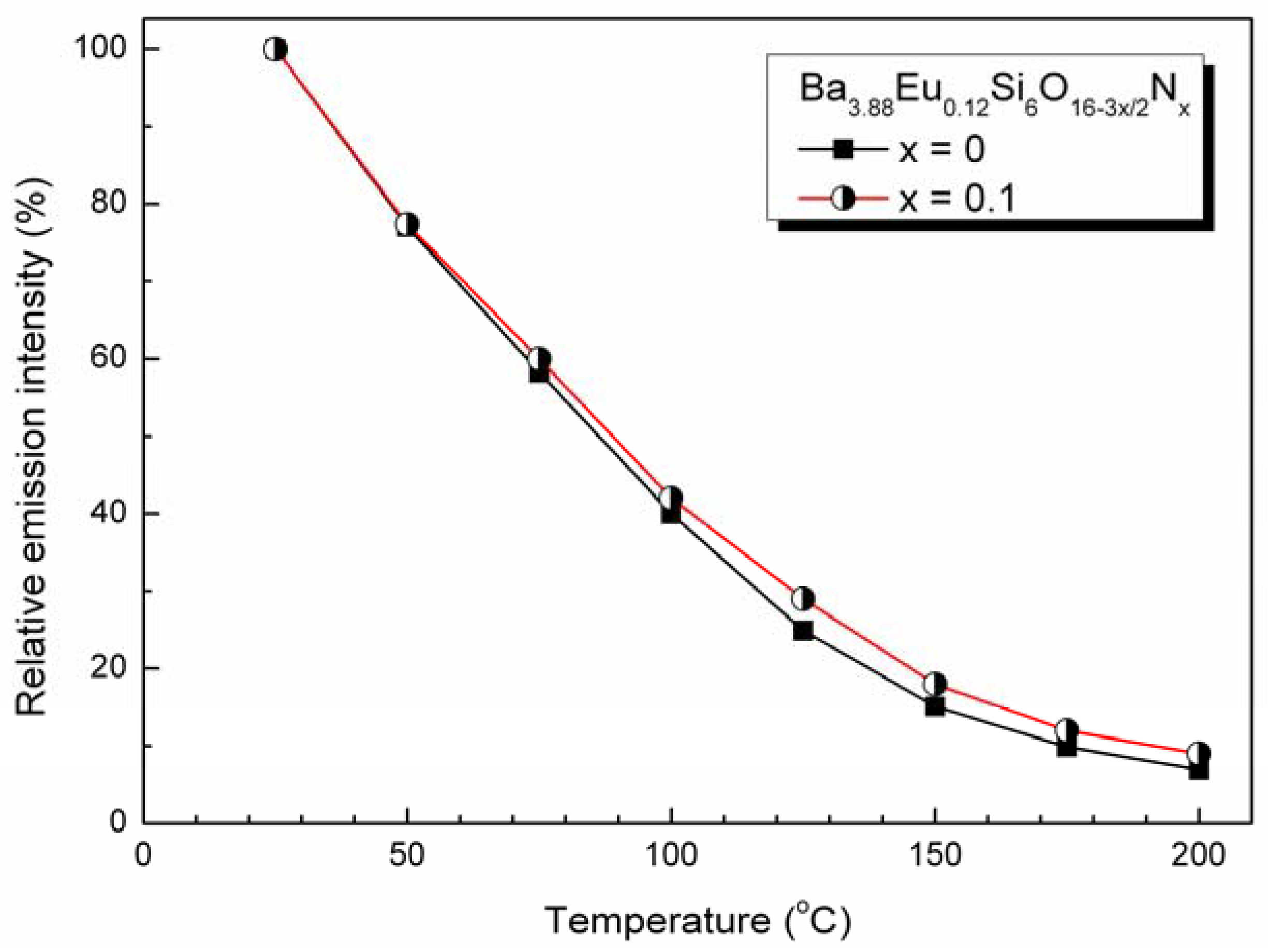
5. Conclusions
Acknowledgements
References
- Li, Y.Q.; de With, G.; Hintzen, H.T. Luminescence properties of Eu2+-doped MAl2-xSixO4-xNx (M = Ca, Sr, Ba) conversion phosphor for white-LED applications. J. Electrochem. Soc. 2006, 153, G278–G282. [Google Scholar] [CrossRef]
- Li, Y.Q.; Hirosaki, N.; Xie, R.-J.; Mitomo, M. Crystal, electronic and luminescence properties of Eu2+-doped Sr2Al2-xSi1+xO7-xNx. Sci. Technol. Adv. Mat. 2007, 8, 607–616. [Google Scholar] [CrossRef]
- Mikami, M.; Imura, H.; Uheda, K.; Jijima, N.; Matsuo, H.; Miyamoto, Y.; Yamamoto, H. Partial Nitridation of A Blue Phosphor, Sr3Al10SiO20:Eu2+, and Its Luminescence Properties (I). In The 56th Japan Society of Applied Physics, Spring Meeting, Tsukuba, Japan, March 30−April 2, 2009; p. 1486.
- Matsuo, H.; Mikami, M.; Uheda, K.; Jijima, N.; Miyamoto, Y.; Yamamoto, H. Partial Nitridation of A Blue Phosphor, Sr3Al10SiO20:Eu2+, and Its Luminescence Properties (II). In The 56th Japan Society of Applied Physics, Spring Meeting, Tsukuba, Japan, March 30−April 2, 2009; p. 1486.
- Hintzen, H.T.; Li, Y.Q. Rare-Earth-Doped Silicon-Aluminum-(Oxy)nitride Materials. In The Encyclopedia of Materials Science and Technology; Buschow, K.H.J., Ed.; Elsevier Science Ltd.: Oxford, UK, 2004; pp. 1–3. [Google Scholar]
- Barry, T.L. Fluorescence of Eu2+-Activated phases in binary alkaline earth orthosilicate systems. J. Electrochem. Soc. 1968, 115, 1181–1184. [Google Scholar] [CrossRef]
- Jenkins, H.G.; McKeag, A.H. Some rare earth activated phosphors. J. Electrochem. Soc. 1950, 97, 415–418. [Google Scholar] [CrossRef]
- Blasse, G.; Wanmaker, W.L.; ter Vrugt, J.W.; Bril, A. Flourescence of Eu2+-activated silicates. Philips Res. Rep. 1968, 23, 189–200. [Google Scholar]
- Poort, S.H.M.; Janssen, W.; Blasse, G. Optical properties of Eu2+-activated orthosilicates and orthophosphates. J. Alloys Compd. 1997, 260, 93–97. [Google Scholar] [CrossRef]
- Poort, S.H.M.; Reijnhoudt, H.M.; van der Kulp, H.O.T.; Blasse, G. Luminescence of Eu2+ in silicate host lattices with alkaline earth ions in a row. J. Alloys Compd. 1996, 241, 75–81. [Google Scholar] [CrossRef]
- Poort, S.H.M.; Meijerink, A.; Blasse, G. Lifetime measurements in Eu2+-doped host lattices. J. Phys. Chem. Solids 1997, 58, 1451–1456. [Google Scholar] [CrossRef]
- Lim, M.A.; Park, J.K.; Kim, C.H.; Park, H.D. Luminescence characteristics of green light emitting Ba2SiO4:Eu2+ phosphor. J. Mater. Sci. Letts. 2003, 22, 1351–1353. [Google Scholar] [CrossRef]
- Kim, J.S.; Jeon, P.E.; Choi, J.C.; Park, H.L. Emission color variation of M2SiO4:Eu2+ (M = Ba, Sr, Ca) phosphors for light-emitting diode. Solid State Commun. 2005, 133, 187–190. [Google Scholar] [CrossRef]
- Yamaga, M.; Masui, Y.; Sakuta, S.; Kodama, N.; Kaminaga, K. Radiative and nonradiative decay processes responsible for long lasting phosphorescence of Eu2+-doped barium silicates. Phys. Rev. B 2005, 71, 205102:1–205102:7. [Google Scholar] [CrossRef]
- Park, J.K.; Lim, M.A.; Choi, K.J.; Kim, C.H. Luminescence characteristics of yellow emitting Ba3SiO5:Eu2+ phosphor. J. Mater. Sci. 2005, 40, 2069–2071. [Google Scholar] [CrossRef]
- Butler; Keith, H. Fluorescent Lamp Phosphors−Technology and Theory; The Pennsylvania State University Press: University Park and London, PA, USA, 1981; pp. 266–283. [Google Scholar]
- Liebau, F. Structural Chemistry of Silicates; Springer: Berlin Heidelberg New York, NY, USA, 1985. [Google Scholar]
- Milman, V.; Winkler, B.; White, J.A.; Pickard, J.; Payne, M.C.; Akhmatskaya, E.V.; Nobes, R.H. Electronic structure, properties, and phase stabilities of inorganic crystals: A pseuopotential plane-wave study. Int. J. Quantum Chem. 2000, 77, 895–910. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio molecular-dynamics simulation of the liquid-metal–amorphous- semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of Ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for Ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Hesse, K.F.; Liebau, F. Crystal chemistry of silica-rich barium silicates. Z. Krist. 1980, 153, 3–17. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System; Report LAUR 86–748; Los Alamos: National Laboratory, NM, USA, 2000. [Google Scholar]
- Toby, B.H. EXPGUI, A Graphical user interface for GSAS. J. Appl. Cryst. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chaleogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Balić-Žunic, T.; Vickovic, I. IVTON-A program for the calculation of geometrical aspects of crystal structures and some crystal chemical applications. J. Appl. Cryst. 1996, 29, 305–306. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer-Verlag: Berlin, Germany, 1994. [Google Scholar]
- Hong, Y.C.; Bang, C.U.; Shin, D.H.; Uhm, H.S. Band gap narrowing of TiO2 by nitrogen doping in atmospheric microwave plasma. Chem. Phys. Lett. 2005, 413, 454–457. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Y.; Fang, Y.; Hirosaki, N.; Xie, R.-J.; Liu, L.; Takeda, T.; Li, X. Crystal and Electronic Structures, Photoluminescence Properties of Eu2+-Doped Novel Oxynitride Ba4Si6O16-3x/2Nx. Materials 2010, 3, 1692-1708. https://doi.org/10.3390/ma3031692
Li Y, Fang Y, Hirosaki N, Xie R-J, Liu L, Takeda T, Li X. Crystal and Electronic Structures, Photoluminescence Properties of Eu2+-Doped Novel Oxynitride Ba4Si6O16-3x/2Nx. Materials. 2010; 3(3):1692-1708. https://doi.org/10.3390/ma3031692
Chicago/Turabian StyleLi, Yuanqiang, Yuan Fang, Naoto Hirosaki, Rong-Jun Xie, Lihong Liu, Takashi Takeda, and Xiaoyun Li. 2010. "Crystal and Electronic Structures, Photoluminescence Properties of Eu2+-Doped Novel Oxynitride Ba4Si6O16-3x/2Nx" Materials 3, no. 3: 1692-1708. https://doi.org/10.3390/ma3031692





