2.1. Diffusion-controlled fabrication technique
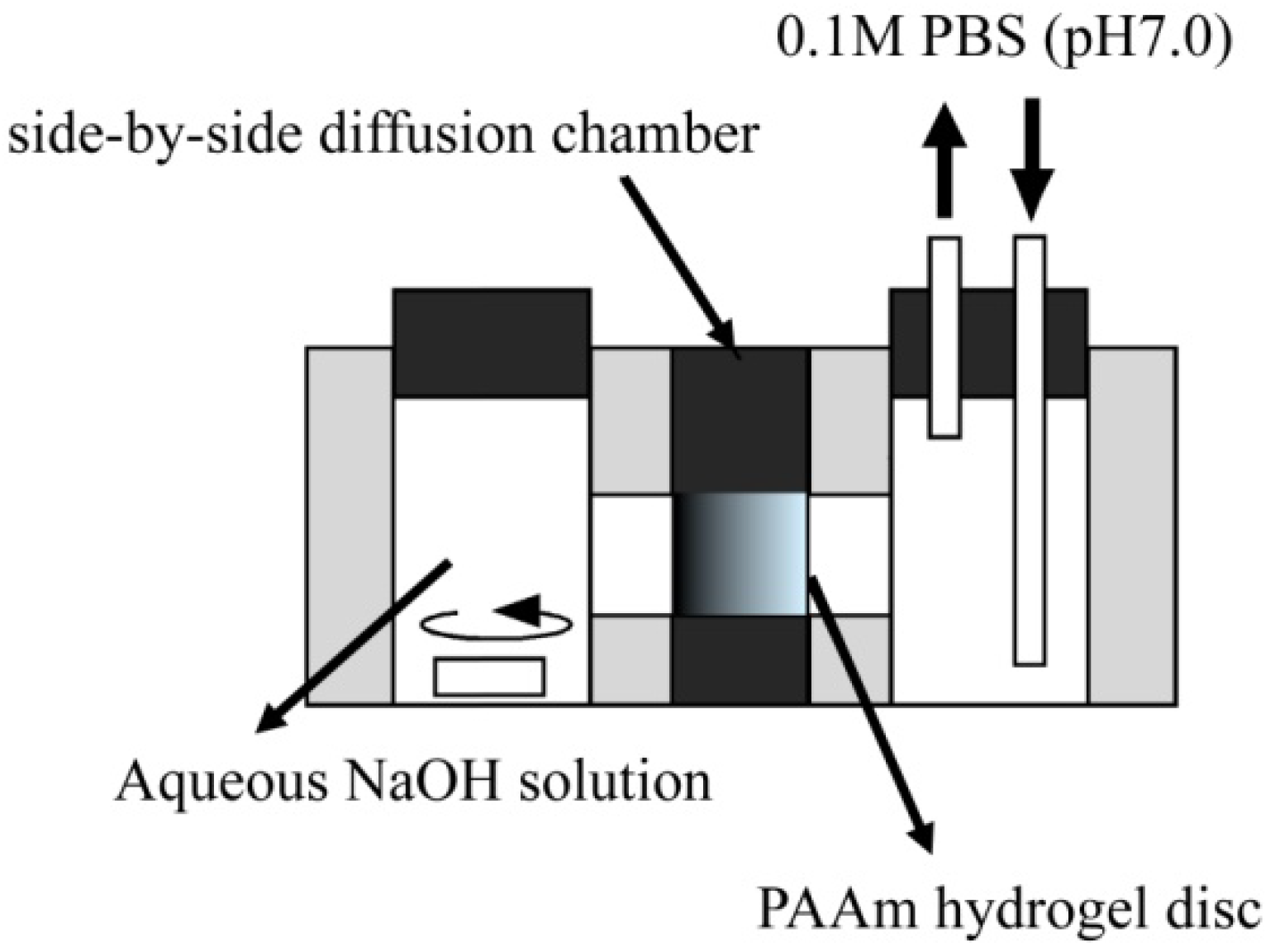
The diffusion-controlled fabrication technique could generate the three-dimensional gradients of functional groups in hydrogels. Carboxyl group gradients in PAAm hydrogel discs were generated by a diffusion-controlled hydrolysis of amide groups, which can be realized by the formation of a sodium hydroxide (NaOH) concentration gradient in the hydrogel (
Figure 1). In the side-by-side diffusion chamber, both the NaOH and 0.1 M phosphate-buffered solution (PBS, pH 7.0) diffuse across the hydrogel disc in opposite directions and are mixed in different ratios based on their local concentration in the hydrogel. The pH value of NaOH solutions after mixing with PBS decreased with an increase in the mixing volume ratio of PBS to NaOH, while the amount of carboxyl groups generated in the hydrogel disc depended on the pH value of mixtures (
Figure 2). This result suggests that the local concentration of NaOH in the hydrogel disc affected the hydrolysis extent of amide groups into carboxyl groups. Therefore, both the initial NaOH concentration and the reaction time for the hydrolysis of amide groups are key to control the concentration gradients of carboxyl groups.
Figure 1.
Schematic representation of a side-by-side diffusion chamber to generate a concentration gradient of carboxyl groups in PAAm hydrogels.
Figure 1.
Schematic representation of a side-by-side diffusion chamber to generate a concentration gradient of carboxyl groups in PAAm hydrogels.
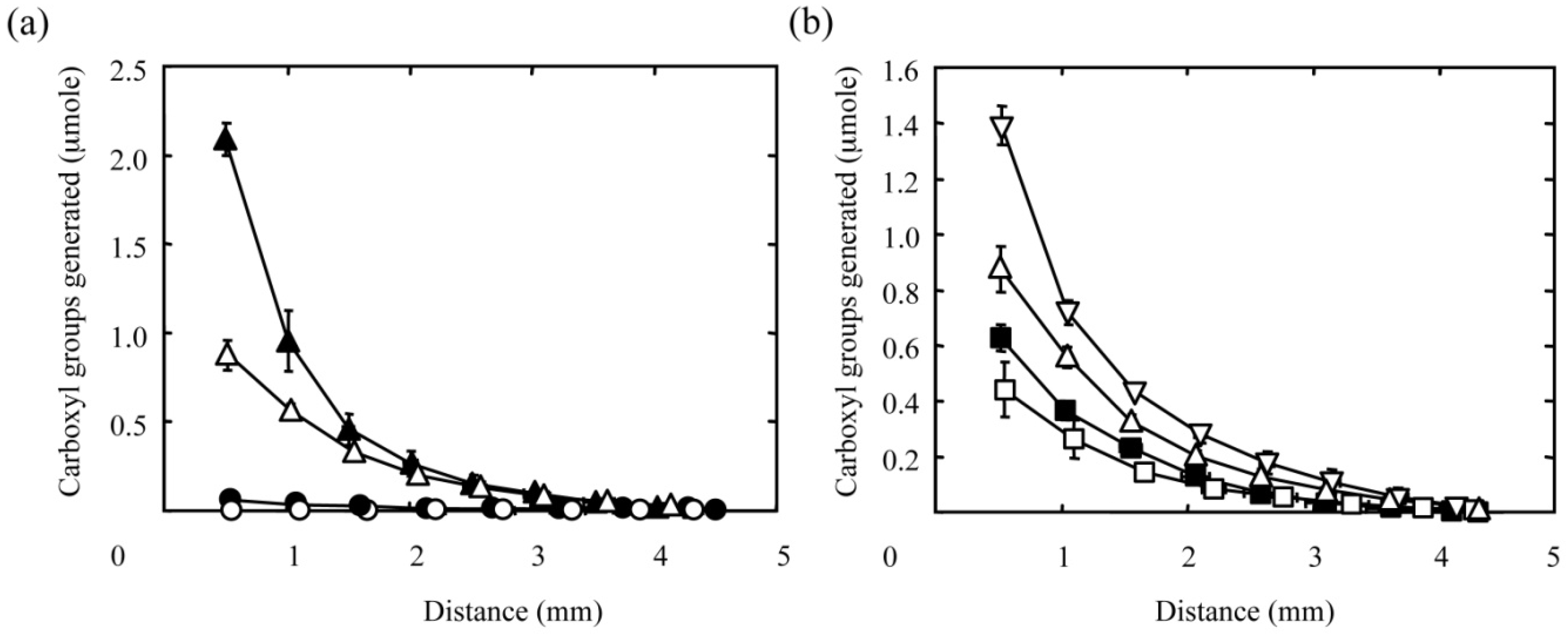
Figure 3 shows the concentration gradients of carboxyl groups generated in PAAm hydrogel discs by the diffusion-controlled hydrolysis of amide groups both with different NaOH concentrations (
Figure 3a) and reaction times (
Figure 3b). Irrespective of the reaction conditions, the amount of carboxyl groups generated decreased gradually with distance from the surface of hydrogel disc facing the NaOH solution. Every curve in
Figure 3a represents a gradient prepared by the hydrolysis reaction for 30 min. It is clear that the increasing concentration of NaOH solution resulted in larger gradients in terms of the amount of carboxyl groups generated in the hydrogel disc. Furthermore, the amount of carboxyl groups generated increased with reaction time (
Figure 3b). These results indicate that the diffusion-controlled hydrolysis under the different reaction conditions allows us to alter the concentration gradients of carboxyl groups in the hydrogel disc.
In general, the Fick’s law will be of considerable practical value in predicting the rate of molecular transports in a multi-component system. However, the profiles of carboxyl group gradients are totally different from that of local NaOH concentrations predicted by the Fick’s law. This is probably due to the hydrolysis reaction simultaneously occurring with the diffusion of NaOH in PAAm hydrogels. Other parameters, such as the acrylamide monomer concentration and crosslinking density of PAAm hydrogels, are considered to be important to modulate the diffusion-controlled hydrolysis. The diffusion chamber meets a technical difficulty in treating PAAm hydrogels with lower Young’s moduli prepared at lower monomer concentrations and crosslinking densities, and further technical development should be made.
Figure 2.
Generation of carboxyl groups in PAAm hydrogels in different concentrations of NaOH solution: (○) the amount of carboxyl groups generated in PAAm hydrogels and (●) the pH value of solutions at different concentrations of NaOH.
Figure 2.
Generation of carboxyl groups in PAAm hydrogels in different concentrations of NaOH solution: (○) the amount of carboxyl groups generated in PAAm hydrogels and (●) the pH value of solutions at different concentrations of NaOH.
Figure 3.
Effect of the NaOH concentration (a) and reaction time (b) on the amount of carboxyl groups generated in PAAm hydrogels by the hydrolysis of amide groups with NaOH solution. (a) The concentration of the NaOH solution is 0.001 (○), 0.01 (●), 0.1 ( △), or 1 M (▲). The reaction time is 30 min. (b) The reaction time is 10 (□), 20 (■), 30(△), or 60 min (▽). The concentration of the NaOH solution is 0.1 M. The X-axis indicates the distance from the surface of hydrogel facing the NaOH solution.
Figure 3.
Effect of the NaOH concentration (a) and reaction time (b) on the amount of carboxyl groups generated in PAAm hydrogels by the hydrolysis of amide groups with NaOH solution. (a) The concentration of the NaOH solution is 0.001 (○), 0.01 (●), 0.1 ( △), or 1 M (▲). The reaction time is 30 min. (b) The reaction time is 10 (□), 20 (■), 30(△), or 60 min (▽). The concentration of the NaOH solution is 0.1 M. The X-axis indicates the distance from the surface of hydrogel facing the NaOH solution.
2.2. Fabrication of hydrogels with a concentration gradient of type I collagen
The carboxyl groups generated in the longitudinal slice of PAAm hydrogel discs were chemically coupled with the amino groups of type I collagen by using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) to prepare the hydrogel slice with a concentration gradient of collagen immobilized. The type I collagen immobilized in the hydrogel slice was stained with Sircol dye reagent for further quantitative analysis.
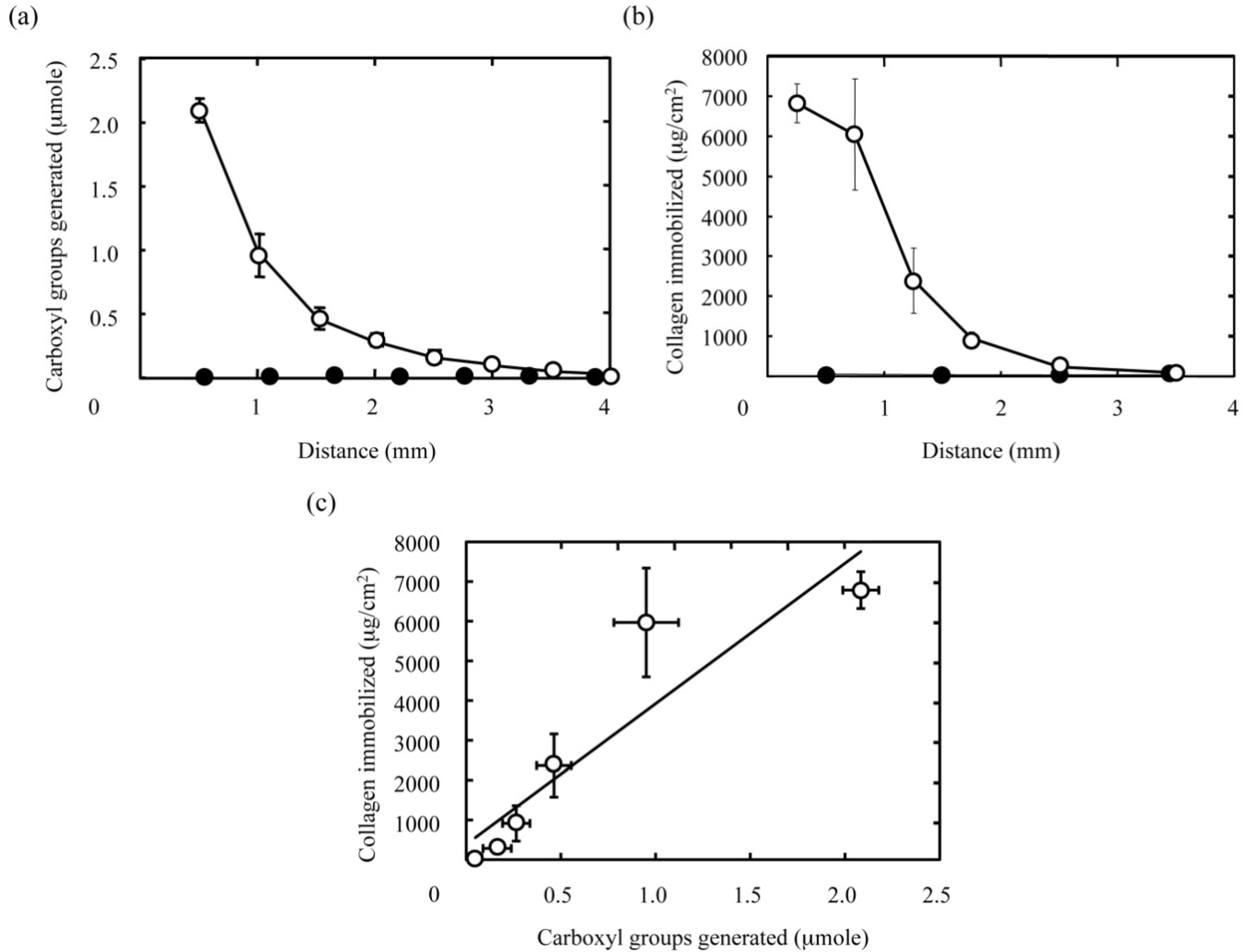
Figure 4 shows the concentration gradient of type I collagen immobilized in the hydrogel slice. As shown in
Figure 4b, the amount of type I collagen immobilized gradually decreased with distance from the surface of hydrogel disc facing the NaOH solution, while type I collagen was not detected in hydrogel discs without carboxyl groups. It is well known that the surface of PAAm-grafted materials exhibits poor protein adsorption [
18]. As a result, non-specific adsorption of type I collagen to the matrix of hydrogels was not observed as seen in
Figure 4b. In other words, we can say that the immobilization reaction is highly limited to the carboxyl groups generated (
Figure 4a). This high selectivity in immobilization leads to the well correlation between the amounts of the collagen immobilized and the carboxyl groups generated as seen in
Figure 4c. Taken together, these results strongly suggest that protein gradients could be introduced into hydrogels by altering the gradient of functional groups in a well-controlled manner.
2.3. Cell responses to hydrogel slices with a concentration gradient of type I collagen
Cell responses to hydrogel slices with a concentration gradient of type I collagen were investigated with L929 mouse fibroblasts. As shown in
Figure 5a, it was found that the cell density significantly decreased with distance from the surface of hydrogel discs facing the NaOH solution. More cells adhered as the type I collagen amount increased (
Figure 5d). Similar to the cell density, the morphology of cells adhered varies with the amount of type I collagen immobilized. The morphology of cells adhered became more spreading with a spindle shape (
Figure 5b). In addition, the cell ellipticity, which was defined as the major to minor axis ratio, was calculated by measuring the length of those axes for the cells attached (
Figure 5c). Significantly higher cell ellipticity was found in the site (1) with the highest amount of type I collagen immobilized (
Figure 5e).
Tamada
et al. have reported that the collagen-immobilized surface facilitates the adhesion, proliferation, and collagen synthesis of fibroblasts
in vitro within three days [
19], suggesting that fibroblasts can secrete their extracellular matrices to create their own microenvironment when they are sitting on a substrate suitable for their phenotype expression. On the basis of this finding, it is conceivable that the initial cell behavior directly influenced by the type I collagen gradients on a substrate could play an important role to determine the following cell fate.
Recently, some researches have demonstrated that two-dimensional concentration gradients of biomolecules affect the alignment and migration of cells
in vitro [
5,
6,
7,
8,
9,
10,
11,
12,
13,
15,
16,
17,
20]. Smith
et al. showed that the drift speed for bovine aortic endothelial cells is faster on the fibronectin gradient substrate than that on the fibronectin constant substrate [
20]. A concentration gradient of Arg-Gly-Asp-Ser (RGDS) peptide on polyethyleneglycol-based hydrogel facilitates fibroblasts alignment along the RGDS-gradient axis and fibroblasts migration in comparison with a constant concentration of the peptide [
16]. Bastmeyer
et al. created ephrinA5 gradients to study the effect of the slope and local concentration of gradients on the growth cone navigation [
7]. However, in this study, we did not observe both the alignment and migration of fibroblasts on the hydrogel slice with a concentration gradient of type I collagen, although the attachment and morphology of fibroblasts were strongly influenced by the collagen gradient.
Figure 4.
The immobilization pattern of type I collagen in PAAm hydrogels with a concentration gradient of carboxyl groups. (a) The concentration gradient of carboxyl groups in PAAm hydrogels generated by the hydrolysis for 30 min with 1 M NaOH (○) and NaOH-free solutions (●). The X-axis indicates the distance from the surface of hydrogel facing the NaOH solution. (b) The amount of collagen immobilized in the corresponding hydrogels to (a). (c) The relationship between the amounts of carboxyl groups generated and collagen immobilized in PAAm hydrogels. The coefficient of determination, R2 value for the least mean square fitting is 0.842.
Figure 4.
The immobilization pattern of type I collagen in PAAm hydrogels with a concentration gradient of carboxyl groups. (a) The concentration gradient of carboxyl groups in PAAm hydrogels generated by the hydrolysis for 30 min with 1 M NaOH (○) and NaOH-free solutions (●). The X-axis indicates the distance from the surface of hydrogel facing the NaOH solution. (b) The amount of collagen immobilized in the corresponding hydrogels to (a). (c) The relationship between the amounts of carboxyl groups generated and collagen immobilized in PAAm hydrogels. The coefficient of determination, R2 value for the least mean square fitting is 0.842.
Pelham
et al. have shown that the cell locomotion and foal adhesions are regulated by the stiffness of PAAm hydrogels [
21]. However, unfortunately, we do not evaluate a variation of the substrate stiffness across the hydrogel. In this study, we used PAAm hydrogels with a homogeneous crosslinking, where there is no variation of the substrate stiffness before the treatments. It is possible that the introduction of carboxyl groups in the hydrogel by the hydrolysis reaction may modify both the swelling and the stiffness of the hydrogel by intermolecular repulsion between the carboxyl groups introduced, while this effect could be shielded by the immobilization of type I collagen. Consistent with the shielding effect, we found that the shape of the hydrogel used in the cell culture experiments is not trapezoidal but rectangle (data not shown). In addition, fibroblasts attach well on collagen-immobilized substrates, while poor fibroblasts adhesion was observed for PAAm-grafted substrates as Tamada
et al. reported [
19]. Taken together, we can say with certainly that the attachment and morphology of fibroblasts were strongly influenced by the type I collagen gradient rather than the variation of the substrate stiffness across the hydrogel.
2.4. Advantages of the developed technique
Recent advancement of lithographic and micro-manipulation technologies and methodologies allows us to create surface-bound gradient structures with a high resolution, a complex pattern, and a well-designed physicochemical property in two-dimension. The gradient structure can function as a high-throughput screening method to facilitate both the fast screening of physicochemical phenomena and cell behaviors. However, there are some technical limitations on the lithographic and micromanipulation technologies and methodologies to generate three-dimensional gradients even with complex and special equipments. It is therefore clear that an easy and simple technology to form protein gradients in three-dimensional cell scaffolds is greatly required for tissue engineering applications.
Fabrication of three-dimensional protein gradients has emerged in the research field of chemotaxis as an
in vitro technique to investigate directed cell migration by soluble biomolecules. The Boyden chamber [
22] has been employed to study chemotaxis assays, although the gradients generated by the chamber were unstable and hence could not be used for extended time periods. Tissue engineering applications require stabilized multiple gradients of immobilized factors in three-dimensional scaffolds. Immobilized protein gradients can mimic the spatial regulation indispensable for the process of tissue regeneration. To create a three-dimensional gradient of proteins, assembling different microspheres containing proteins with a gradient mixing ratio [
14] and mixing different solutions by using a gradient maker normally used to make polyacrylamide gels for electrophoresis [
15,
16,
17], have been investigated. However, there still remain the complex processes in those methods, such as the fabrication of microspheres containing proteins and the synthesis of prepolymers conjugating proteins.
On the contrary, our diffusion-controlled fabrication technique is an easy and simple method based on a side-by-side diffusion chamber, which is applicable to several material types, such as hydrogels, sponges, and non-woven fabrics. We have already succeeded in fabricating sponges and non-woven fabrics with a concentration gradient of both the RGDS peptide and the growth factors, respectively (our unpublished data). In addition, by changing reaction conditions, it is practically possible to alter the profile of protein gradients. A technique similar to our method has been developed to generate immobilization gradients of enzymes in a porous three-dimensional silk fibroin scaffold using the principles of diffusion [
23]. Taken together with our data, the diffusion-controlled fabrication technique could be extended to immobilize a variety of proteins and small molecules in several types of porous materials, thereby offering new options in the fields of chemotaxis and tissue engineering.
Figure 5.
The attachment and morphology of L929 cells on PAAm hydrogels with a concentration gradient of type I collagen. (a) Effect of the concentration gradient of collagen immobilized in the PAAm hydrogel on the cell density. * p < 0.05; significance against the cell density of the plot (2) in the figure. ** p < 0.05; significance against the cell density of the plot (3) in the figure. (b) Phase contrast microscopic pictures of cells attached at the sites which correspond to the plots (1), (2), and (3) in
Figure 5a. Bars correspond to 100 μm. (c) Effect of the concentration gradient of collagen immobilized in the PAAm hydrogel on the cell ellipticity calculated by image analyses for the microscopic pictures of cells (
Figure 5b). * p < 0.05; significance against the cell ellipticity at the site of 2 mm and longer in distance from the surface of hydrogel facing the NaOH solution. (d) Effect of the amount of type I collagen immobilized in the PAAm hydrogel on the cell density. (e) Effect of the amount of type I collagen immobilized in the PAAm hydrogel on the cell ellipticity.
Figure 5.
The attachment and morphology of L929 cells on PAAm hydrogels with a concentration gradient of type I collagen. (a) Effect of the concentration gradient of collagen immobilized in the PAAm hydrogel on the cell density. * p < 0.05; significance against the cell density of the plot (2) in the figure. ** p < 0.05; significance against the cell density of the plot (3) in the figure. (b) Phase contrast microscopic pictures of cells attached at the sites which correspond to the plots (1), (2), and (3) in
Figure 5a. Bars correspond to 100 μm. (c) Effect of the concentration gradient of collagen immobilized in the PAAm hydrogel on the cell ellipticity calculated by image analyses for the microscopic pictures of cells (
Figure 5b). * p < 0.05; significance against the cell ellipticity at the site of 2 mm and longer in distance from the surface of hydrogel facing the NaOH solution. (d) Effect of the amount of type I collagen immobilized in the PAAm hydrogel on the cell density. (e) Effect of the amount of type I collagen immobilized in the PAAm hydrogel on the cell ellipticity.
![Materials 03 02393 g005]()