Light Converting Inorganic Phosphors for White Light-Emitting Diodes
Abstract
:1. Introduction
2. Principle of White Light Generating in LEDs
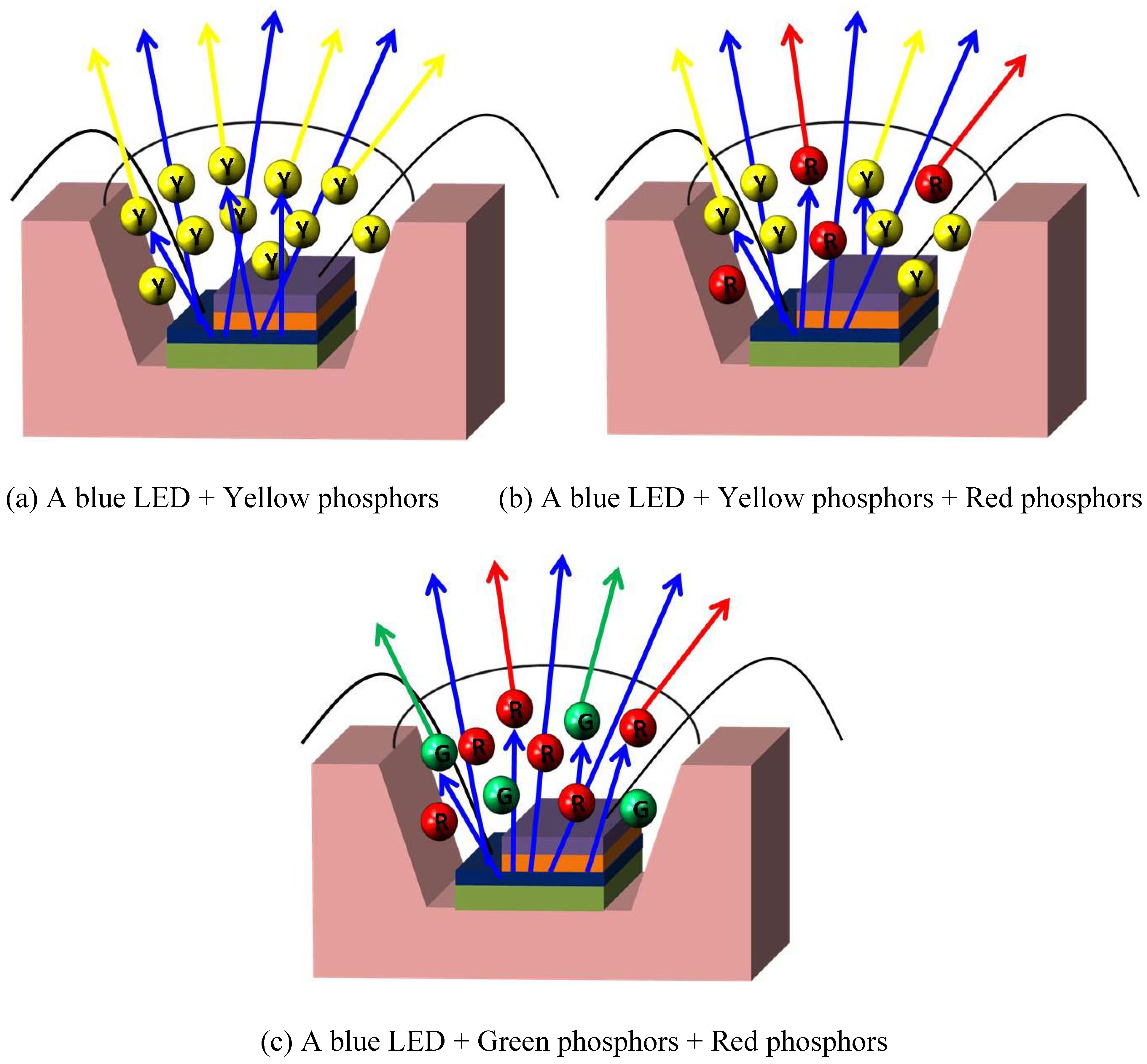
3. Converting Phosphors for LEDs
3.1. Yellow phosphors
3.1.1. YAG:Ce3+

| Atoms | x | y | z | Fraction | Uiso |
|---|---|---|---|---|---|
| Ce | 0.125 | 0 | 0.25 | 0.023 | 0.0164(2) |
| Y | 0.125 | 0 | 0.25 | 0.977 | 0.0164(2) |
| Al | 0 | 0 | 0 | 1 | 0.0197(7) |
| Al | 0.375 | 0 | 0.25 | 1 | 0.0171(5) |
| O | -0.0298(2) | 0.0506(2) | 0.1493(2) | 1 | 0.0172(8) |
| Space group: Ia-3d (cubic) | Reliability factors | Interatomic distances(Å) | |||
| Cell parameters: | Rp = 10.02% | Ce-O | 2.300(3) | ||
| a = 12.0136(9)Å | Rwp = 13.69% | 2.440(3) | |||
| b = 12.0136(9)Å | χ2 = 2.109 | Al-O | 1.928(3) | ||
| c = 12.0136(9)Å | 1.772(2) | ||||
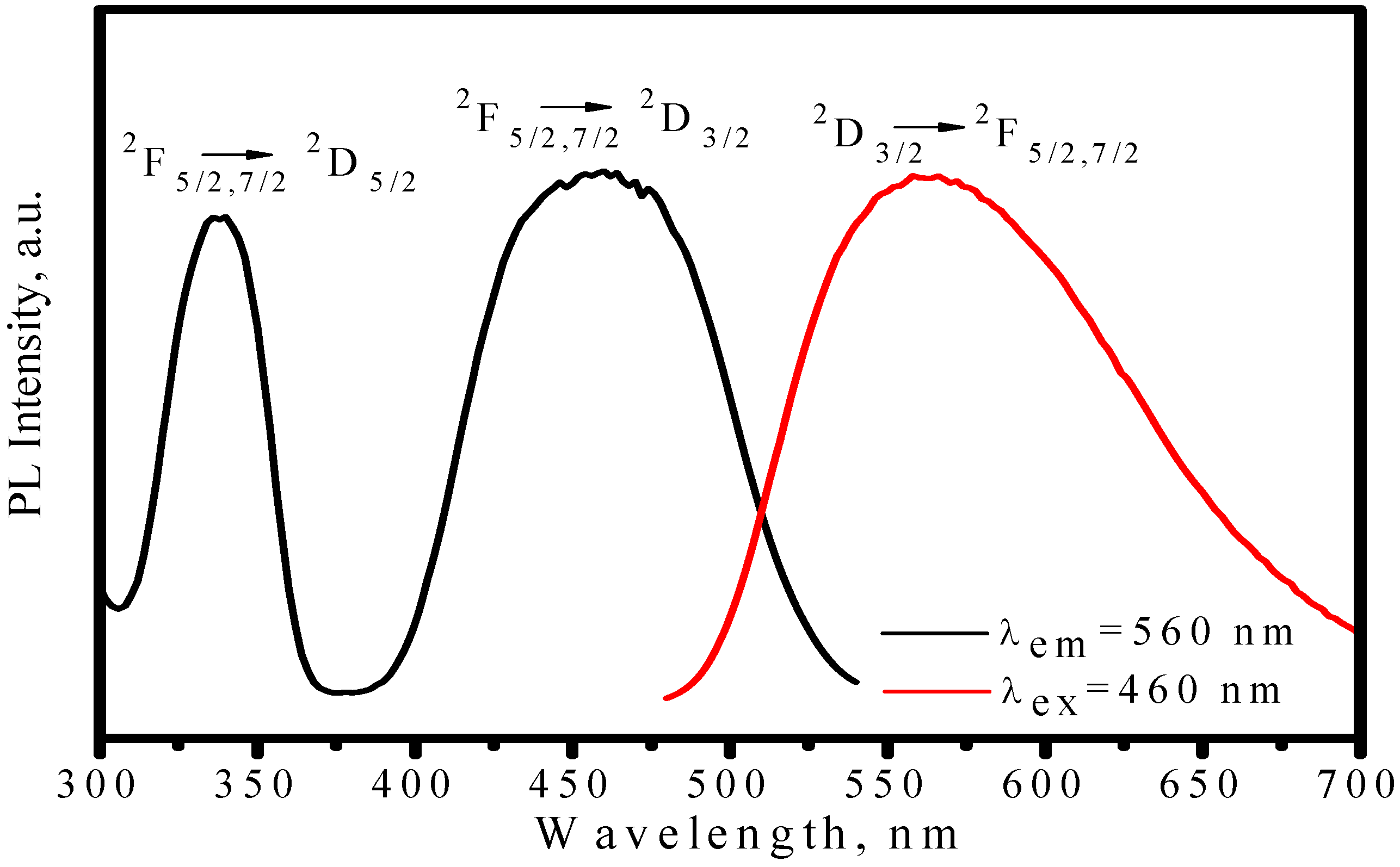

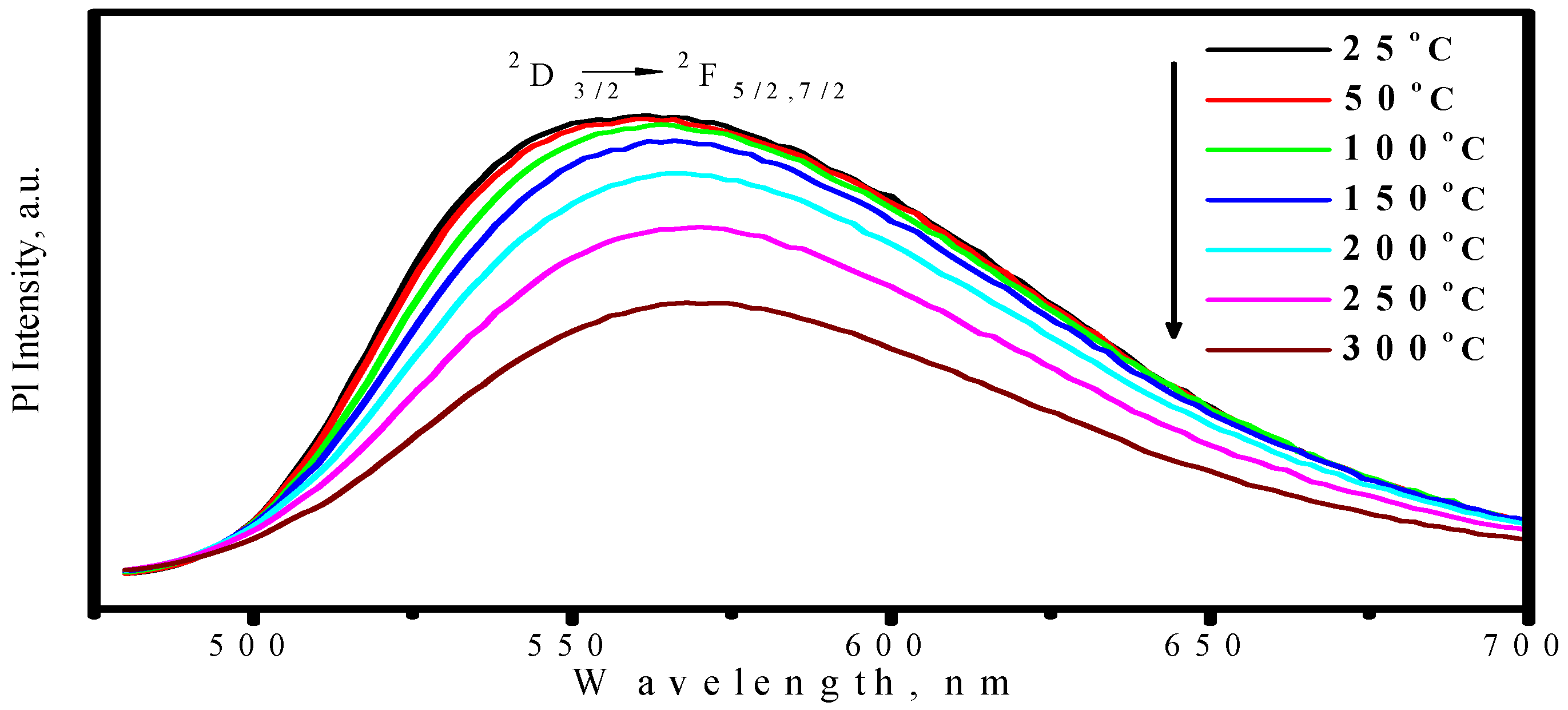

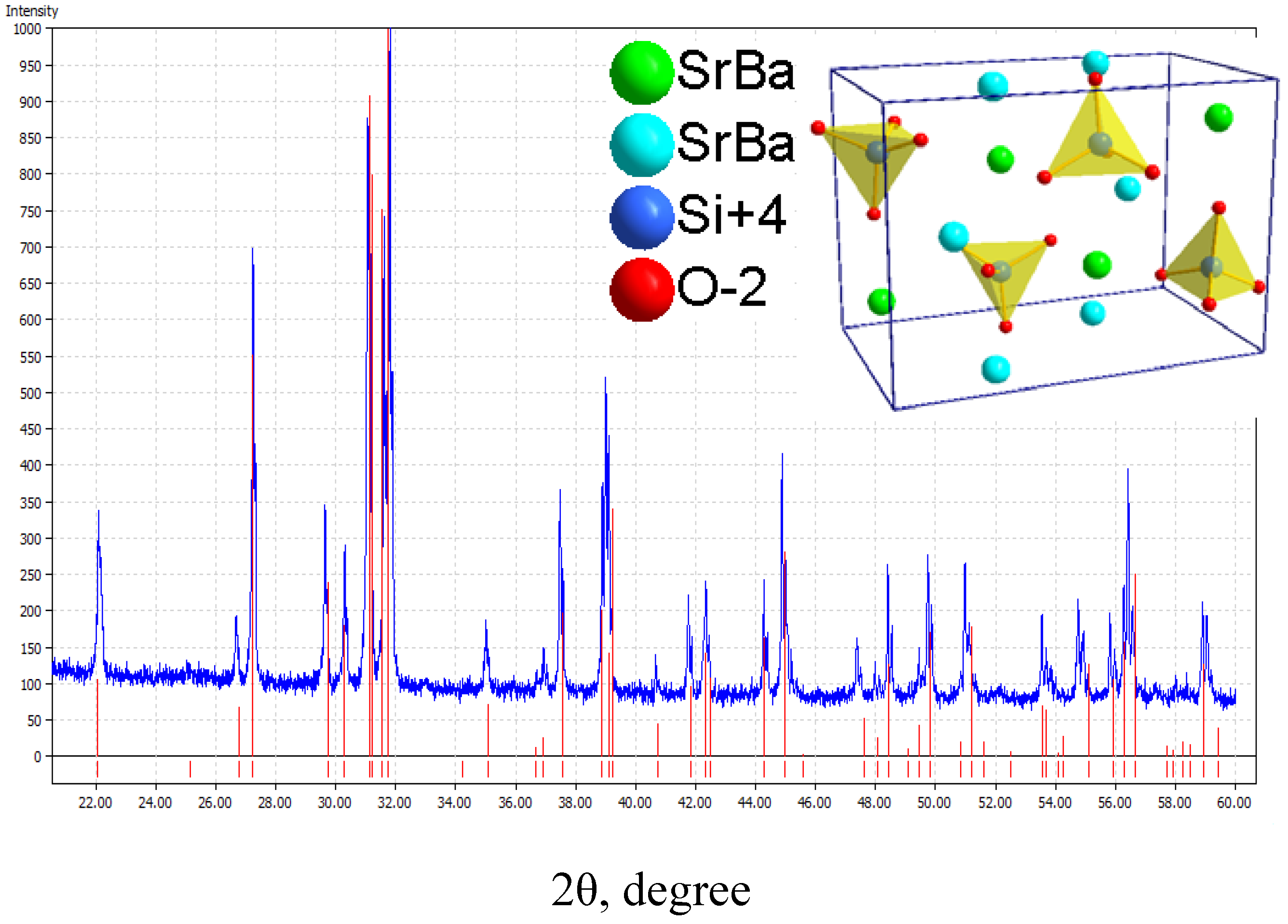
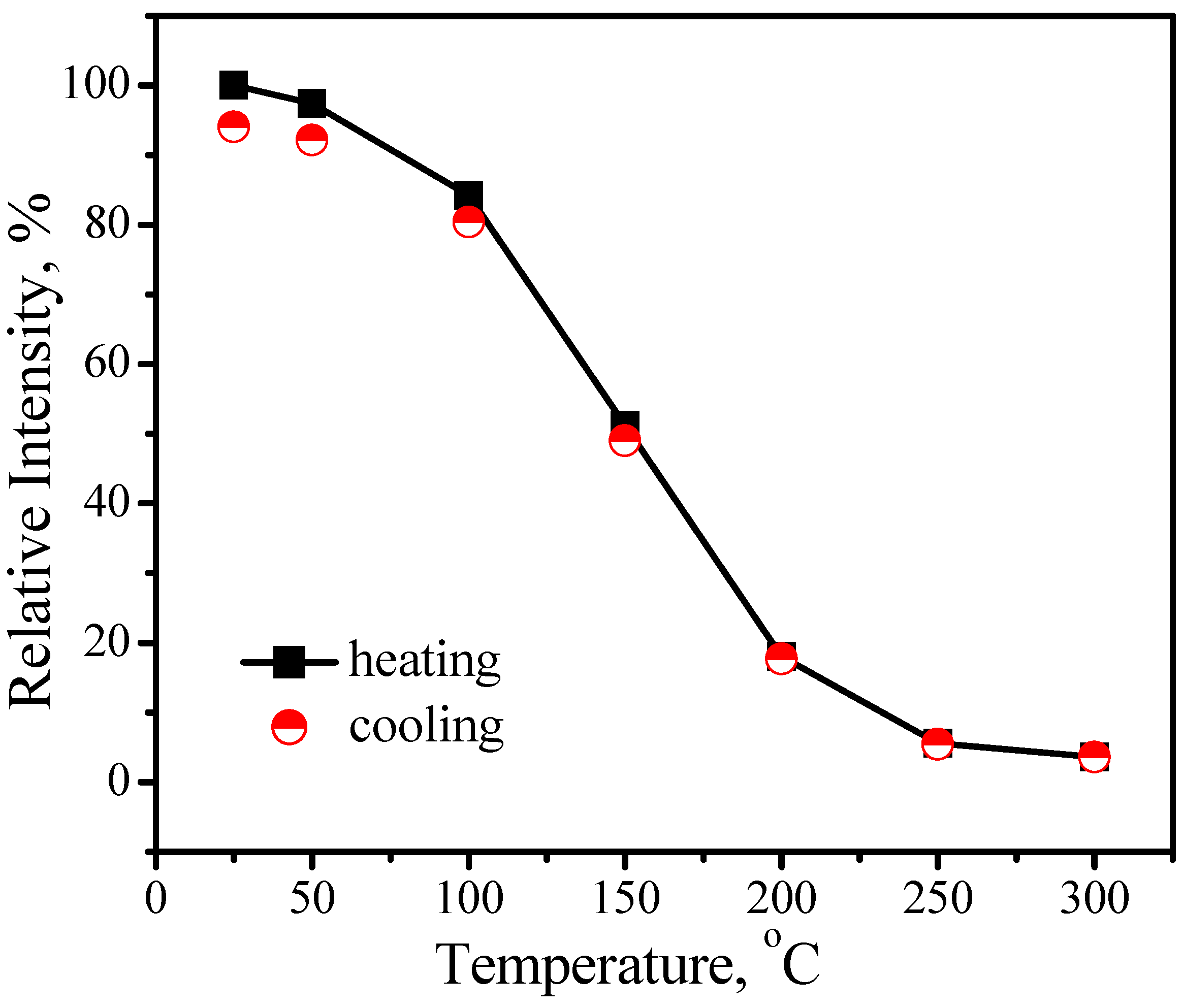
3.1.2. (Sr,Ba)SiO4:Eu2+
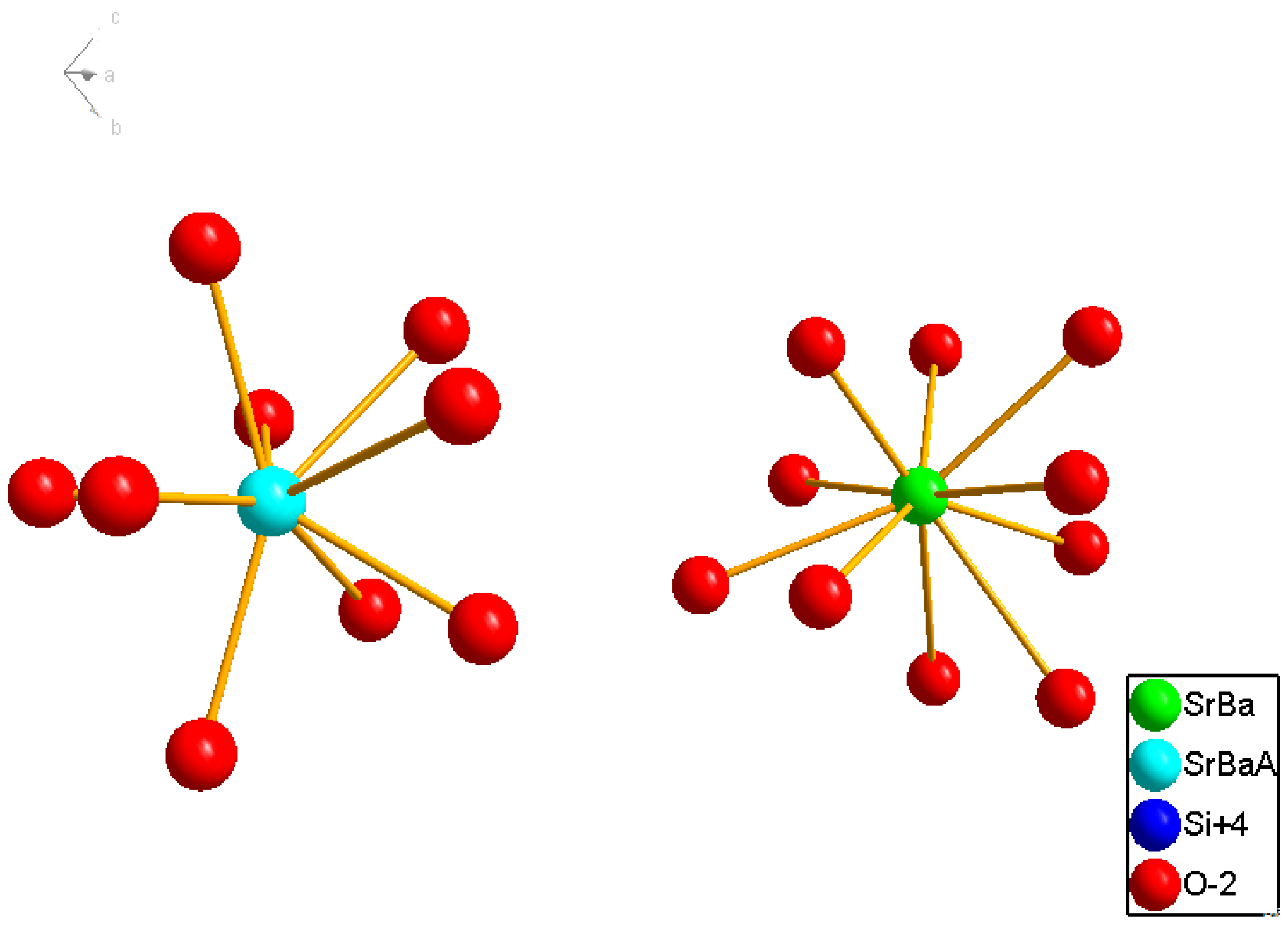
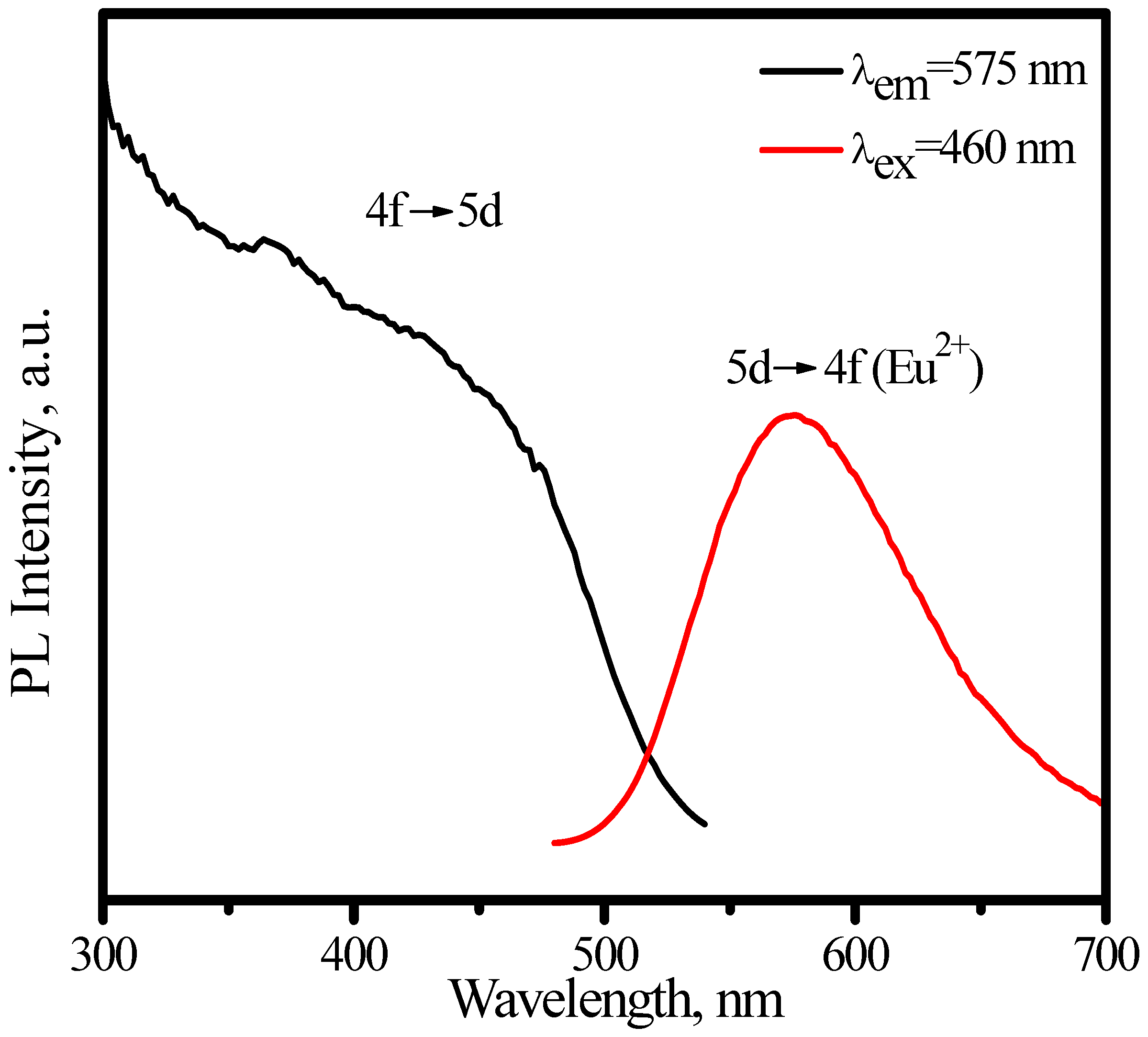
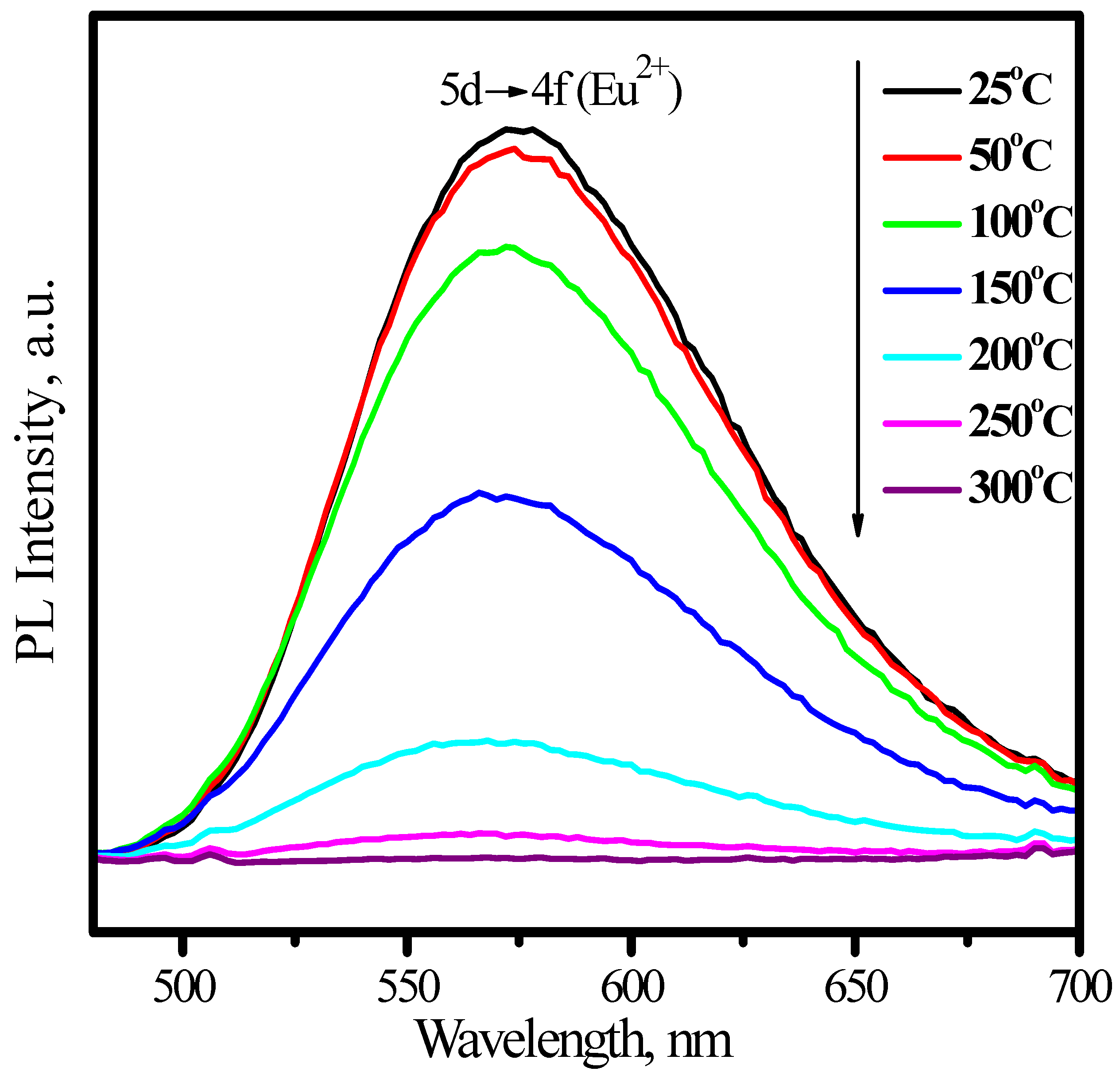
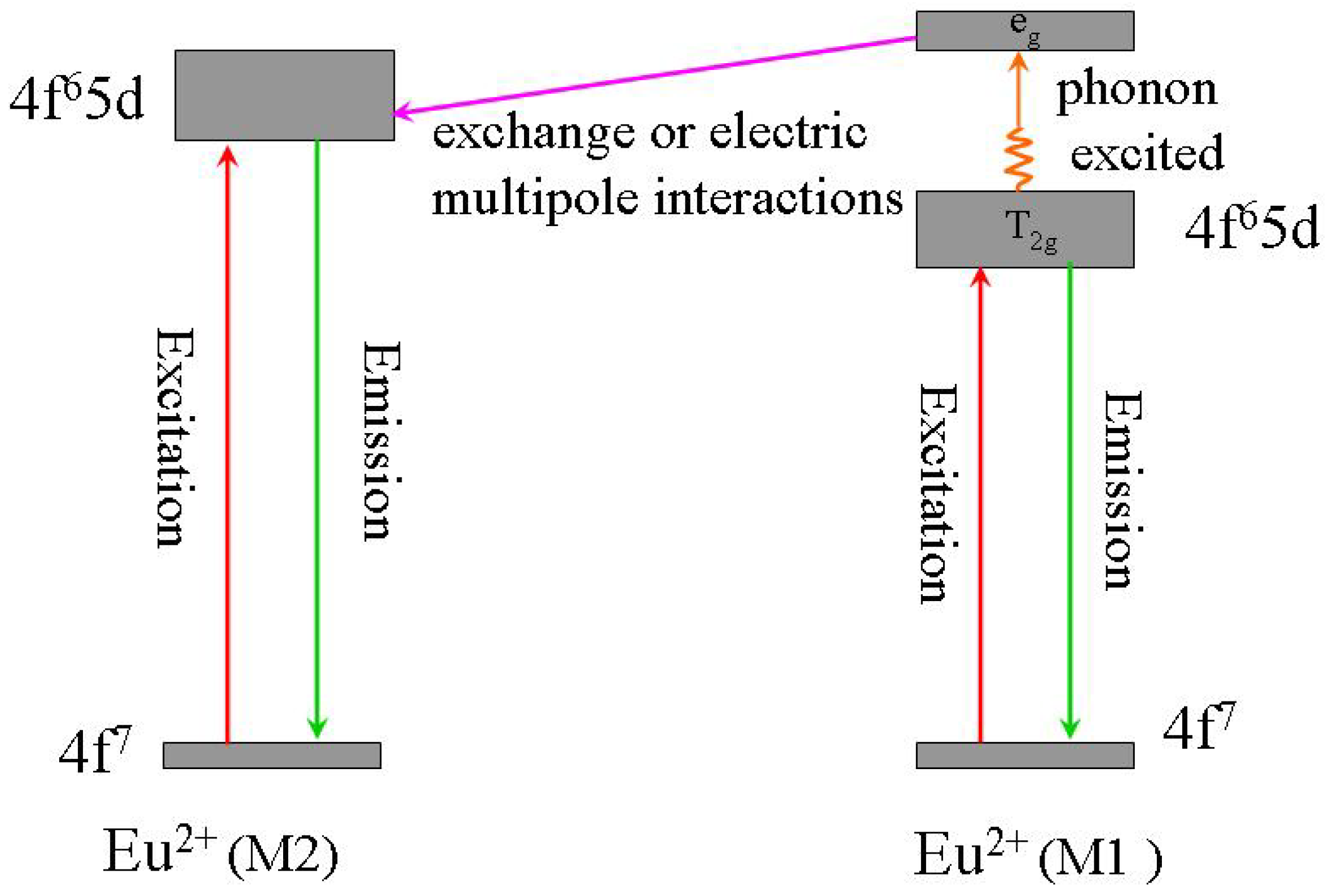
3.2. Green phosphors
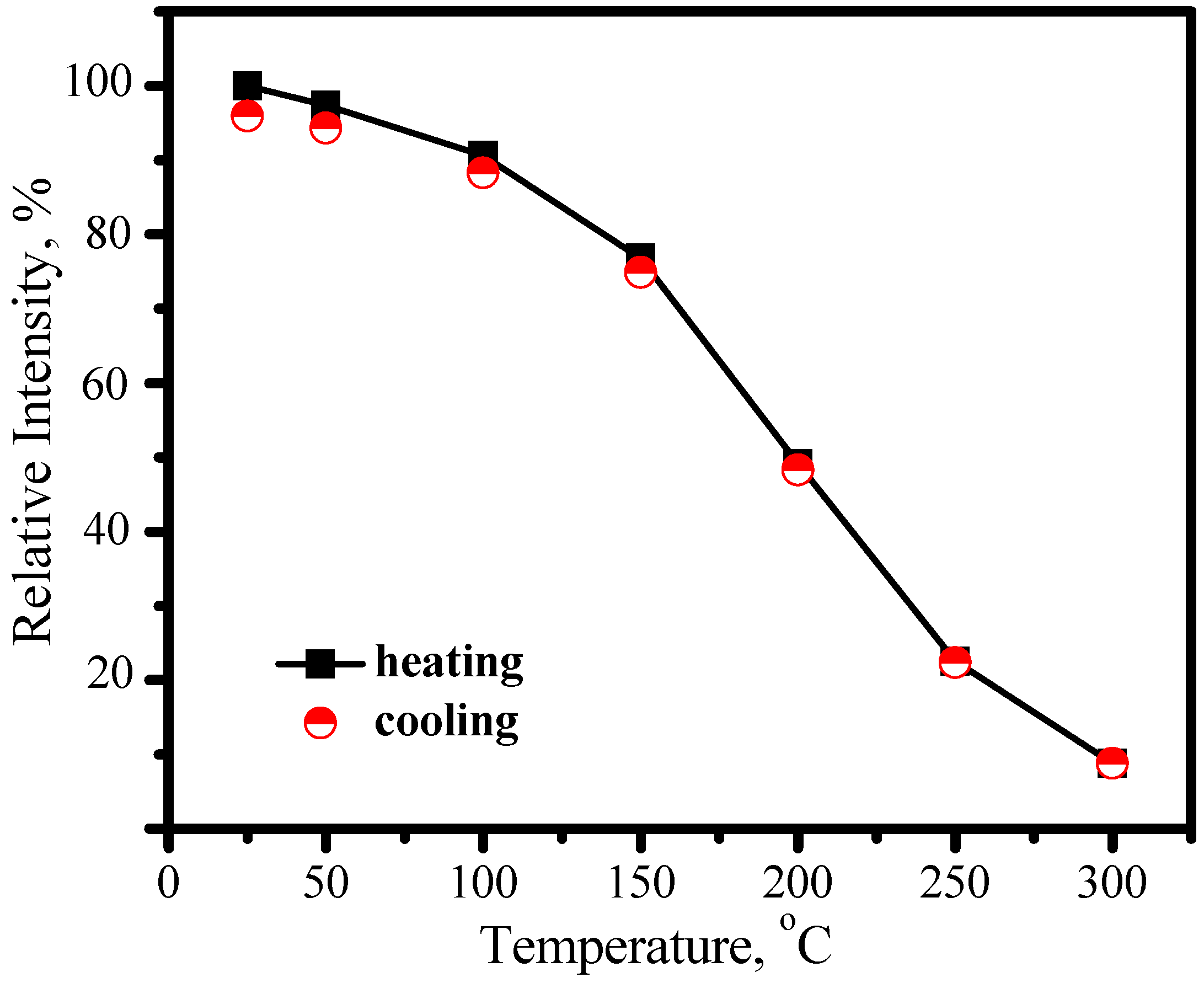
3.2.1. (Ba,Sr)SiO4:Eu2+
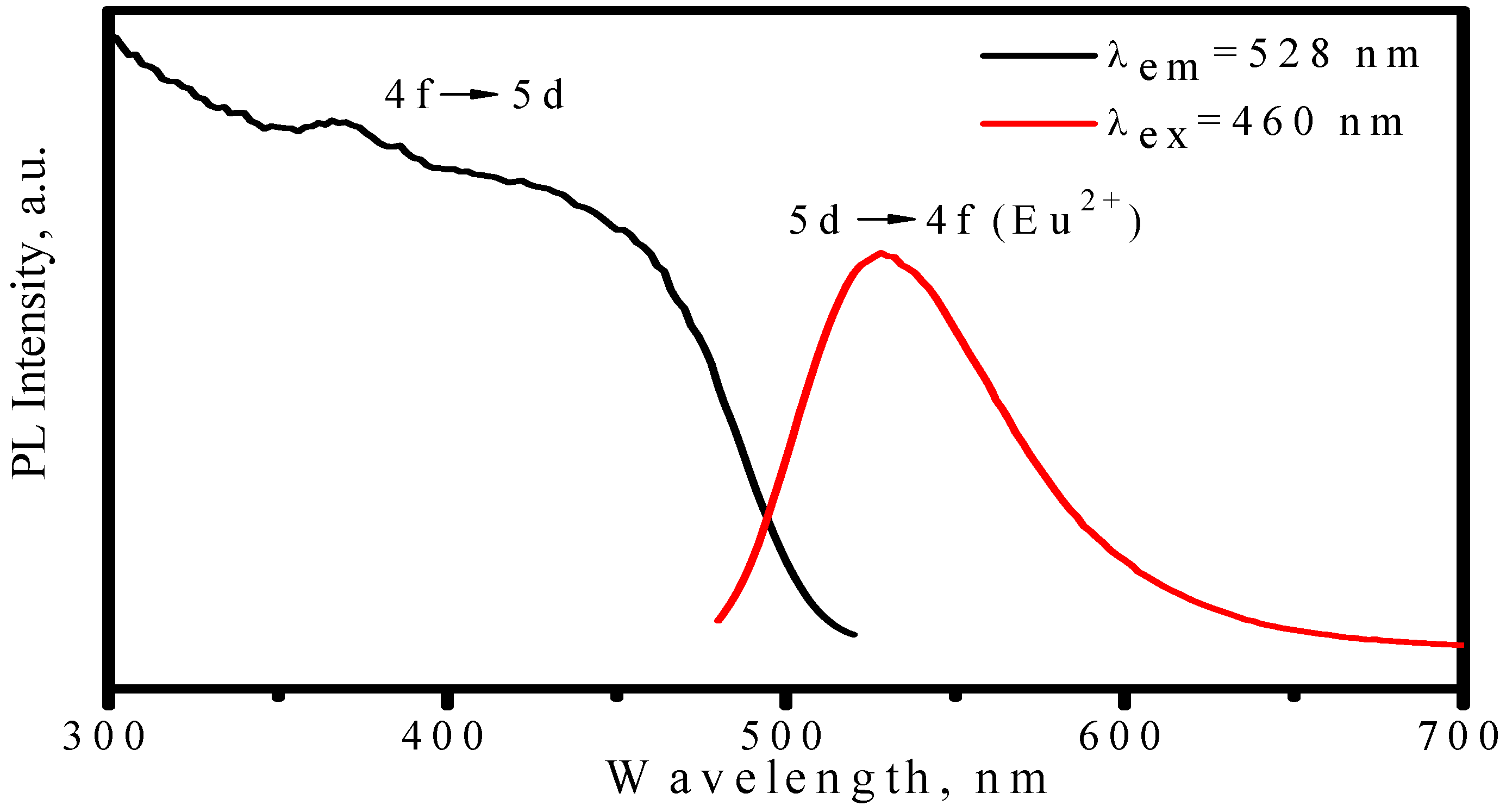

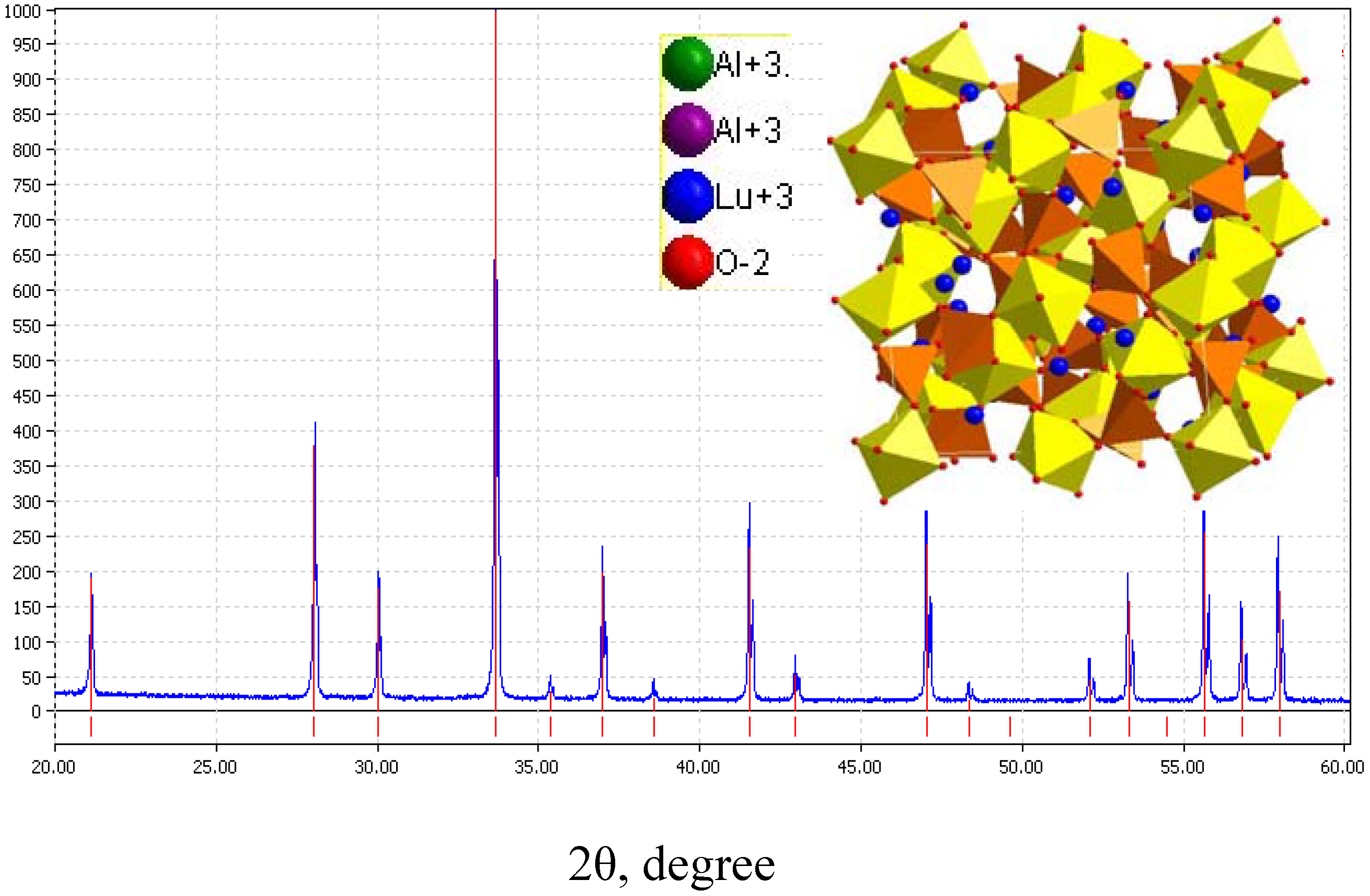
3.2.2. LuAG:Ce3+

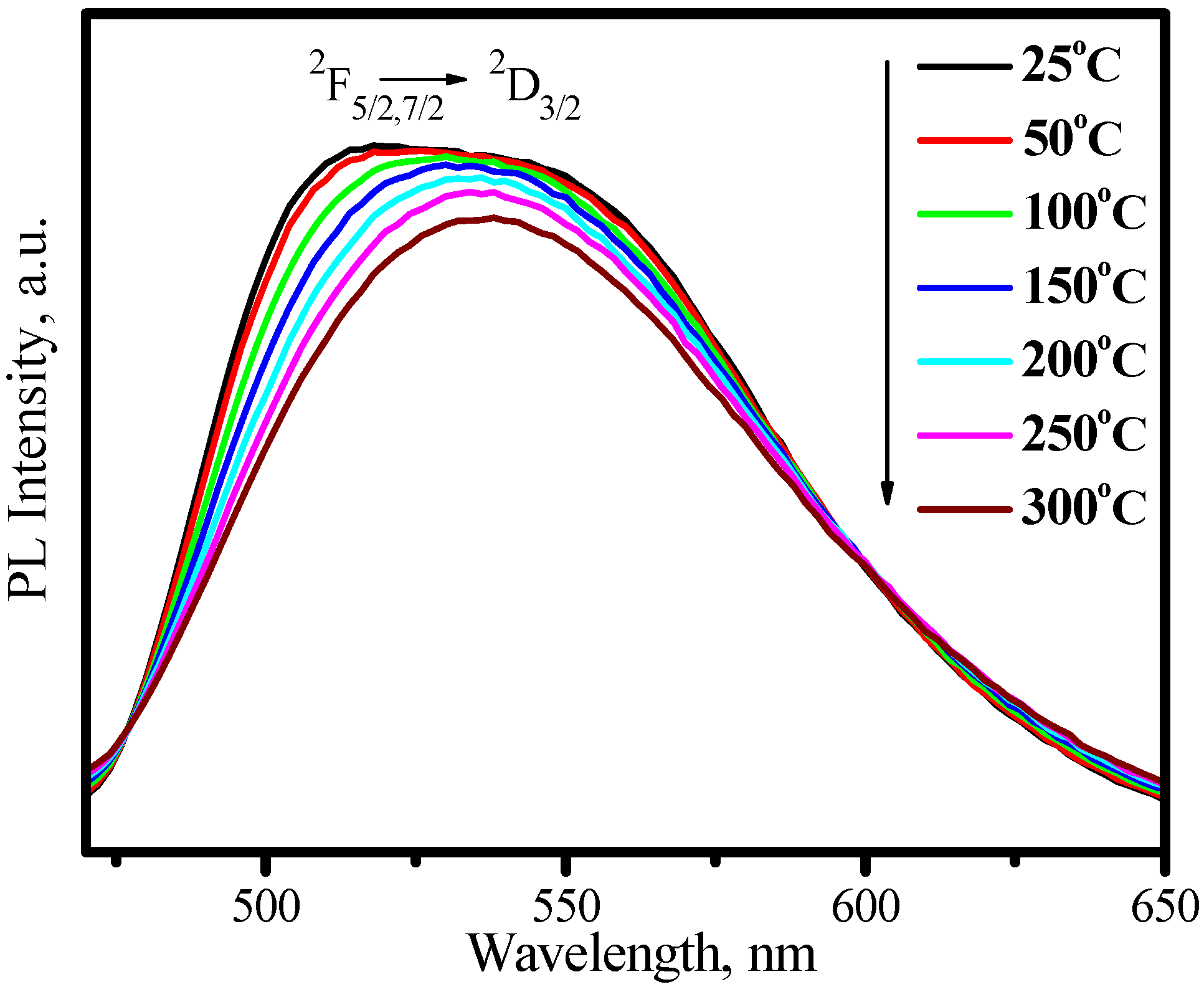
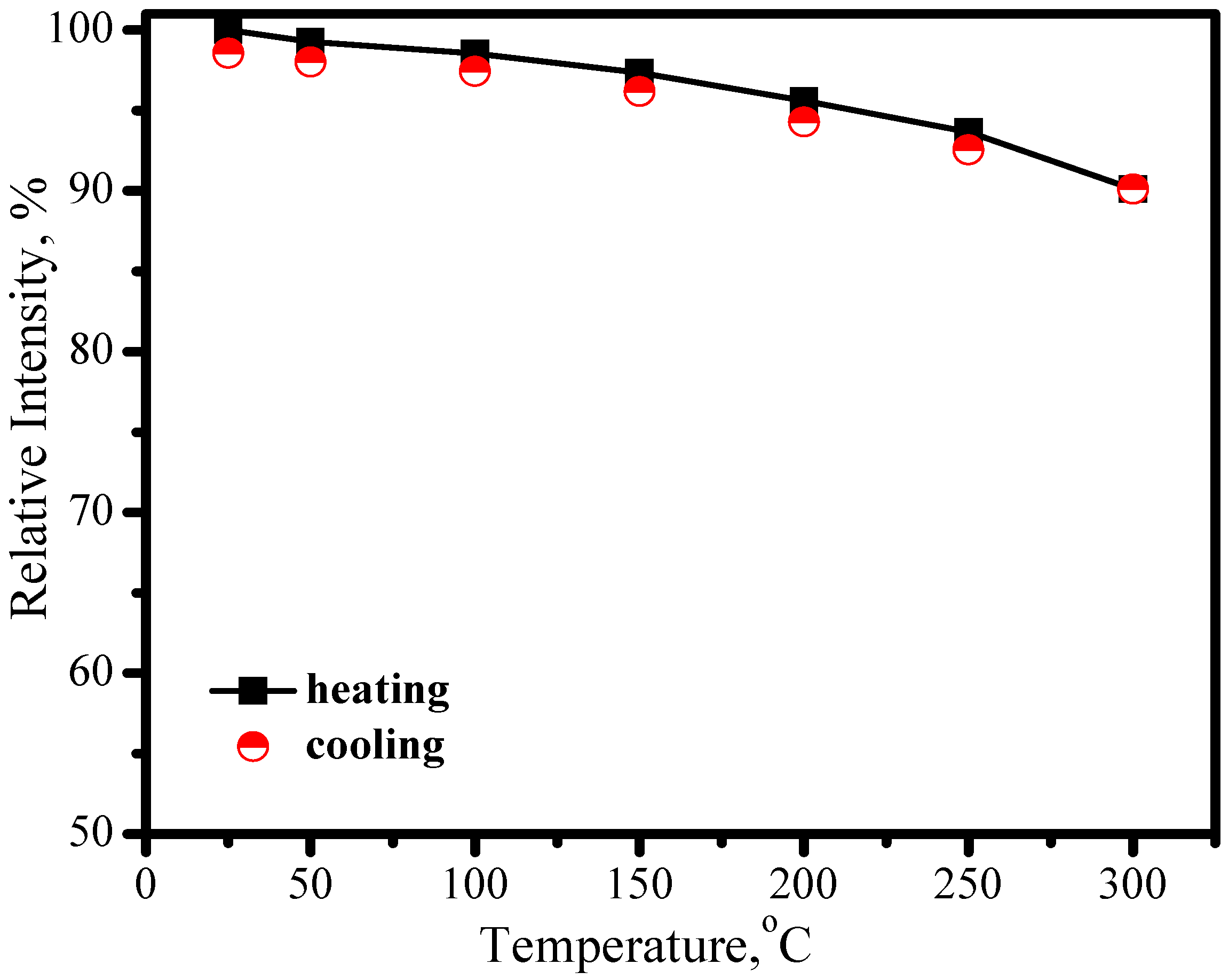
3.3. Red phosphors
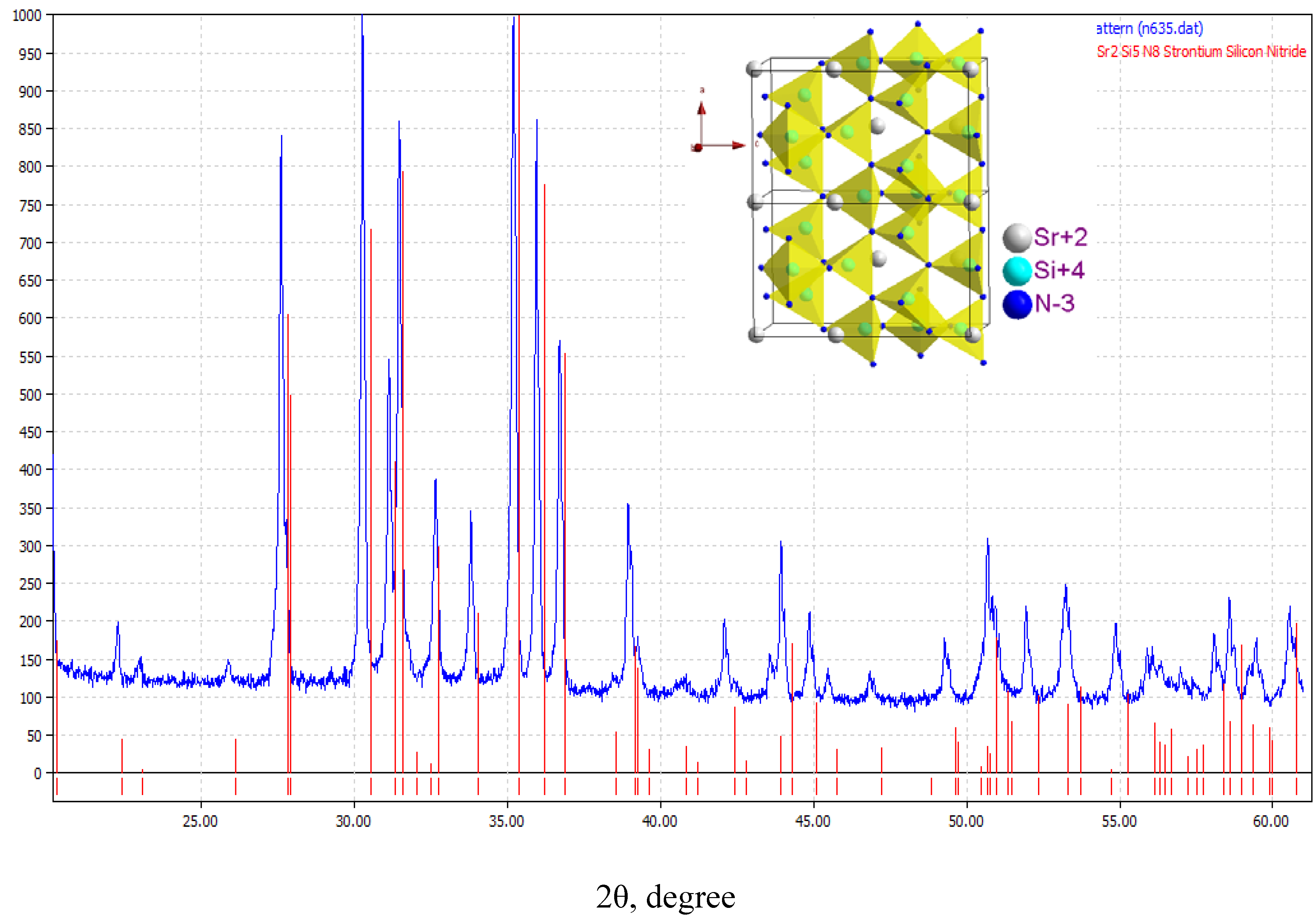
3.3.1. (Sr,Ba)2Si5N8:Eu2+
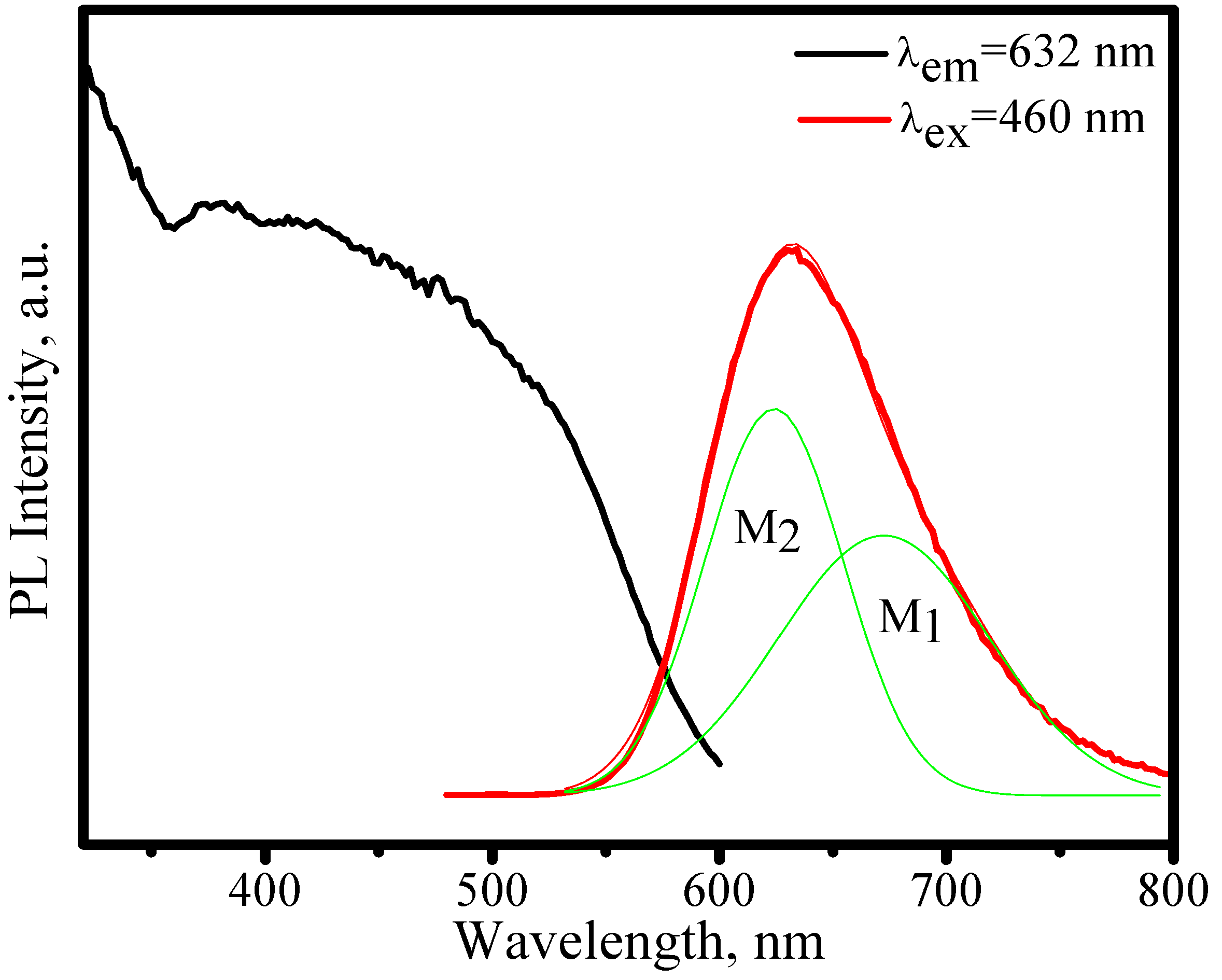
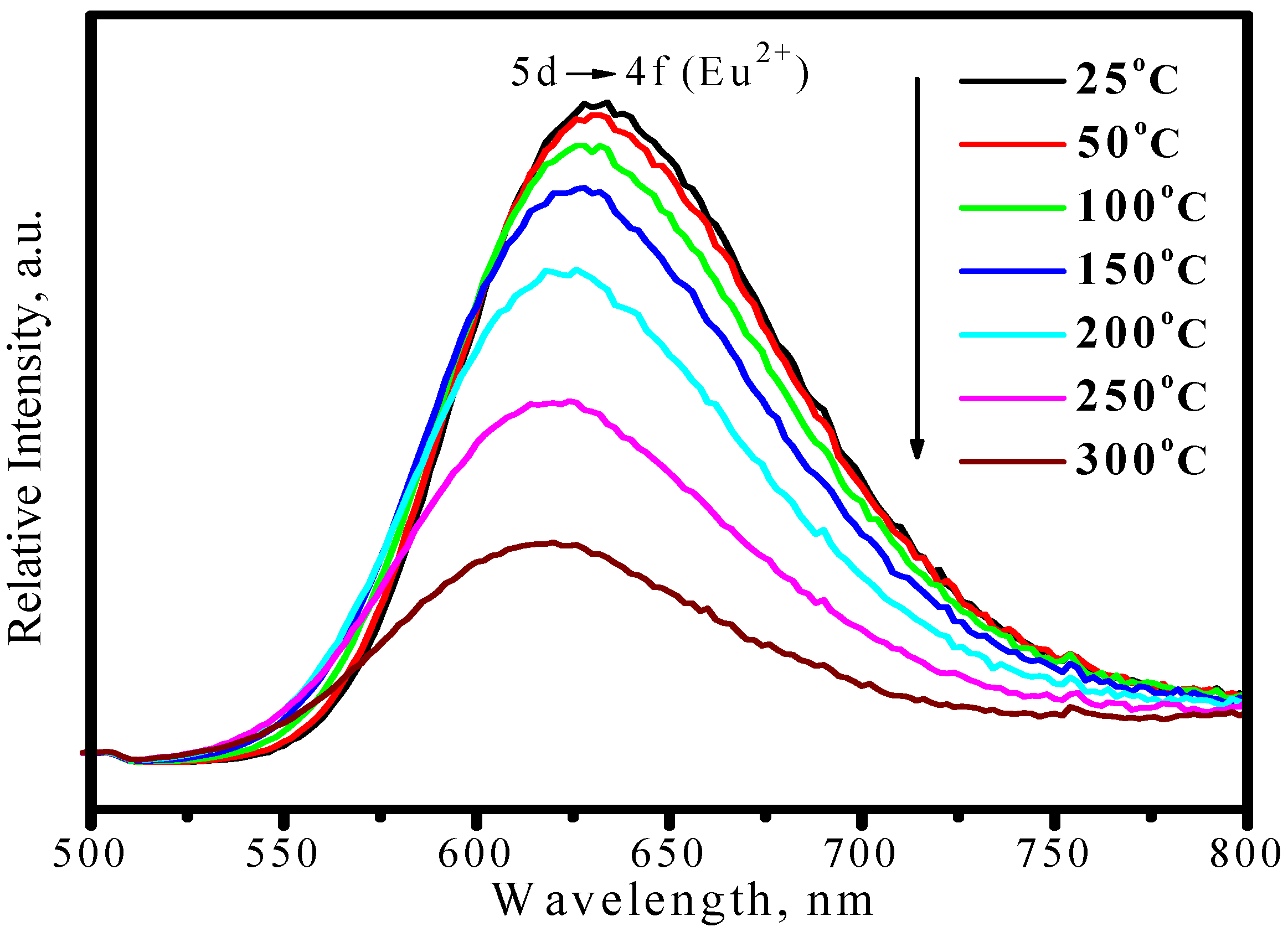
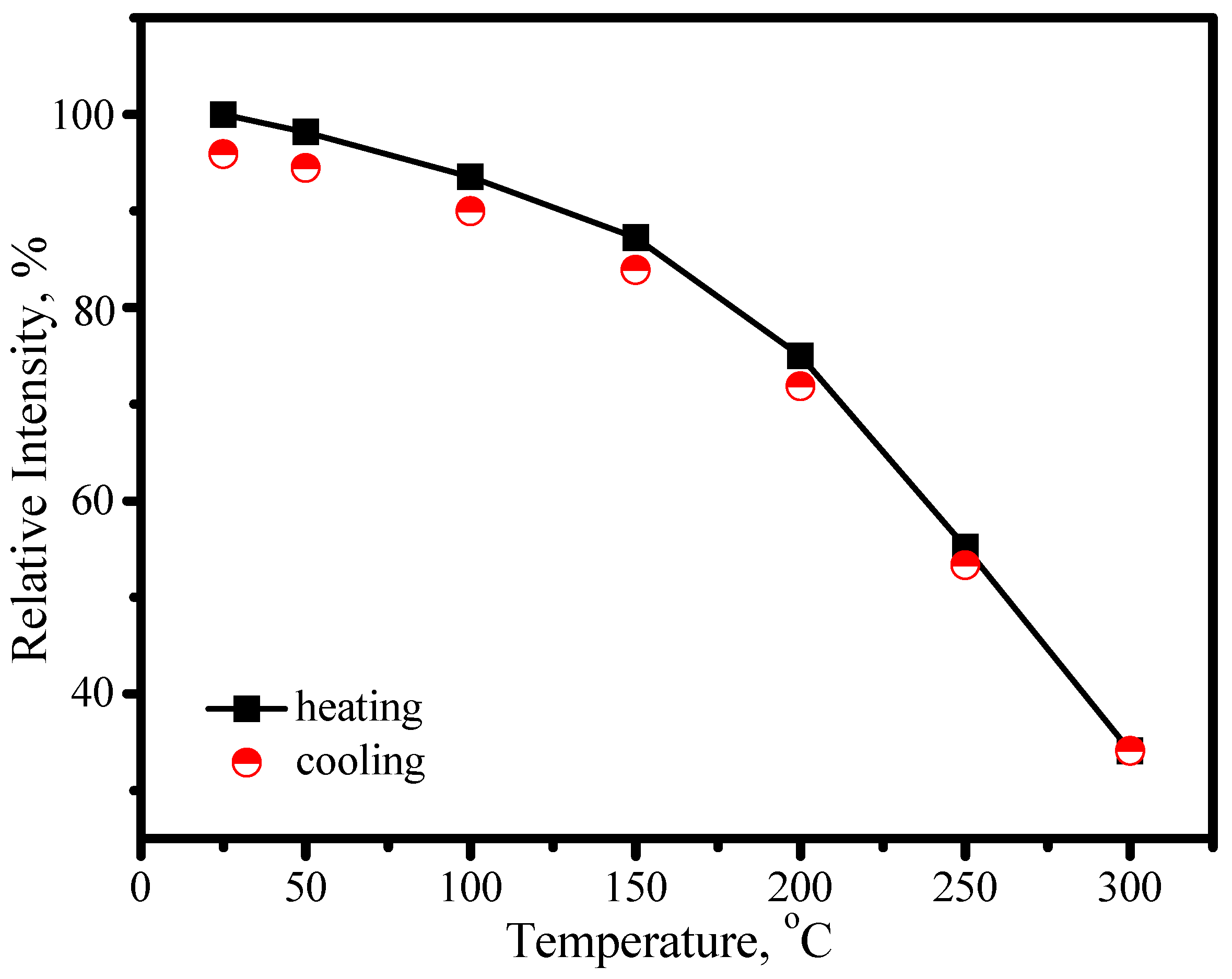
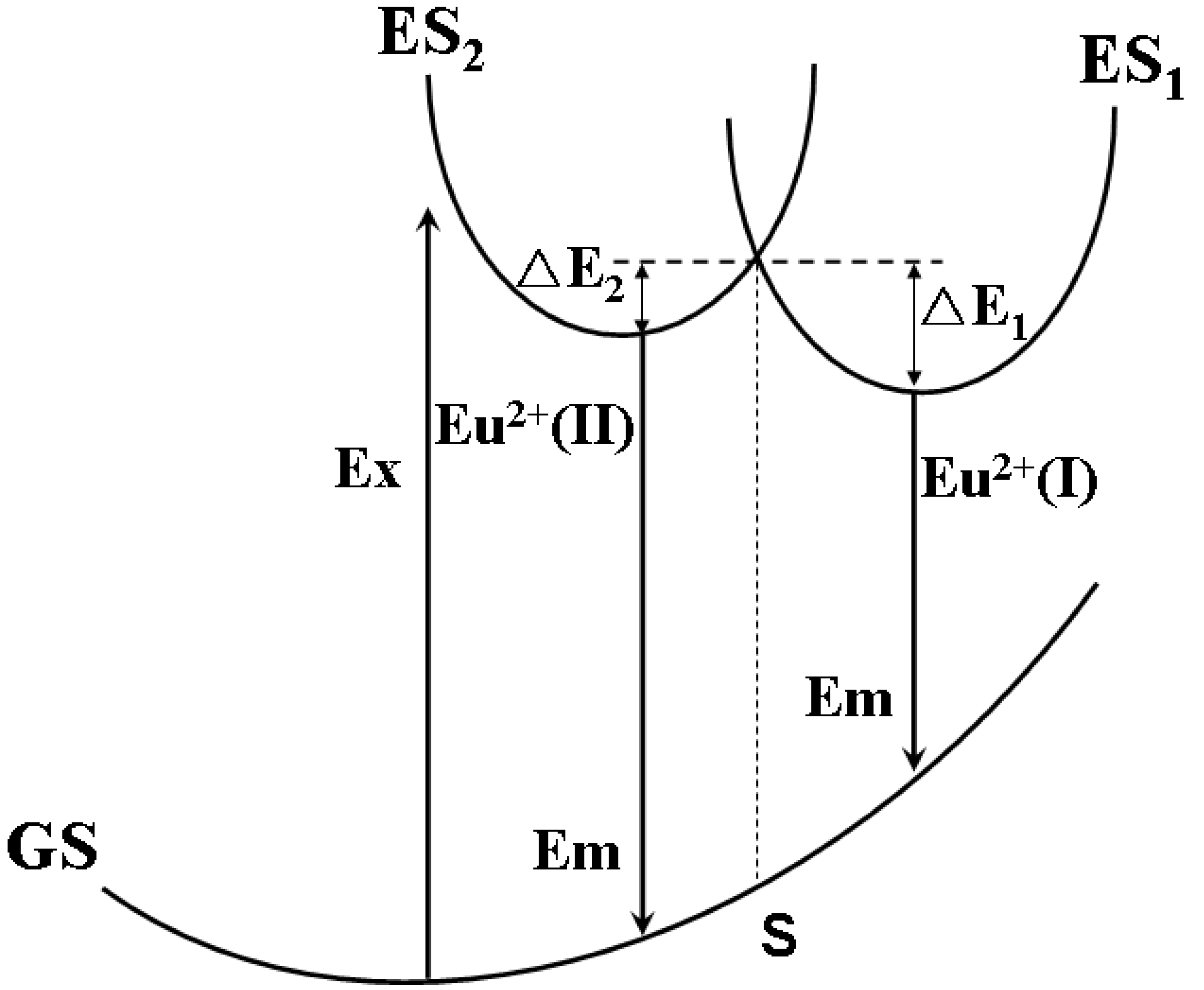
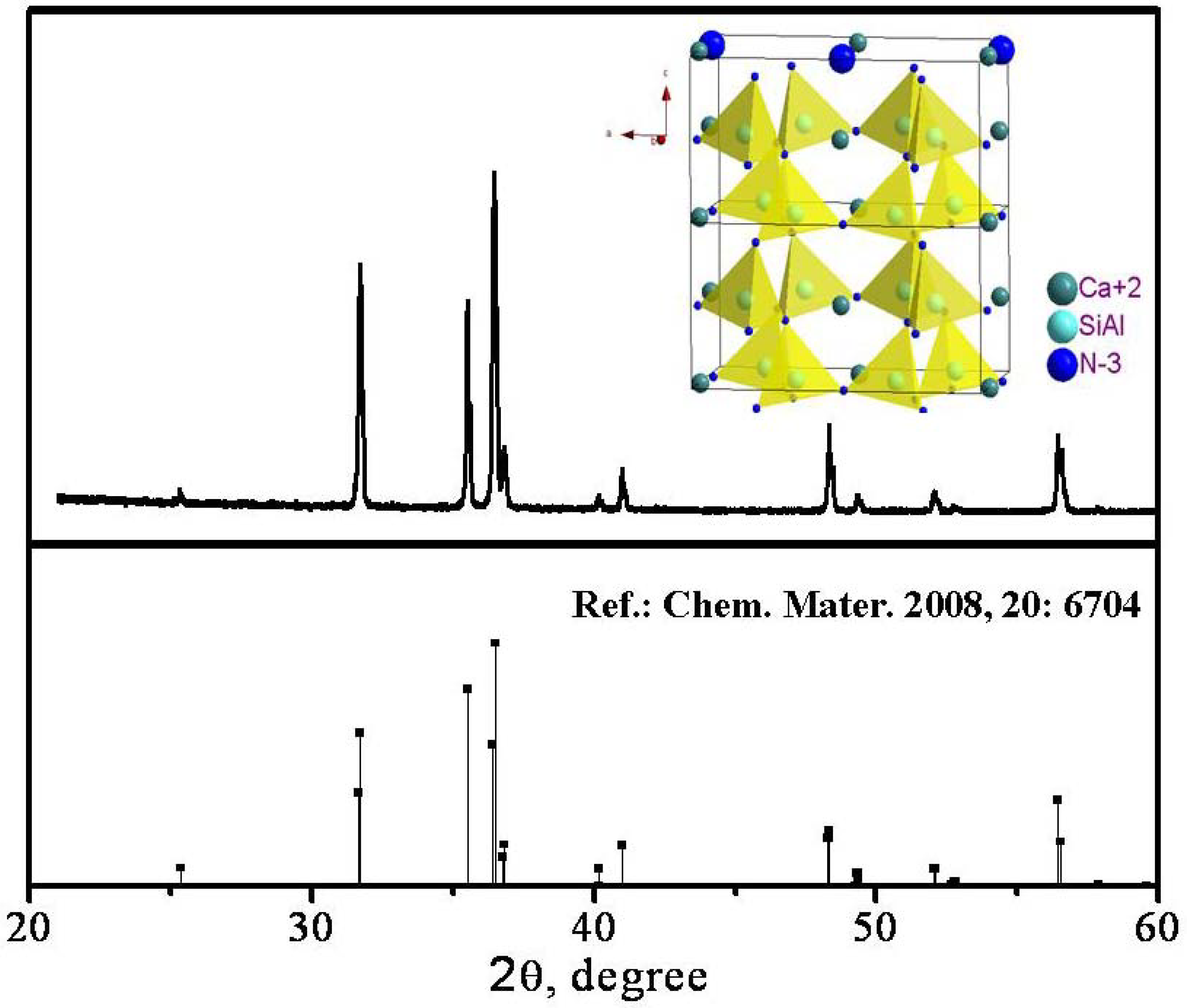
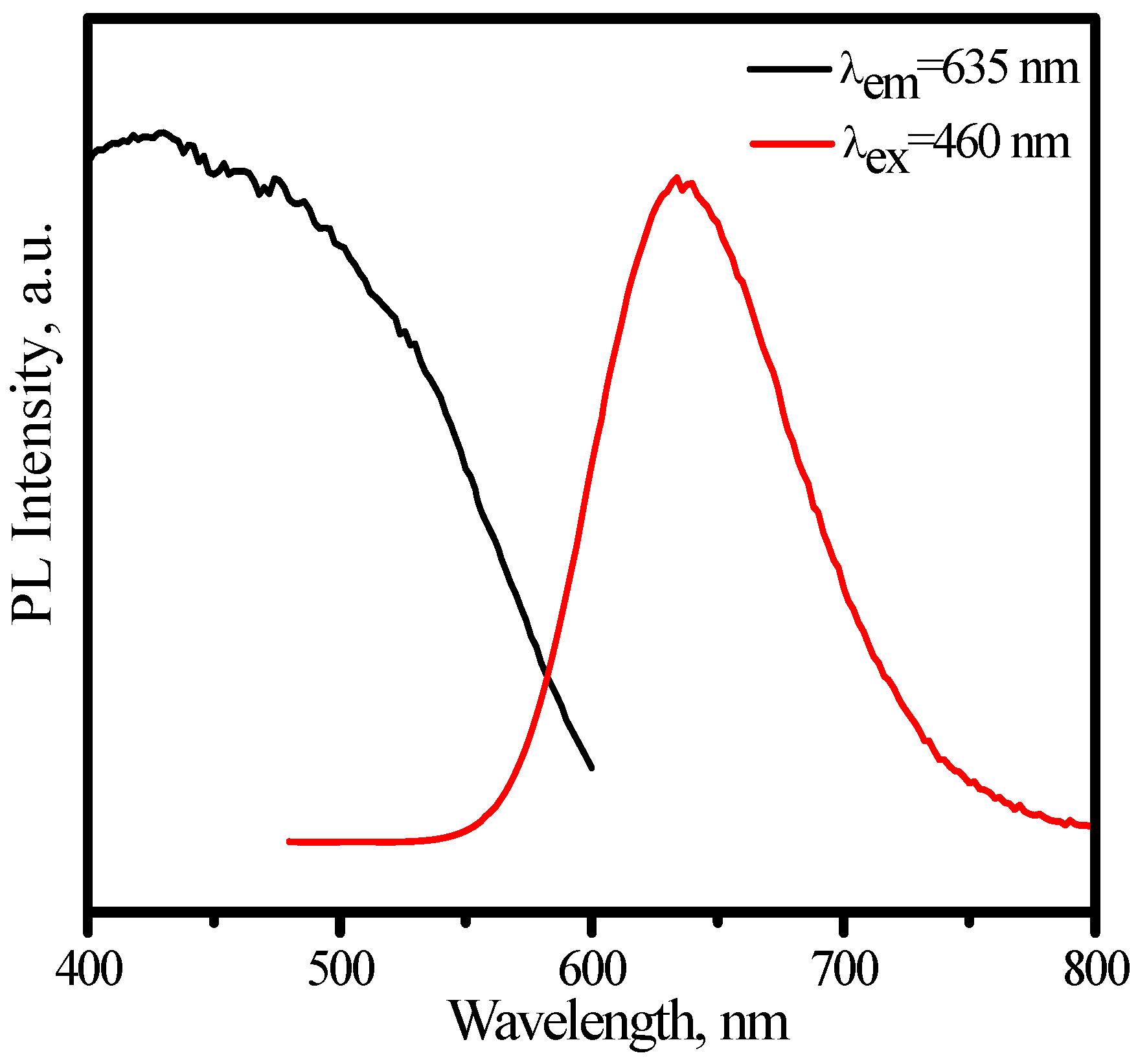
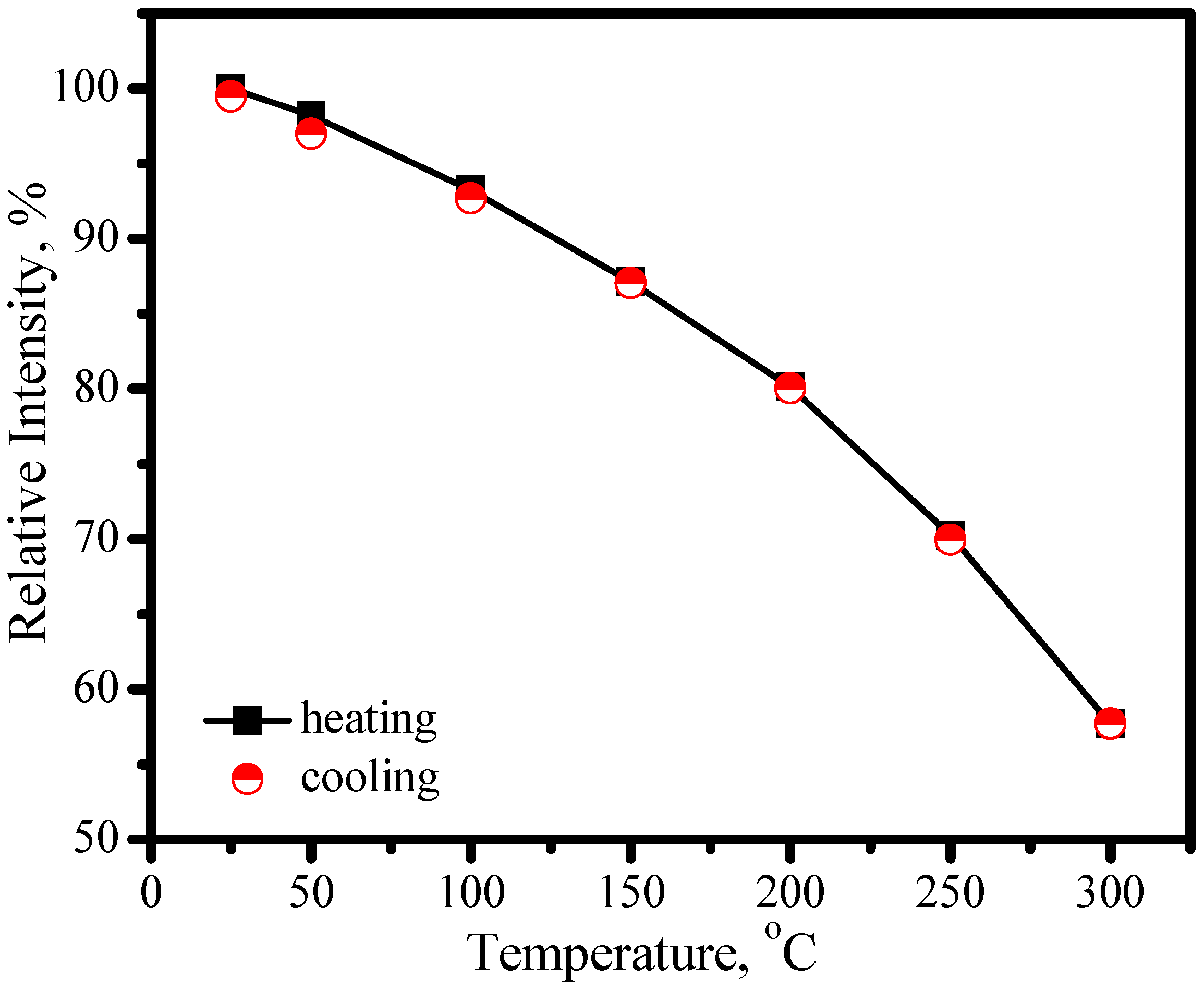
3.3.2. (Sr,Ca)SiAlN3:Eu2+

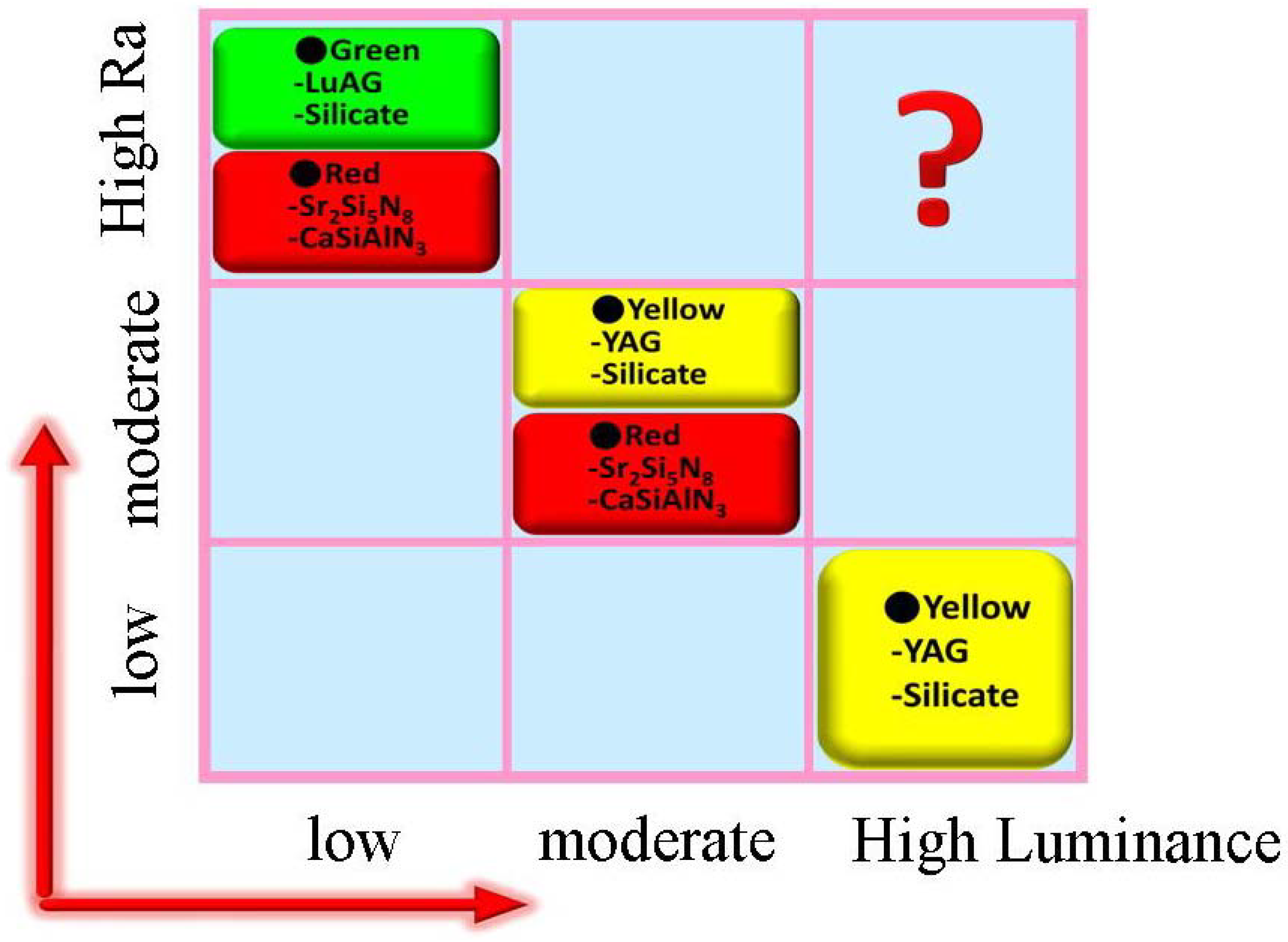
4. Conclusions
Acknowledgements
References and Notes
- Henini, M. The blue laser diode: The complete story (2nd and extended edition): S. Nakamura, S. Pearton, G. Fasol; Springer-Verlag Berlin Heidelberg New York, 368 pages, ISBN: 3-540-66505-6. Microelectr. J. 2001, 32, 177. [Google Scholar] [CrossRef]
- Kovac, J.; Peternai, L.; Lengyel, O. Advanced light emitting diodes structures for optoelectronic applications. Thin Solid Films 2003, 433, 22–26. [Google Scholar] [CrossRef]
- Nakamura, S.; Senoh, M.; Mukai, T. P-GaN/N-InGaN/N-GaN double-heterostructure blue-light-emitting diodes. Jpn. J. App. Phys. 1993, 32, L8–L11. [Google Scholar] [CrossRef]
- Nakamura, S. InGaN-based laser diodes (In Japanese). Nikkei Electron. 1994, 602, 93. [Google Scholar]
- Nakamura, S.; Mukai, T.; Senoh, M. Candela-class high-brightness InGaN/AlGaN double-heterostructure blue-light-emitting diodes. Appl. Phys. Lett. 1994, 64, 1687–1689. [Google Scholar] [CrossRef]
- Nakamura, S.; Fasol, G. The Blue Laser Diode: GaN Based Light Emitters and Lasers; Springer: Berlin, Germany, 1997; p. 216. [Google Scholar]
- Liu, J.; Lian, H.; Sun, J.; Shi, C. Characterization and properties of green emitting Ca3SiO4Cl2:Eu2+ powder phosphor for white light-emitting diodes. Chem. Lett. 2005, 34, 1340–1341. [Google Scholar] [CrossRef]
- Schubert, E.F.; Kim, J.K. Solid-state light sources getting smart. Science 2005, 308, 1274–11278. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.S.; Liu, Y.H.; Bagkar, N.C.; Hu, S.F. Enhanced luminescence of SrSi2O2N2:Eu2+ phosphors by codoping with Ce3+, Mn2+, and Dy3+ ions. Appl. Phys. Lett. 2007, 91, 061119. [Google Scholar] [CrossRef]
- Mancic, L.; Marinkovic, K.; Marinkovic, B.A.; Dramicanin, M.; Milosevic, O. YAG:Ce3+ nanostructured particles obtained via spray pyrolysis of polymeric precursor solution. J. Eur. Ceram. Soc. 2010, 30, 577–582. [Google Scholar] [CrossRef]
- Li, Y.X.; Min, Y.L.; Zhou, X.Z.; You, X.Z. Coating and stability of YAG:Ce phosphor synthesized using inorganic-organic hybrid gel method. Chin. J. Inorg. Chem. 2003, 19, 1169–1174. [Google Scholar]
- Lu, C.H.; Hong, H.C.; Jaganathan, R. Sol–gel synthesis and photoluminescent properties of cerium-ion doped yttrium aluminium garnet powders. J. Mater. Chem. 2002, 12, 2525–2530. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, J.; Yu, M.; Wang, S.B.; Zhang, H.J. Synthesis-dependent luminescence properties of Y3Al5O12:Re3+ (Re = Ce, Sm, Tb) phosphors. Mater. Lett. 2002, 56, 628–636. [Google Scholar] [CrossRef]
- Shi, S.K.; Wang, J.Y. Combustion synthesis of Eu3+ activated Y3Al5O12 phosphor nanoparticles. J. Alloys Compounds 2001, 327, 82–86. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. Generalized Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR 86−748; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994. [Google Scholar]
- Chiang, C.C.; Tsai, M.S.; Hon, M.H. Luminescent properties of cerium-activated garnet series phosphor: Structure and temperature effects. J. Electrochem. Soc. 2008, 155, B517–B520. [Google Scholar] [CrossRef]
- Jacobs, R.R.; Krupke, W.F.; Weber, M.F. Measurement of excited-state-absorption loss for Ce3+ in Y3Al5O12 and implications for tunable 5d→4f rare-earth lasers. Appl. Phys. Lett. 1978, 33, 410–412. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, G.; Xu, J.; Zhao, G.; Su, F.; Su, L.; Zhang, G.; Zhang, D.; Li, H.; Si, J. Luminescence studies of Ce:YAG using vacuum ultraviolet synchrotron radiation. Mater. Res. Bull. 2006, 41, 1959–1963. [Google Scholar] [CrossRef]
- Catti, M.; Gazzoni, G.; Ivaldi, G. Structures of twinned β-Sr2SiO4 and of α'-Sr1.9Ba0.1SiO4. Acta Crystallogr. C 1983, 39, 29–34. [Google Scholar] [CrossRef]
- Höppe, H.A.; Lutz, H.; Morys, P.; Schnick, W.; Seilmeier, A. Luminescence in Eu2+-doped Ba2Si5N8: Fluorescence, thermoluminescence, and upconversion. J. Phys. Chem. Solids 2000, 61, 2001–2006. [Google Scholar] [CrossRef]
- van Krevel, J.W.H.; Hintzen, H.T.; Metselaar, R.; Meijerink, A. Long wavelength Ce3+ emission in Y–Si–O–N materials. J. Alloys Compounds 1998, 268, 272–277. [Google Scholar] [CrossRef]
- van Krevel, J.W.H.; van Rutten, J.W.T.; Mandal, H.; Hintzen, H.T.; Metselaar, R. Luminescence properties of terbium-, cerium-, or europium-doped α-sialon materials. J. Solid State Chem. 2002, 165, 19–24. [Google Scholar] [CrossRef]
- Xie, R.J.; Mitomo, M.; Uheda, K.; Xu, F.F.; Akimune, Y. Preparation and luminescence spectra of Calcium- and Rare-Earth (R = Eu, Tb, and Pr)-codoped α-SiAlON ceramics. J. Am. Ceram. Soc. 2002, 85, 1229–1234. [Google Scholar] [CrossRef]
- Uheda, K.; Takizawa, H.; Endo, T.; Yamane, H.; Shimada, M.; Wang, C.M.; Mitomo, M. Synthesis and luminescent property of Eu3+-doped LaSi3N5 phosphor. J. Lumin. 2000, 87–89, 967–969. [Google Scholar] [CrossRef]
- Li, Y.Q.; van Steen, J.E.J.; van Krevel, J.W.H.; Botty, G.; Delsing, A.C.A.; DiSalvo, F.J.; de With, G.; Hintzen, H.T. Luminescence properties of red-emitting M2Si5N8:Eu2+ (M = Ca, Sr, Ba) LED conversion phosphors. J. Alloys Compd. 2006, 417, 273–279. [Google Scholar] [CrossRef]
- Huppertz, H.; Schenick, W. Eu2Si5N8 and EuYbSi4N7. The first nitridosilicates with a divalent rare earth metal. Acta Crystallogr. C 1997, 53, 1751–1753. [Google Scholar] [CrossRef]
- Duan, C.J.; Otten, W.M.; Delsing, A.C.A.; Hintzen, H.T. Preparation and photoluminescence properties of Mn2+-activated M2Si5N8 (M = Ca, Sr, Ba) phosphors. J. Solid State Chem. 2008, 181, 751–757. [Google Scholar] [CrossRef]
- Piao, X.; Horikawa, T.; Hanzawa, H.; Machida, K.I. Characterization and luminescence properties of Sr2Si5N8:Eu2+ phosphor for white light-emitting-diode illumination. Appl. Phys. Lett. 2006, 88, 161908. [Google Scholar]
- Sohn, K.S.; Lee, S.; Xie, R.J.; Hirosaki, N. Time-resolved photoluminescence analysis of two-peak emission behavior in Sr2Si5N8:Eu2+. Appl. Phys. Lett. 2009, 95, 121903. [Google Scholar] [CrossRef]
- Huang, P.; Cui, C.E; Wang, S. Synthesis and characterization of Sr3Al2O6:Eu2+, Dy3+ phosphors prepared by sol-gel-combustion processing. Chin. Phys. B 2009, 18, 4524. [Google Scholar] [CrossRef]
- Uheda, K.; Hirosaki, N.; Yamamoto, Y.; Naito, A.; Nakajima, T.; Yamamoto, H. Luminescence properties of a red phosphor, CaAlSiN3:Eu2+, for white light-emitting diodes. Electrochem. Solid-State Lett. 2006, 9, H22–H25. [Google Scholar] [CrossRef]
- Uheda, K.; Hirosaki, N.; Yamamoto, H. Host lattice materials in the system Ca3N2–AlN–Si3N4 for white light emitting diode. Phy. Status Solidi A 2006, 203, 2712–2717. [Google Scholar] [CrossRef]
- Li, Y.Q.; Hirosaki, N.; Xie, R.J.; Takeda, T.; Mitomo, M. Yellow-orange-emitting CaAlSiN3:Ce3+ phosphor: Structure, photoluminescence, and application in white LEDs. Chem. Mater. 2008, 20, 6704–6714. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, L.; Lin, C.-C.; Yeh, C.-W.; Liu, R.-S. Light Converting Inorganic Phosphors for White Light-Emitting Diodes. Materials 2010, 3, 2172-2195. https://doi.org/10.3390/ma3032172
Chen L, Lin C-C, Yeh C-W, Liu R-S. Light Converting Inorganic Phosphors for White Light-Emitting Diodes. Materials. 2010; 3(3):2172-2195. https://doi.org/10.3390/ma3032172
Chicago/Turabian StyleChen, Lei, Chun-Che Lin, Chiao-Wen Yeh, and Ru-Shi Liu. 2010. "Light Converting Inorganic Phosphors for White Light-Emitting Diodes" Materials 3, no. 3: 2172-2195. https://doi.org/10.3390/ma3032172




