Hydrophobic Modification of Layered Clays and Compatibility for Epoxy Nanocomposites
Abstract
:1. Introduction
2. Organic Modification of Layered Clays
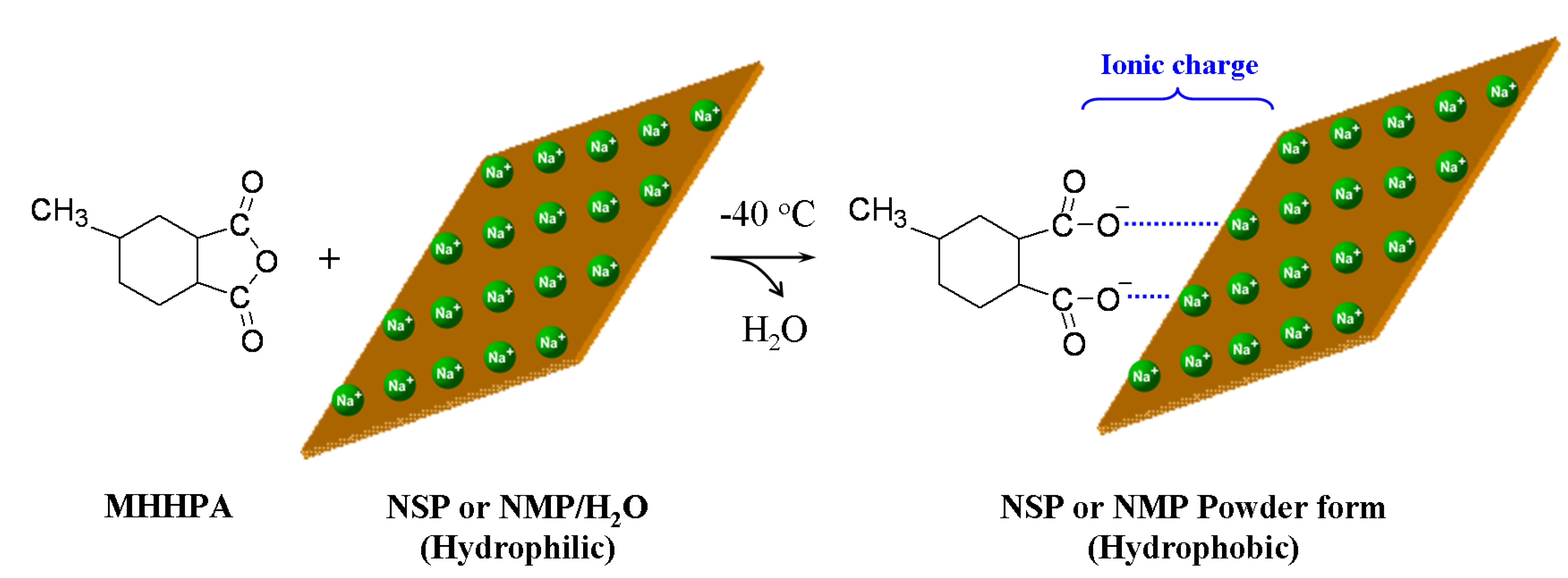
2.1. Modification of cationic sodium MMT and synthetic fluorinated Mica
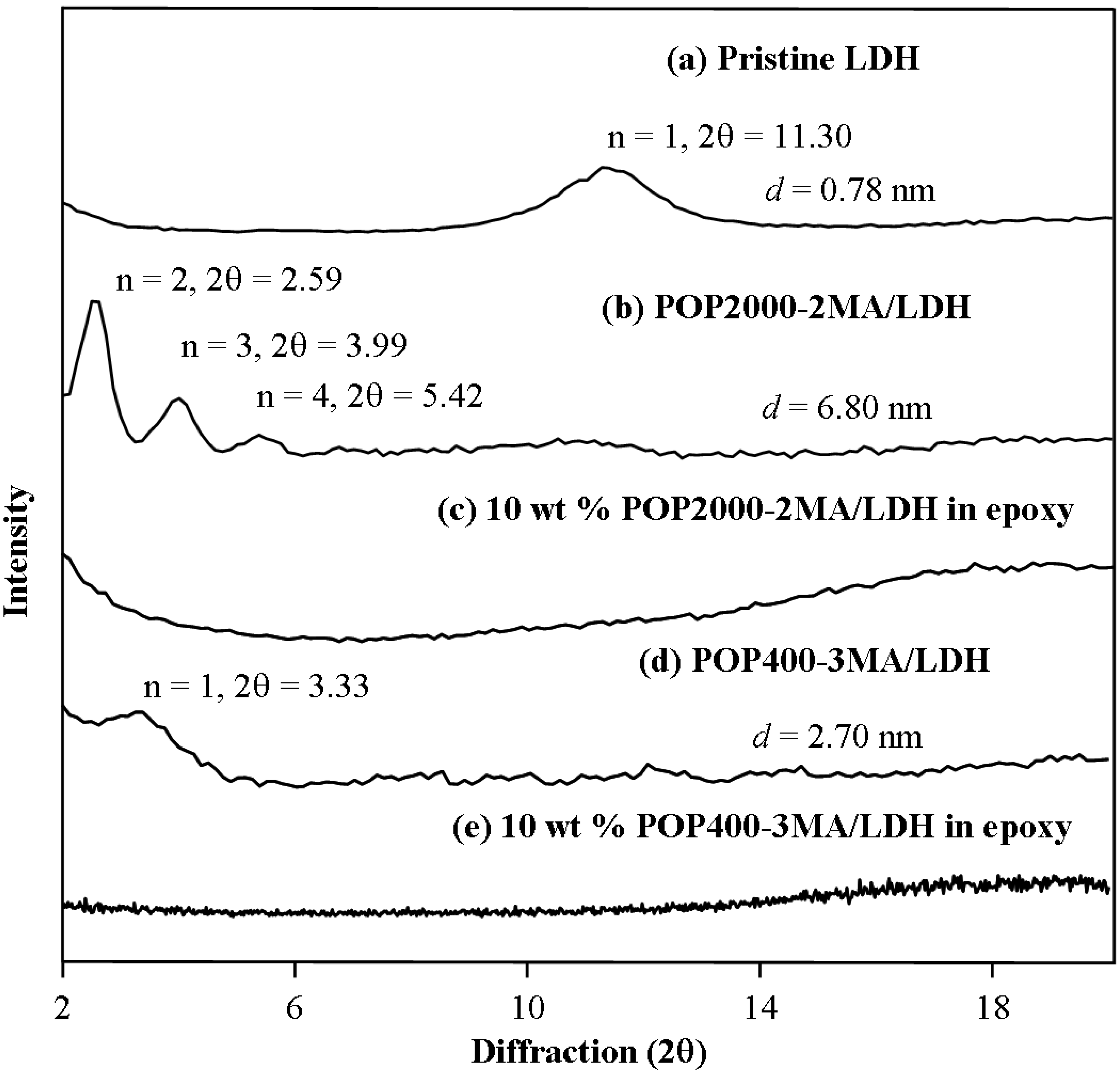
2.2. Modification of anionic Al-Mg LDH
2.3. Exfoliation of MMT and Mica with multifunctional amine copolymers
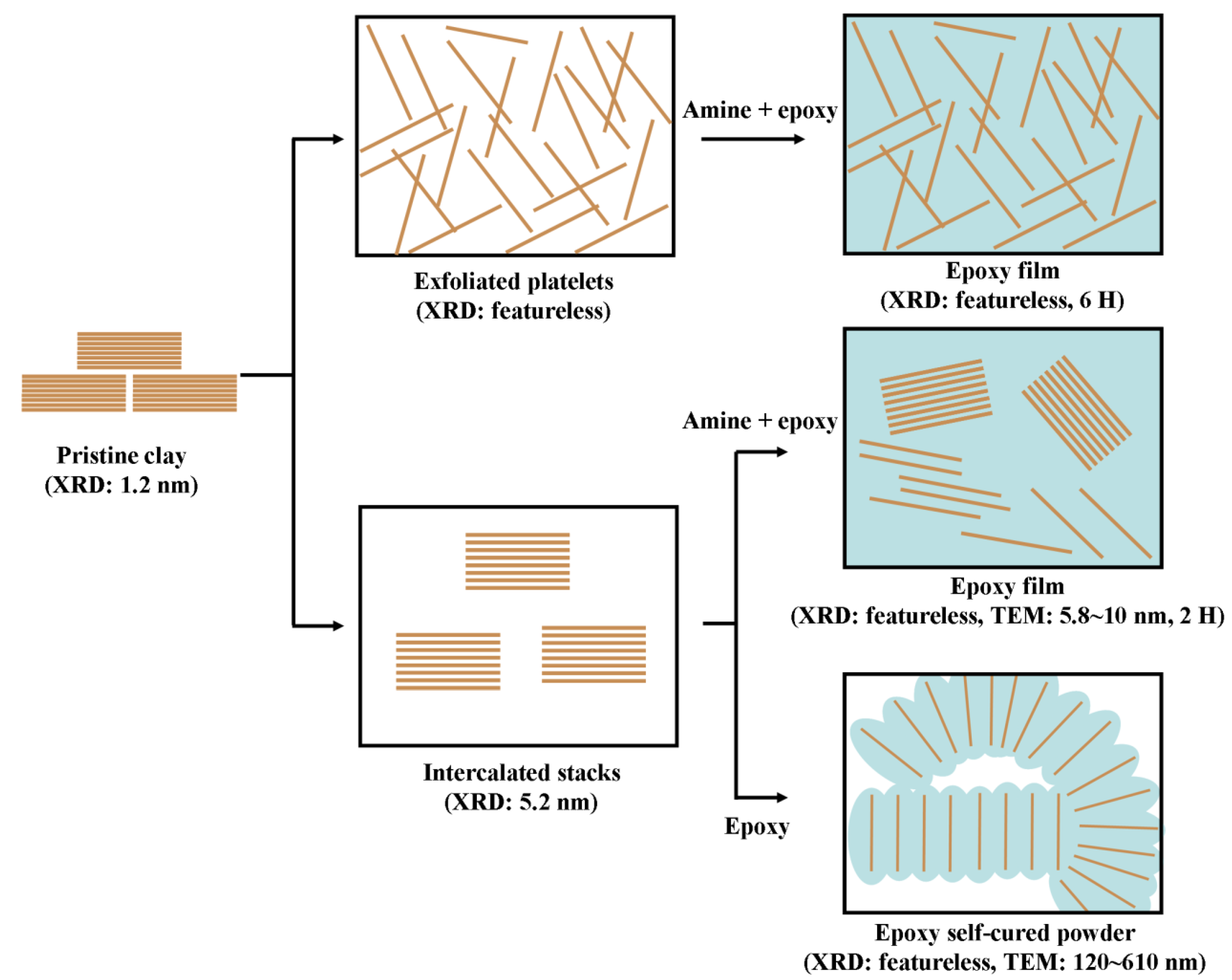
3. Layered Clays for Epoxy Nanocomposites
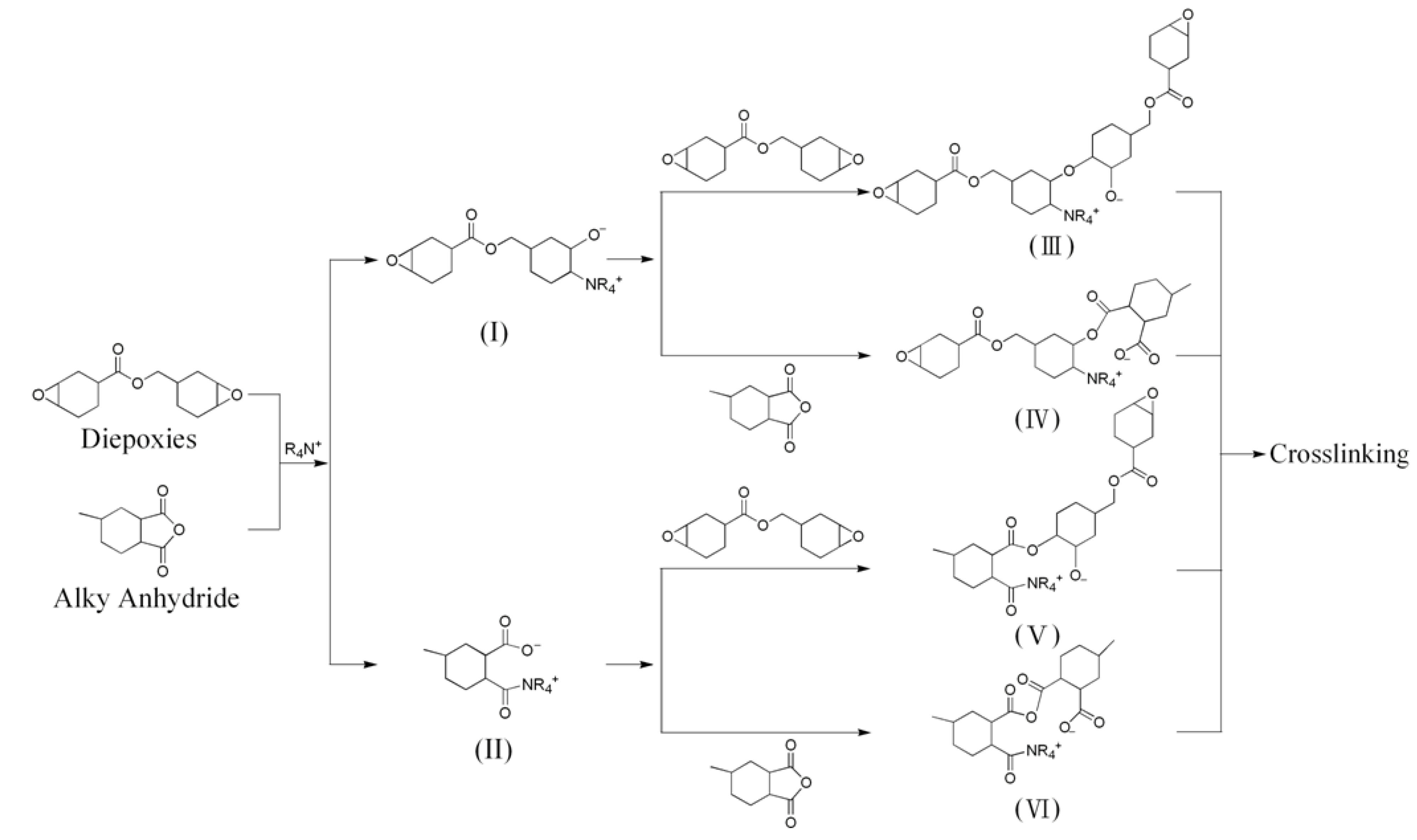
3.1. Intercalated and exfoliated MMT clays for epoxies
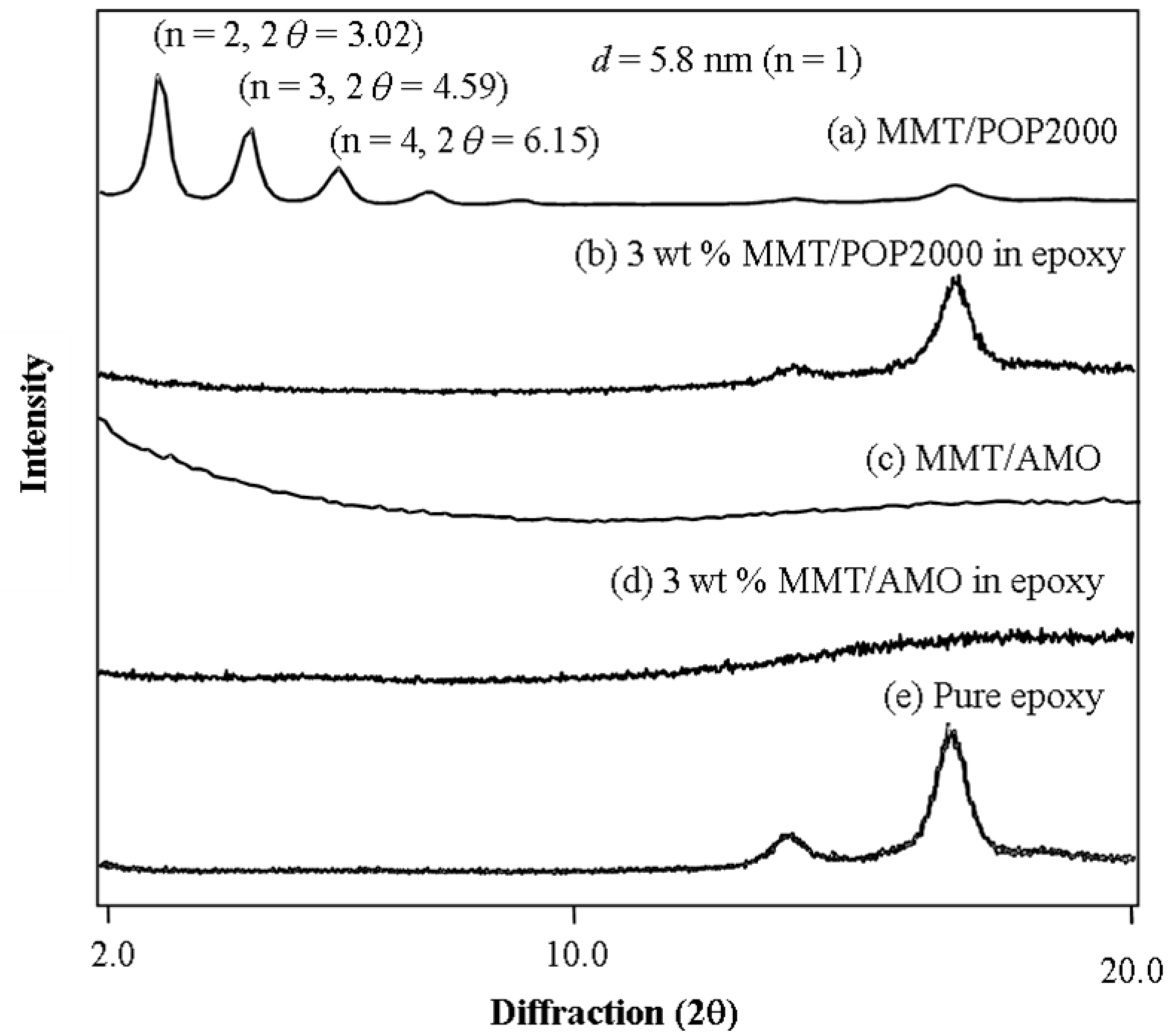
| Organoclay | MMT content (wt %) a | Hardness (H) | Transparency (%) b | CTE (μm/m °C) c | Tg (°C) d |
|---|---|---|---|---|---|
| None | 0 | 2 | 60.0 | 66.9 | 113.3 |
| MMT/POP2000 | 0.5 | 4 | 59.3 | 52.0 | 137.9 |
| (XRD = 58 Å) | 1 | 5 | 58.2 | 33.5 | 136.2 |
| MMT/POP copolymer | 0.5 | 6 | 57.5 | 47.0 | 140.4 |
| (XRD, featureless) | 1 | 7 | 56.1 | 37.5 | 138.8 |
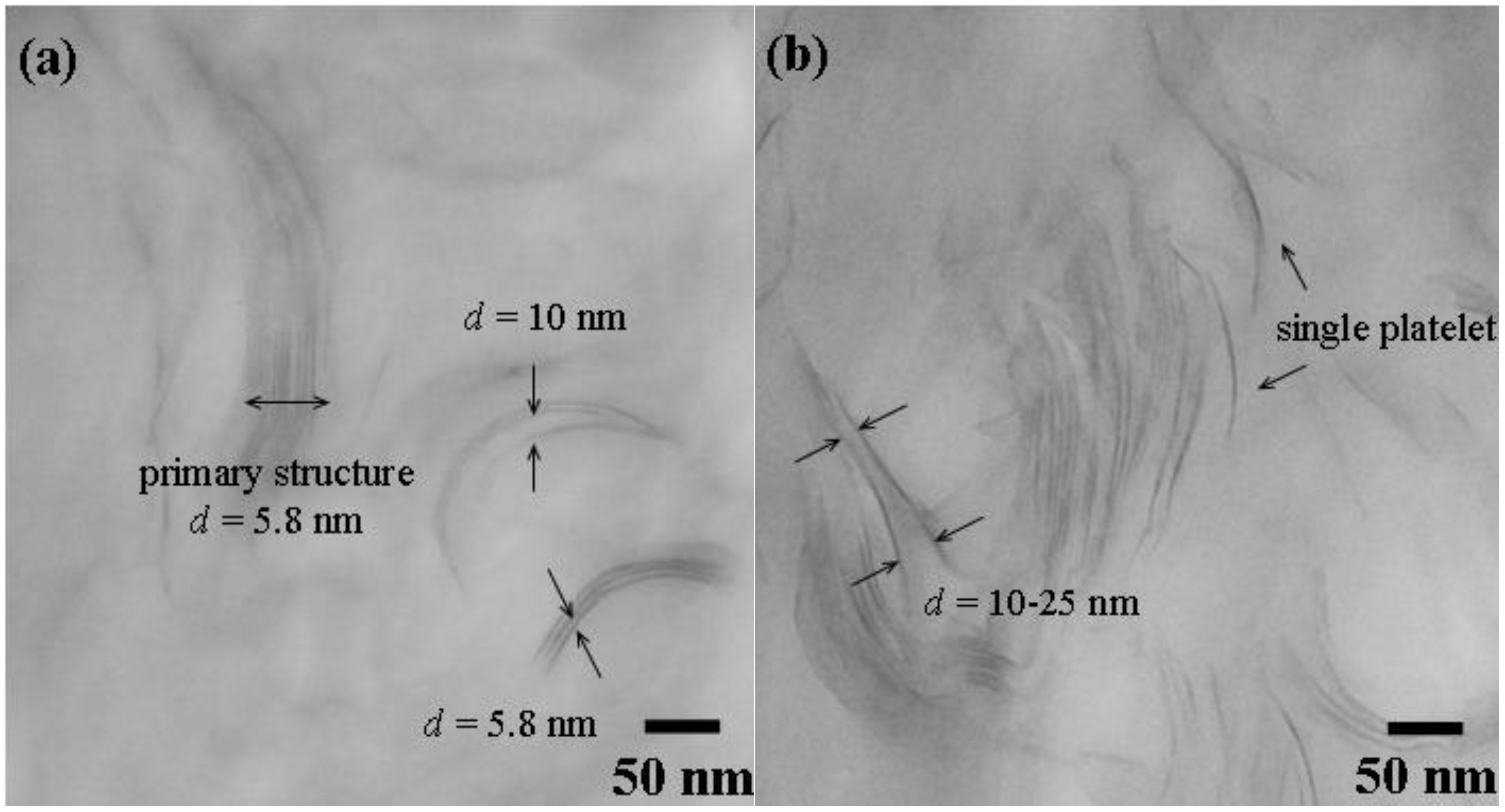
3.2. Comparison of platelet size of the exfoliated clay for epoxies
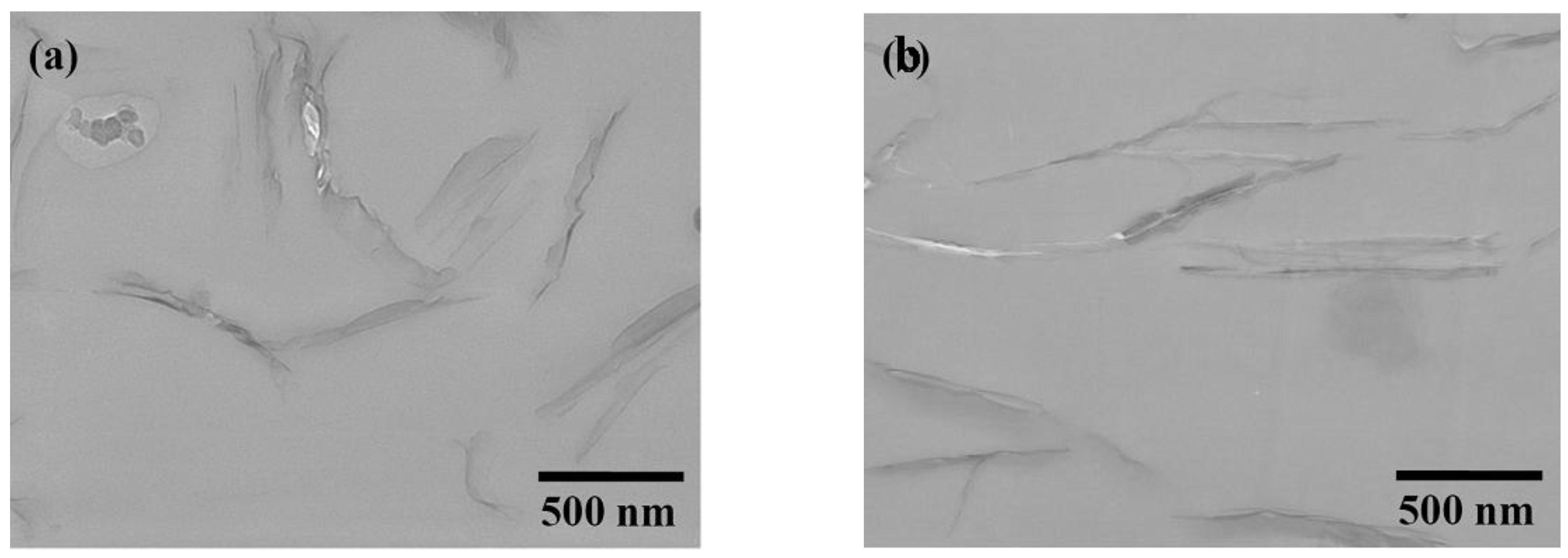

| Silicate platelets | Loading (wt %) a | CTE (μm/m °C) b | Hardness (H) c | Transparency (%) d |
|---|---|---|---|---|
| None | 0 | 82 | 4 | 85 |
| NSP | 0.1 | 78 | 4 | 84 |
| 0.5 | 37 | 7 | 77 | |
| NMP | 0.1 | 71 | 4 | 84 |
| 0.5 | 36 | 8 | 80 |
3.3. Comparison of spherical silica and silicate platelets for epoxie
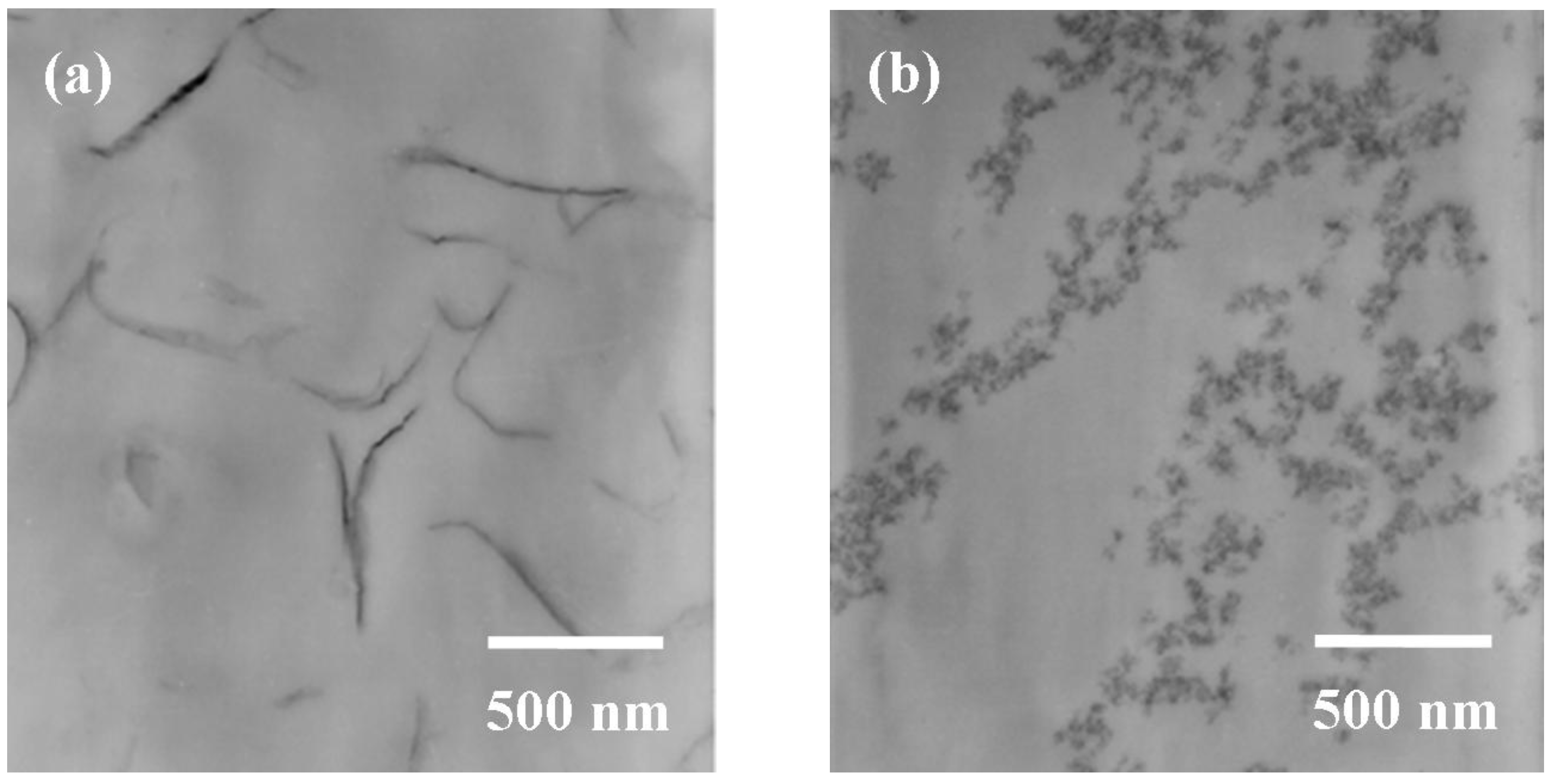
| Silicates | Loading (%) | CTE (μm/m °C) a | Hardness b (H) | Transparency c (%) |
|---|---|---|---|---|
| None | 0 | 98 | 2 | 61 |
| SiO2 | 1 | 80 | 3 | 54 |
| SiO2 | 5 | 72 | 4 | 45 |
| Silicate platelets | 0.5 | 90 | 4 | 55 |
| Silicate platelets | 1 | 95 | 5 | 48 |
3.4. Nanocomposites from one-component curing with organically modified LDH, MMT and Mica
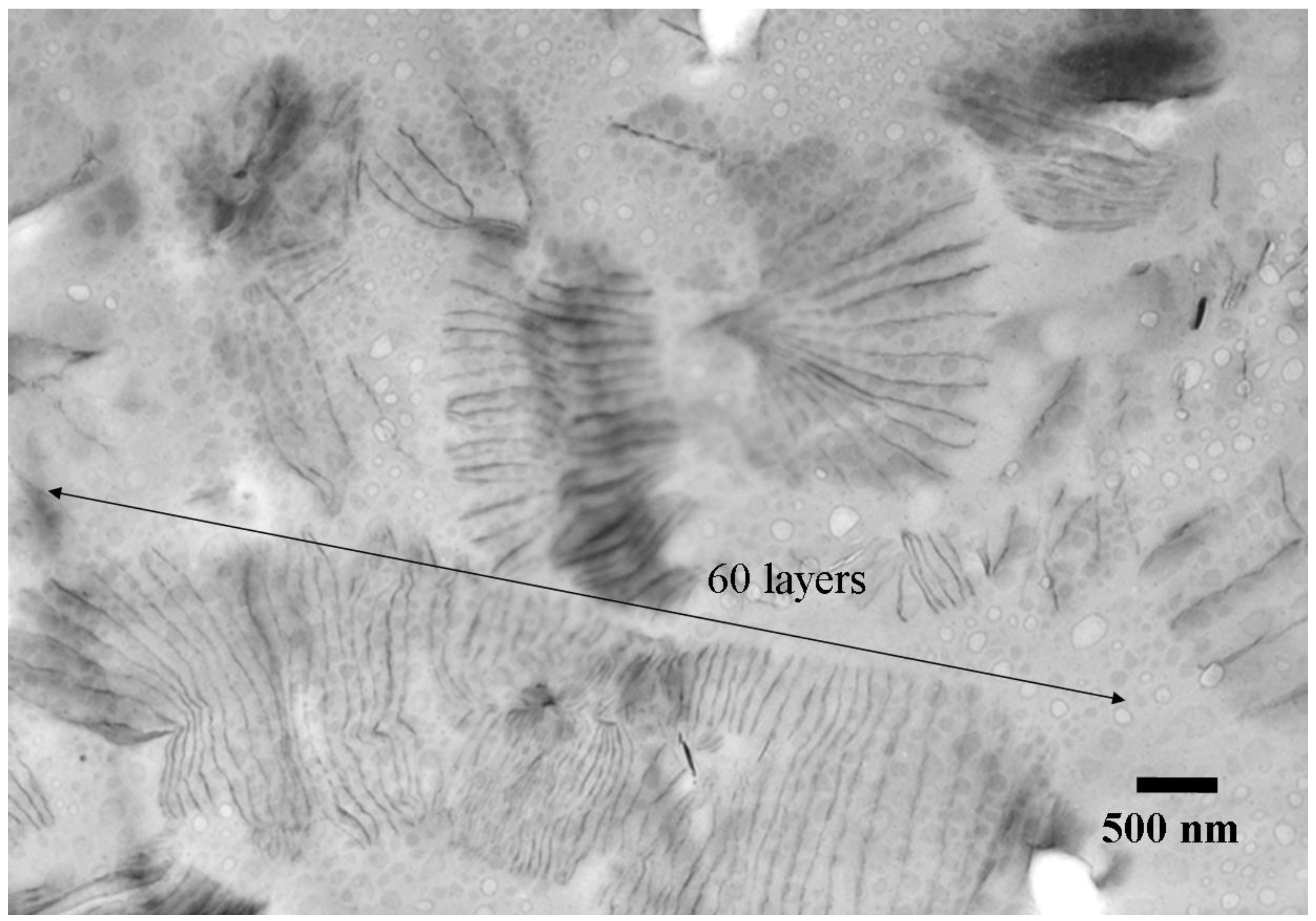
4. Conclusions
Acknowledgements
References and Notes
- Okada, A.; Usuki, A. The chemistry of polymer-clay hybrids. Mater. Sci. Eng. C 1995, 3, 109–115. [Google Scholar] [CrossRef]
- Okada, A.; Usuki, A. Polymer-layered silicate nanocomposites: an overview. Macromol. Mater. Eng. 2006, 291, 1449–1476. [Google Scholar] [CrossRef]
- Porter, D.; Metcalfe, E.; Thomas, M.J.K. Polymer layered silicate nanocomposites. Fire Mater. 2000, 24, 45–52. [Google Scholar]
- Wang, Z.; Pinnavaia, T.J. Hybrid organic-inorganic nanocomposites: exfoliation of magadiite nanolayers in an elastomeric epoxy polymer. Chem. Mater. 1998, 10, 1820–1826. [Google Scholar] [CrossRef]
- Gilman, J.W. Flammability and thermal stability studies of polymer layered-silicate_clay/nanocomposites. Appl. Clay Sci. 1999, 15, 31–49. [Google Scholar] [CrossRef]
- Theng, B.K.G. The Chemistry of Clay-Organic Reactions, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Van Olphen, H. An Introduction to Clay Colloid Chemistry, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Theng, B.K.G. Formation and Properties of Clay-Polymer Complexes; Elsevier: New York, NY, USA, 1979. [Google Scholar]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Usuki, A.; Hasegawa, N.; Kadoura, H.; Okamoto, T. Three-dimensional observation of structure and morphology in nylon-6/clay nanocomposite. Nano Lett. 2001, 1, 271–272. [Google Scholar] [CrossRef]
- Sparks, D.L. Environmental Soil Chemistry; John Wiley & Sons, Ltd.: New York, NY, USA, 2003. [Google Scholar]
- Ajjou, A.N.; Harouna, D.; Detellier, C.; Alper, H. Cation-exchanged montmorillonite catalyzed hydration of styrene derivatives. J. Mol. Catal. A: Chem. 1997, 126, 55–60. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, T.; Zhi, L.; Yan, Y.; Yu, Y. Synthesis of novolac/layered silicate nanocomposites by reaction exfoliation using acid-modified montmorillonite. Macromol. Rapid Commun. 2002, 23, 44–48. [Google Scholar] [CrossRef]
- Tateyama, H.; Nishimura, S.; Tsunematsu, K.; Jinnai, K.; Adachi, Y.; Kimura, M. Synthesis of expandable fluorine mica from talc. Clays Clay Miner. 1992, 40, 180–185. [Google Scholar] [CrossRef]
- Kodama, T.; Higuchi, T.; Shimizu, T.; Shimizu, K.; Komarneni, S.; Hoffbauer, W.; Schneider, H. Synthesis of Na-2-mica from metakaolin and its cation exchange properties. J. Mater. Chem. 2001, 11, 2072–2077. [Google Scholar] [CrossRef]
- Rives, V.; Ulibarri, M.A. Layered double hydroxides (LDH) intercalated with metal coordination compounds and oxometalates. Coord. Chem. Rev. 1999, 181, 61–120. [Google Scholar] [CrossRef]
- Rives, V. Characterisation of layered double hydroxides and their decomposition products. Mater. Chem. Phys. 2002, 75, 19–25. [Google Scholar] [CrossRef]
- Khan, A.I.; O’Hare, D. Intercalation chemistry of layered double hydroxides: recent developments and applications. J. Mater. Chem. 2002, 12, 3191–3198. [Google Scholar] [CrossRef]
- Aisawa, S.; Takahashi, S.; Ogasawara, W.; Umetsu, Y.; Narita, E. Direct intercalation of amino acids into layered double hydroxides by coprecipitation. J. Solid State Chem. 2001, 162, 52–62. [Google Scholar] [CrossRef]
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: an overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Z.K.; Yin, J.; Wan, X.Y.; Qi, Z.E. Preparation and properties of hybrids of organo-soluble polyimide and montmorillonite with various chemical surface modification methods. Polymer 1999, 40, 4407–4414. [Google Scholar] [CrossRef]
- Maiti, M.; Bandyopadhyay, A.; Bhowmick, A.K. Preparation and characterization of nanocomposites based on thermoplastic elastomers from rubber-plastic blends. J. Appl. Polym. Sci. 2006, 99, 1645–1656. [Google Scholar] [CrossRef]
- Subramani, S.; Lee, J.Y.; Choi, S.W.; Kim, J.H. Waterborne trifunctionalsilane-terminated polyurethane nanocomposite with silane-modified clay. J. Polym. Sci. B: Polym. Phys. 2007, 45, 2747–2761. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Jiang, D.D.; Giannelis, E.P. Clay−organosiloxane hybrids: a route to cross-linked clay particles and clay monoliths. Chem. Mater. 2004, 16, 2404–2410. [Google Scholar]
- Hotta, Y.; Taniguchi, M.; Inukai, K.; Yamagishi, A. Formation of thin films of clay−organic complexes with an application as an electrode modifier. Langmuir 1996, 12, 5195–5201. [Google Scholar]
- Wang, K.; Wang, L.; Wu, J.; Chen, L.; He, C. Preparation of highly exfoliated epoxy/clay nanocomposites by “slurry compounding”: process and mechanisms. Langmuir 2005, 21, 3613–3618. [Google Scholar]
- Szabo, A.; Gournis, D.; Karakassides, M.A.; Petridis, D. Clay−aminopropylsiloxane compositions. Chem. Mater. 1998, 10, 639–645. [Google Scholar]
- Hotta, Y.; Inukai, K.; Taniguchi, M.; Nakata, M.; Yamagishi, A. A clay self-assembled on a gold surface as studied by atomic force nicroscopy and electrochemistry. Langmuir 1997, 13, 6697–6703. [Google Scholar]
- Chou, C.C.; Chiang, M.L.; Lin, J.J. Unusual intercalation of cationic smectite clays with detergent-ranged carboxylic ions. Macromol. Rapid Commun. 2005, 26, 1841–1845. [Google Scholar] [CrossRef]
- Mahadevaiah, N.; Venkataramani, B.; Prakash, B.S.J. Restrictive entry of aqueous molybdate species into surfactant modified montmorillonite-a breakthrough curve study. Chem. Mater. 2007, 19, 4606–4612. [Google Scholar]
- Vaia, R.A.; Teukolsky, R.K.; Giannelis, E.P. Interlayer structure and molecular environment of alkylammonium layered silicates. Chem. Mater. 1994, 6, 1017–1022. [Google Scholar] [CrossRef]
- Hotta, S.; Paul, D.R. Nanocomposites formed from linear low density polyethylene and organoclays. Polymer 2004, 45, 7639–7654. [Google Scholar] [CrossRef]
- Zeng, C.; Lee, L.J. Poly(methyl methacrylate) and polystyrene/clay nanocomposites prepared by in-situ polymerization. Macromolecules 2001, 34, 4098–4103. [Google Scholar]
- Fu, X.; Qutubuddin, S. Polymer–clay nanocomposites: exfoliation of organophilic montmorillonite nanolayers in polystyrene. Polymer 2001, 42, 807–813. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Synthesis of polystyrene–clay nanocomposites. Mater. Lett. 2000, 42, 12–15. [Google Scholar] [CrossRef]
- Imai, Y.; Nishimura, S.; Abe, E.; Tateyama, H.; Abiko, A.; Yamaguchi, A.; Aoyama, T.; Taguchi, H. High-modulus poly(ethylene terephthalate)/expandable fluorine mica nanocomposites with a novel reactive compatibilizer. Chem. Mater. 2002, 14, 477–479. [Google Scholar]
- Chang, J.H.; Jang, T.G.; Ihn, K.J.; Lee, W.K.; Sur, G.S. Poly(vinyl alcohol) nanocomposites with different clays: pristine clays and organoclays. J. Appl. Polym. Sci. 2003, 90, 3208–3214. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, C.I.; Choi, W.M.; Lee, J.W.; Lim, J.G.; Park, O.O.; Kim, J.M. Synthesis and material properties of syndiotactic polystyrene/organophilic clay nanocomposites. J. Appl. Polym. Sci. 2004, 92, 2144–2150. [Google Scholar] [CrossRef]
- Ijdo, W.L.; Pinnavaia, T.J. Solid solution formation in amphiphilic organic−inorganic clay heterostructures. Chem. Mater. 1999, 11, 3227–3231. [Google Scholar]
- Maiti, P.; Yamada, K.; Okamoto, M.; Ueda, K.; Okamoto, K. New polylactide/layered silicate nanocomposites: role of organoclays. Chem. Mater. 2002, 14, 4654–4661. [Google Scholar]
- Xie, W.; Xie, R.; Pan, W.P.; Hunter, D.; Koene, B.; Tan, L.S.; Vaia, R. Thermal stability of quaternary phosphonium modified montmorillonites. Chem. Mater. 2002, 14, 4837–4845. [Google Scholar]
- Bottino, F.A.; Fabbri, E.; Fragala, I.L.; Malandrino, G.; Orestano, A.; Pilati, F.; Pollicino, A. Polystyrene-clay nanocomposites prepared with polymerizable imidazolium surfactants. Macromol. Rapid Commun. 2003, 24, 1079–1084. [Google Scholar] [CrossRef]
- Fox, D.M.; Maupin, P.H.; Harris, R.H., Jr.; Gilman, J.W.; Eldred, D.V.; Katsoulis, D.; Trulove, P.C.; DeLong, H.C. Use of a polyhedral oligomeric silsesquioxane (POSS)-imidazolium cation as an organic modifier for montmorillonite. Langmuir 2007, 23, 7707–7714. [Google Scholar]
- Wang, Z.M.; Chung, T.C.; Gilman, J.W.; Manias, E. Melt-processable syndiotactic polystyrene/montmorillonite nanocomposites. J. Polym. Sci. B: Polym. Phys. 2003, 41, 3173–3187. [Google Scholar] [CrossRef]
- Gilman, J.W.; Awad, W.H.; Davis, R.D.; Shields, J.; Harris, R.H., Jr.; Davis, C.; Morgan, A.B.; Sutto, T.; Callahan, E.J.; Trulove, P.C.; DeLong, H.C. Polymer/layered silicate nanocomposites from thermally stable trialkylimidazolium-treated montmorillonite. Chem. Mater. 2002, 14, 3776–3785. [Google Scholar]
- Lin, J.J.; Cheng, I.J.; Wang, R.; Lee, R.J. Tailoring basal spacings of montmorillonite by poly(oxyalkylene)diamine intercalation. Macromolecules 2001, 34, 8832–8834. [Google Scholar]
- Lin, J.J.; Cheng, I.J.; Chou, C.C. Critical conformational change of poly(oxypropylene)diamines in layered aluminosilicate confinement. Macromol. Rapid Commun. 2003, 24, 492–495. [Google Scholar] [CrossRef]
- Chou, C.C.; Shieu, F.S.; Lin, J.J. Copolymer-layered silicate hybrid surfactants from the intercalation of montmorillonite with amphiphilic copolymers. Macromolecules 2003, 36, 2187–2189. [Google Scholar]
- Chou, C.C.; Chang, Y.C.; Chiang, M.L.; Lin, J.J. Conformational change of trifunctional poly(oxypropylene)amines intercalated within a layered silicate confinement. Macromolecules 2004, 37, 473–477. [Google Scholar]
- Lin, J.J.; Hsu, Y.C.; Chou, C.C. Copolymer-layered silicate hybrid surfactants from the intercalation of montmorillonite with amphiphilic copolymers. Langmuir 2003, 19, 5184–5187. [Google Scholar] [CrossRef]
- Lin, J.J.; Chen, Y.M. Amphiphilic properties of poly(oxyalkylene)amine-intercalated smectite aluminosilicates. Langmuir 2004, 20, 4261–4264. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Chou, C.C.; Lin, J.L. Lengthy rod formation from a poly(oxyalkylene)amine-intercalated smectite clay by a self-aligning mechanism. Macromol. Rapid Commun. 2004, 25, 1109–1112. [Google Scholar] [CrossRef]
- Lin, J.J.; Chu, C.C.; Chou, C.C.; Shieu, F.S. Self-assembled nanofibers from random silicate platelets. Adv. Mater. 2005, 17, 301–304. [Google Scholar] [CrossRef]
- Lin, J.J.; Chu, C.C.; Chiang, M.L.; Tsai, W.C. Manipulating assemblies of high-aspect-ratio clays and fatty amine salts to form surfaces exhibiting a lotus effect. Adv. Mater. 2006, 18, 3248–3252. [Google Scholar] [CrossRef]
- Chu, C.C.; Chiang, M.L.; Tsai, C.M.; Lin, J.J. Exfoliation of montmorillonite clay by mannich polyamines with multiple quaternary salts. Macromolecules 2005, 38, 6240–6243. [Google Scholar]
- Lin, J.J.; Chu, C.C.; Chiang, M.L.; Tsai, W.C. First isolation of individual silicate platelets from clay exfoliation and their unique self-assembly into fibrous arrays. J. Phys. Chem. B 2006, 110, 18115–18120. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Chang, Y.C.; Cheng, I.J. Novel mechanism for layered silicate clay intercalation by poly(propylene oxide)-segmented carboxylic acid. Macromol. Rapid Commun. 2004, 25, 508–512. [Google Scholar] [CrossRef]
- Lin, J.J.; Chen, Y.M.; Yu, M.H. Hydrogen-bond driven intercalation of synthetic fluorinated mica by poly(oxypropylene)-amidoamine salts. Col. Surf. A: Physicochem. Eng. Aspects 2007, 302, 162–167. [Google Scholar] [CrossRef]
- Lin, J.J.; Juang, T.Y. Intercalation of layered double hydroxides by poly(oxyalkylene)-amidocarboxylates: tailoring layered basal spacing. Polymer 2004, 45, 7887–7893. [Google Scholar] [CrossRef]
- Wang, M.S.; Pinnavaia, T.J. Clay-Polymer Nanocomposites formed from acidic derivatives of montmorillonite and an epoxy resin. Chem. Mater. 1994, 6, 468–474. [Google Scholar] [CrossRef]
- Lan, T.; Kaviratna, P.D.; Pinnavaia, T.J. Epoxy self-polymerization in smectite clays. J. Phys. Chem. Solids 1996, 57, 1005–1010. [Google Scholar] [CrossRef]
- Ishida, H.; Campbell, S.; Blackwell, J. General approach to nanocomposite preparation. Chem. Mater. 2000, 12, 1260–1267. [Google Scholar] [CrossRef]
- Muzny, C.D.; Butler, B.D.; Hanley, H.J.M.; Tsvetkov, F.; Peiffer, D.G. Clay platelet dispersion in a polymer matrix. Mater. Lett. 1996, 28, 379–384. [Google Scholar] [CrossRef]
- Akelah, A.; El-Deen, N.S.; Hiltner, A.; Baer, E.; Moet, A. Organophilic rubber-montmorillonite nanocomposites. Mater. Lett. 1995, 22, 97–102. [Google Scholar] [CrossRef]
- Ntsihlele, E.S.; Pizzi, A. Cross-linked coatings by co-reaction of isocyanate-methoxymethyl melamine systems. J. Appl. Polym. Sci. 1995, 55, 153–161. [Google Scholar] [CrossRef]
- Kato, M.; Usuki, A.; Okada, A. Synthesis of polypropylene oligomer-clay intercalation compounds. J. Appl. Polym. Sci. 1997, 66, 1781–1785. [Google Scholar] [CrossRef]
- Tyan, H.L.; Liu, Y.C.; Wei, K.H. Thermally and mechanically enhanced clay/polyimide nanocomposite via reactive organoclay. Chem. Mater. 1999, 11, 1942–1947. [Google Scholar]
- Agag, T.; Takeichi, T. Polybenzoxazine–montmorillonite hybrid nanocomposites: synthesis and characterization. Polymer 2000, 41, 7083–7090. [Google Scholar] [CrossRef]
- Kawasumi, M.; Hasegawa, N.; Kato, M.; Usuki, A.; Okada, A. Preparation and mechanical properties of polypropylene-clay hybrids. Macromolecules 1997, 30, 6333–6338. [Google Scholar] [CrossRef]
- Hasegawa, N.; Okamoto, H.; Kawasumi, M.; Usuki, A. Preparation and mechanical properties of polystyrene–clay hybrids. J. Appl. Polym. Sci. 1999, 74, 3359–3364. [Google Scholar] [CrossRef]
- Ogata, N.; Kawakage, S.; Ogihara, T. Structure and thermal/mechanical properties of poly(ethylene oxide) clay mineral blends. Polymer 1997, 38, 5115–5118. [Google Scholar] [CrossRef]
- Jiří, K. Clay and man: clay raw materials in the service of man. Appl. Clay Sci. 1995, 10, 275–335. [Google Scholar] [CrossRef]
- Huang, J.C.; Zhu, Z.K.; Yin, J.; Qian, X.F.; Sun, Y.Y. Poly(etherimide)/montmorillonite nanocomposites prepared by melt intercalation: morphology, solvent resistance properties and thermal properties. Polymer 2001, 42, 873–877. [Google Scholar] [CrossRef]
- Yano, K.; Usuki, A.; Okada, A.; Kurauchi, T.; Kamigaito, O. Synthesis and properties of polyimide-clay hybrid. J. Polym. Sci. A: Polym. Chem. 1993, 31, 2493–2498. [Google Scholar] [CrossRef]
- Frisk, P.; Laurent, J. Nanocomposite polymer container. US Patent 5972448, 1999. [Google Scholar]
- Jan, I.N.; Lee, T.M.; Chiou, K.C.; Lin, J.J. Comparisons of physical properties of intercalated and exfoliated clay/epoxy nanocomposites. Ind. Eng. Chem. Res. 2005, 44, 2086–2090. [Google Scholar] [CrossRef]
- Chiu, C.W.; Cheng, W.T.; Wang, Y.P.; Lin, J.J. Fine dispersion of hydrophobic silicate platelets in anhydride-cured epoxy nanocomposites. Ind. Eng. Chem. Res. 2007, 46, 7384–7388. [Google Scholar] [CrossRef]
- Chu, C.C.; Lin, J.J.; Shiu, C.R.; Kwan, C.C. Fine dispersion and property differentiation of nanoscale silicate platelets and spheres in epoxy nanocomposites. Polym. J. 2005, 37, 239–246. [Google Scholar] [CrossRef]
- Chan, Y.N.; Juang, T.Y.; Liao, Y.L.; Dai, S.A.; Lin, J.J. Preparation of clay/epoxy nanocomposites by layered-double-hydroxide initiated self-polymerization. Polymer 2008, 49, 4796–4801. [Google Scholar] [CrossRef]
- Chan, Y.N.; Dai, S.A.; Lin, J.J. Simultaneous occurrence of self-assembling silicate skeletons to worm-like microarrays and epoxy ring-opening polymerization. Macromolecules 2009, 42, 4362–4365. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, J.-J.; Chan, Y.-N.; Lan, Y.-F. Hydrophobic Modification of Layered Clays and Compatibility for Epoxy Nanocomposites. Materials 2010, 3, 2588-2605. https://doi.org/10.3390/ma3042588
Lin J-J, Chan Y-N, Lan Y-F. Hydrophobic Modification of Layered Clays and Compatibility for Epoxy Nanocomposites. Materials. 2010; 3(4):2588-2605. https://doi.org/10.3390/ma3042588
Chicago/Turabian StyleLin, Jiang-Jen, Ying-Nan Chan, and Yi-Fen Lan. 2010. "Hydrophobic Modification of Layered Clays and Compatibility for Epoxy Nanocomposites" Materials 3, no. 4: 2588-2605. https://doi.org/10.3390/ma3042588




