Use of Temporary Implantable Biomaterials to Reduce Leg Pain and Back Pain in Patients with Sciatica and Lumbar Disc Herniation
Abstract
:1. Introduction
2. Discussion
2.1. Sciatica
2.2. Lumbar Back Pain
- 1)
- induces degeneration of nerve fibers;
- 2)
- increases discharge of nerve fibers;
- 3)
- attracts inflammatory cells (cellular mediators of pain) and;
- 4)
- induces increased intraneural capillary permeability.
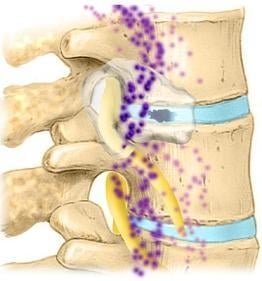
2.3. Lumbar Disc: Anatomy
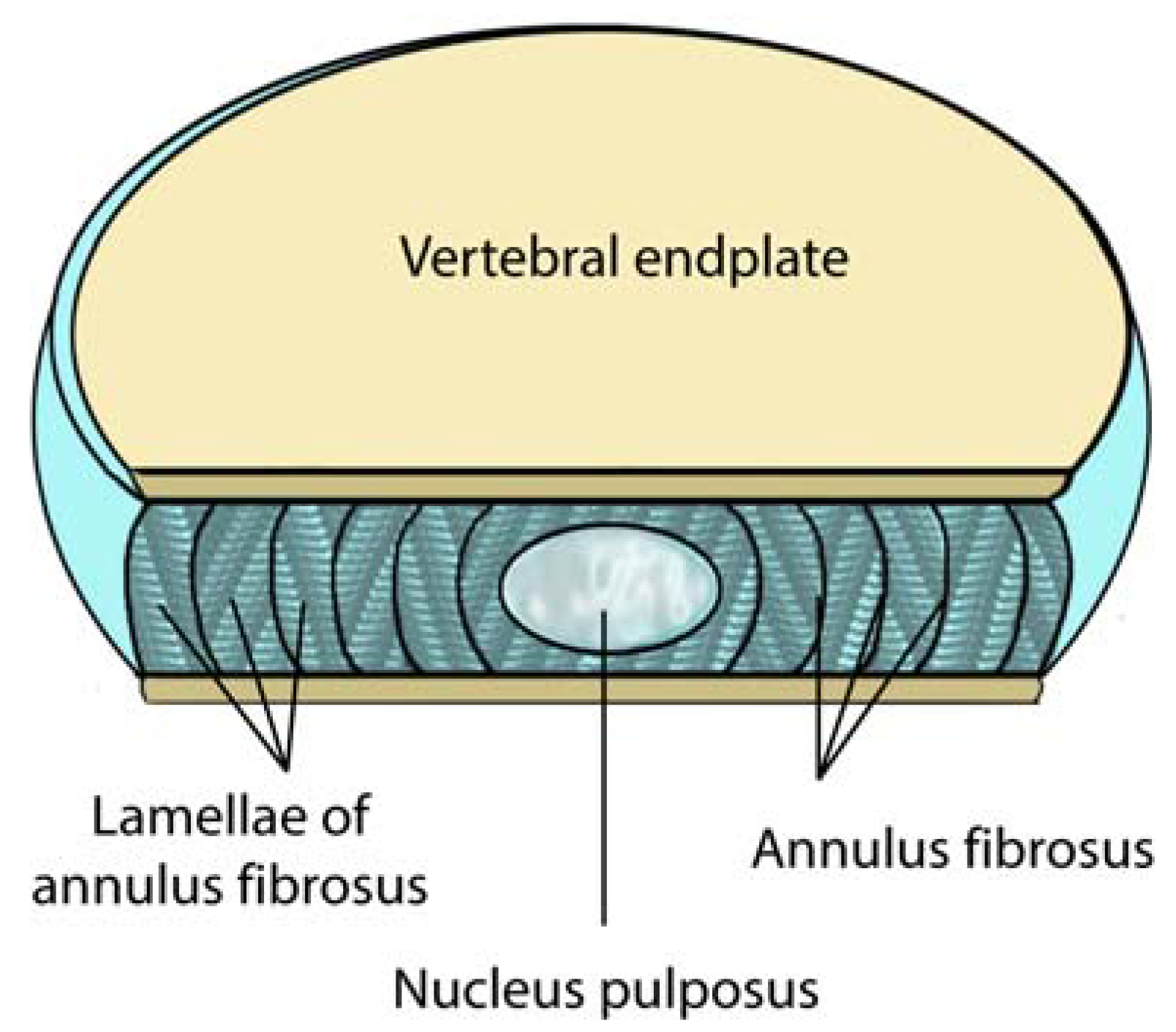
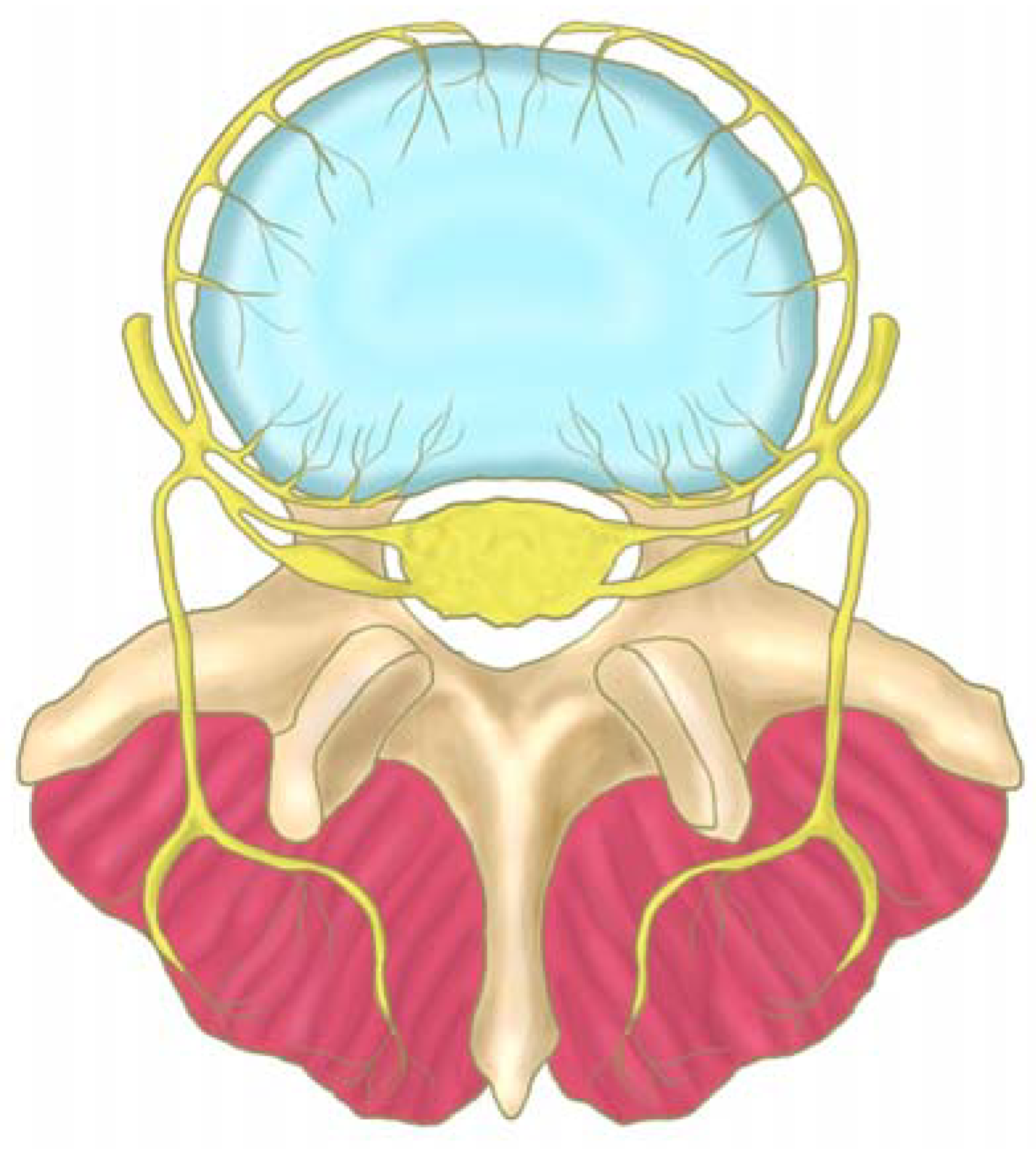
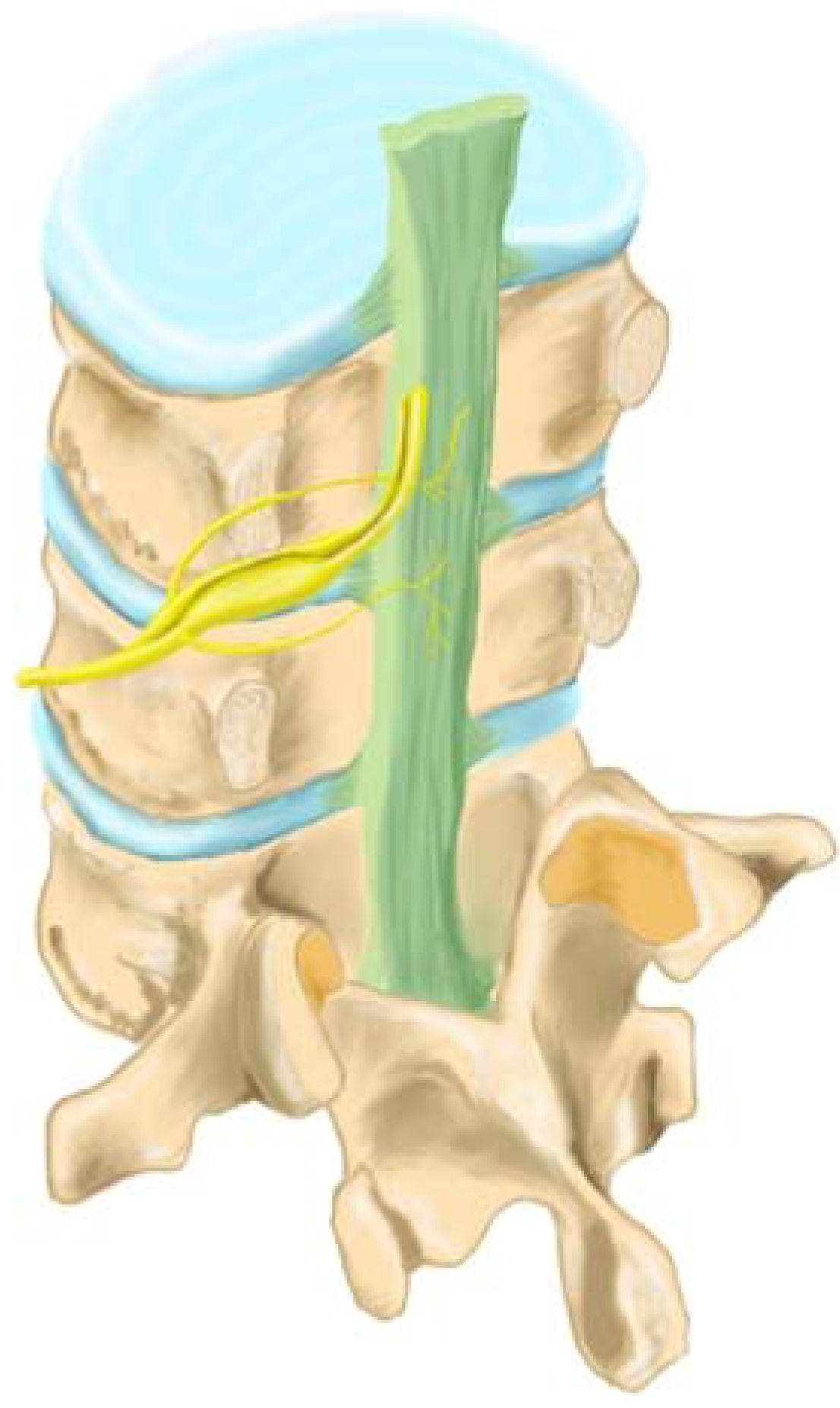
2.4. Sensory Innervation of the Disc
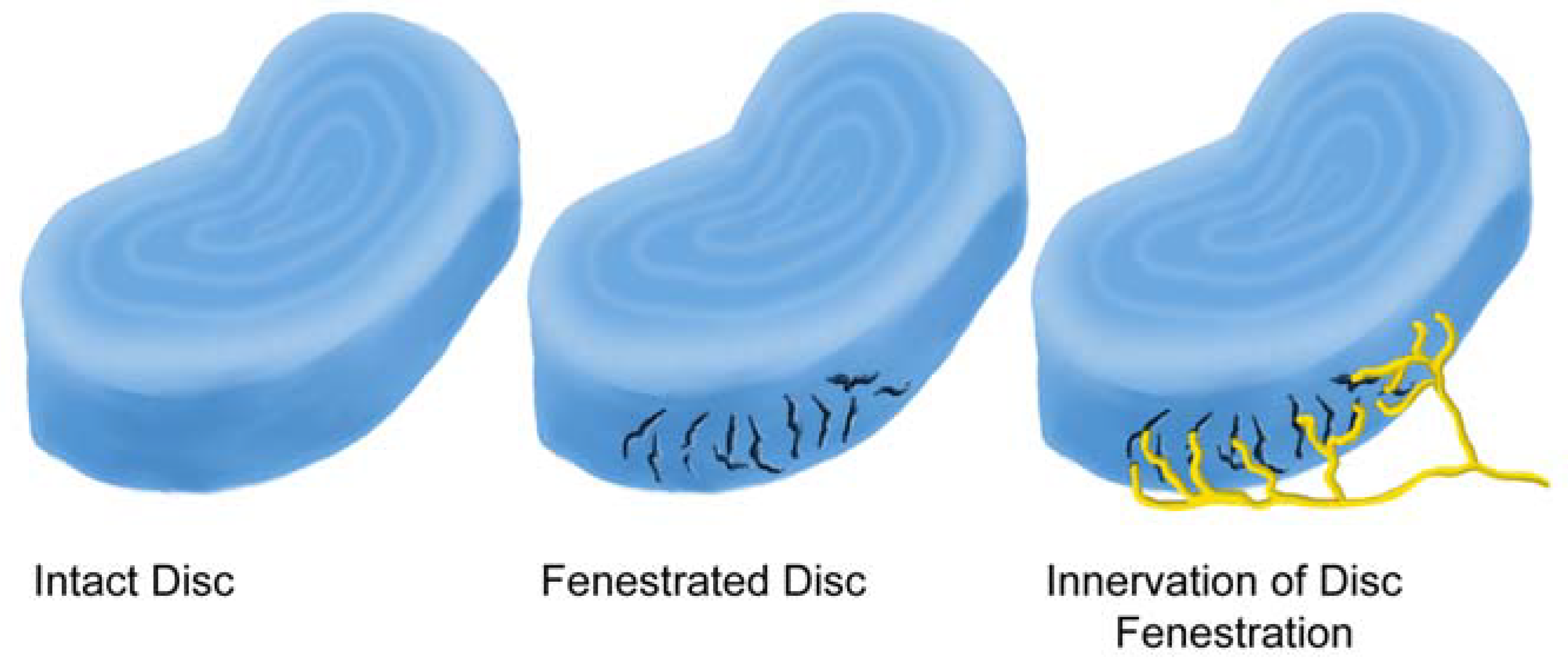
2.5. Sensory Innervation of the Epidural Space
2.6. Sensitization of Sensory Nerves
2.7. Post Operative Pain
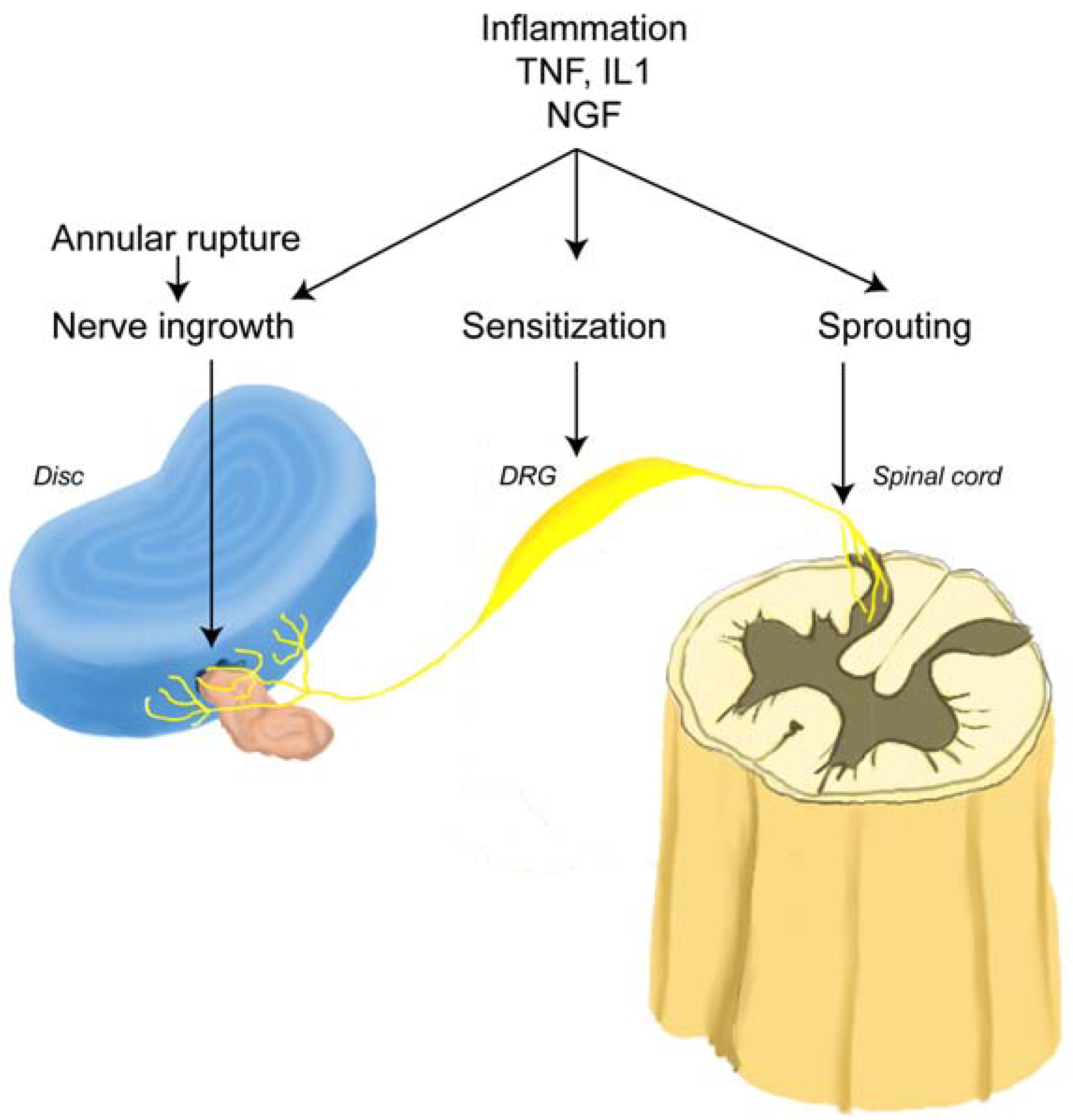
2.8. Pain Mediators during Herniation and Following Decompression Surgery
2.9. Cellular Pain Mediators
2.9.1. Neutrophils
2.9.2. Macrophages
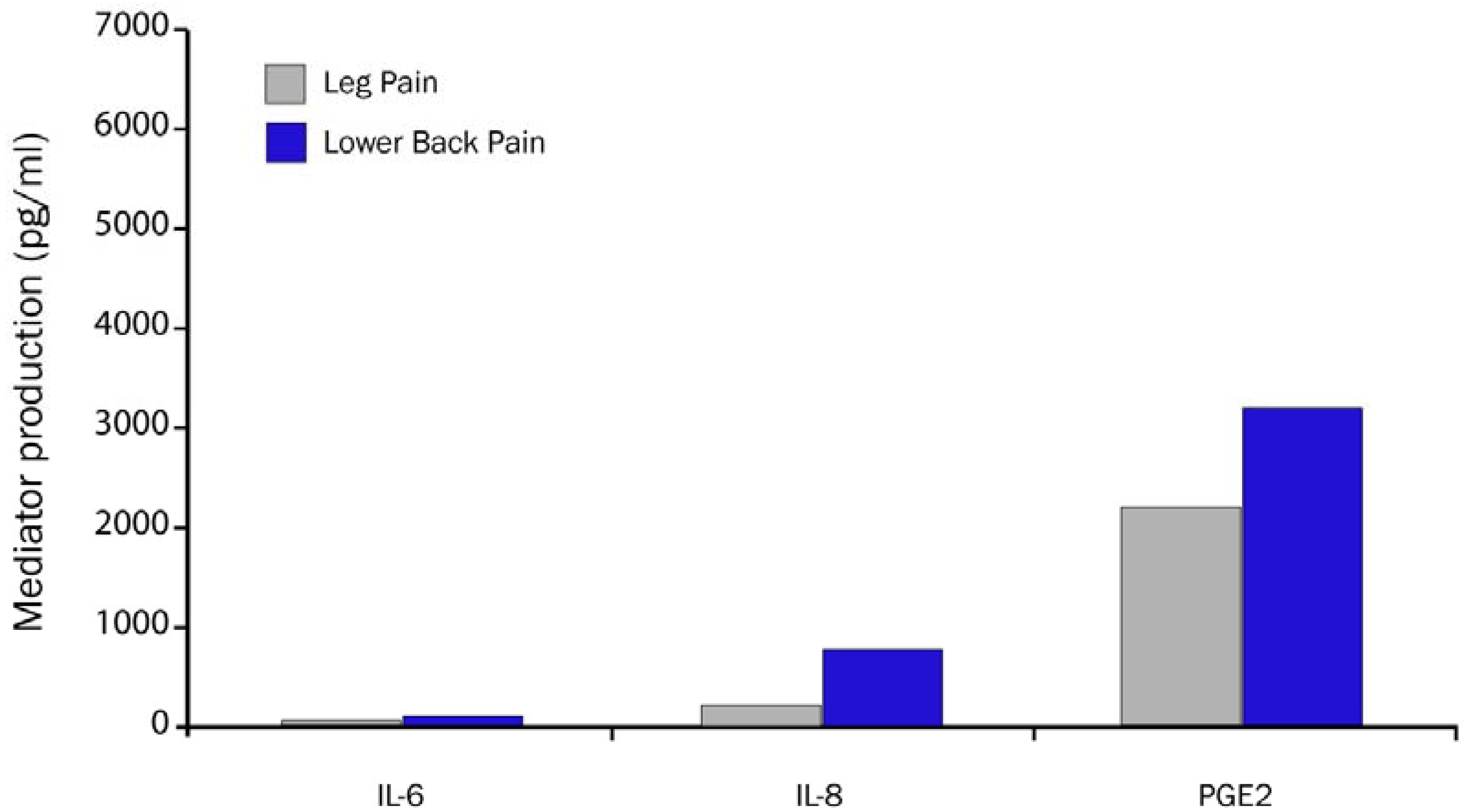
2.10. Biochemical Pain Mediators Identified in the Epidural Space in Patients with LBP
2.11. Annular Leakage and Pain Sensitization
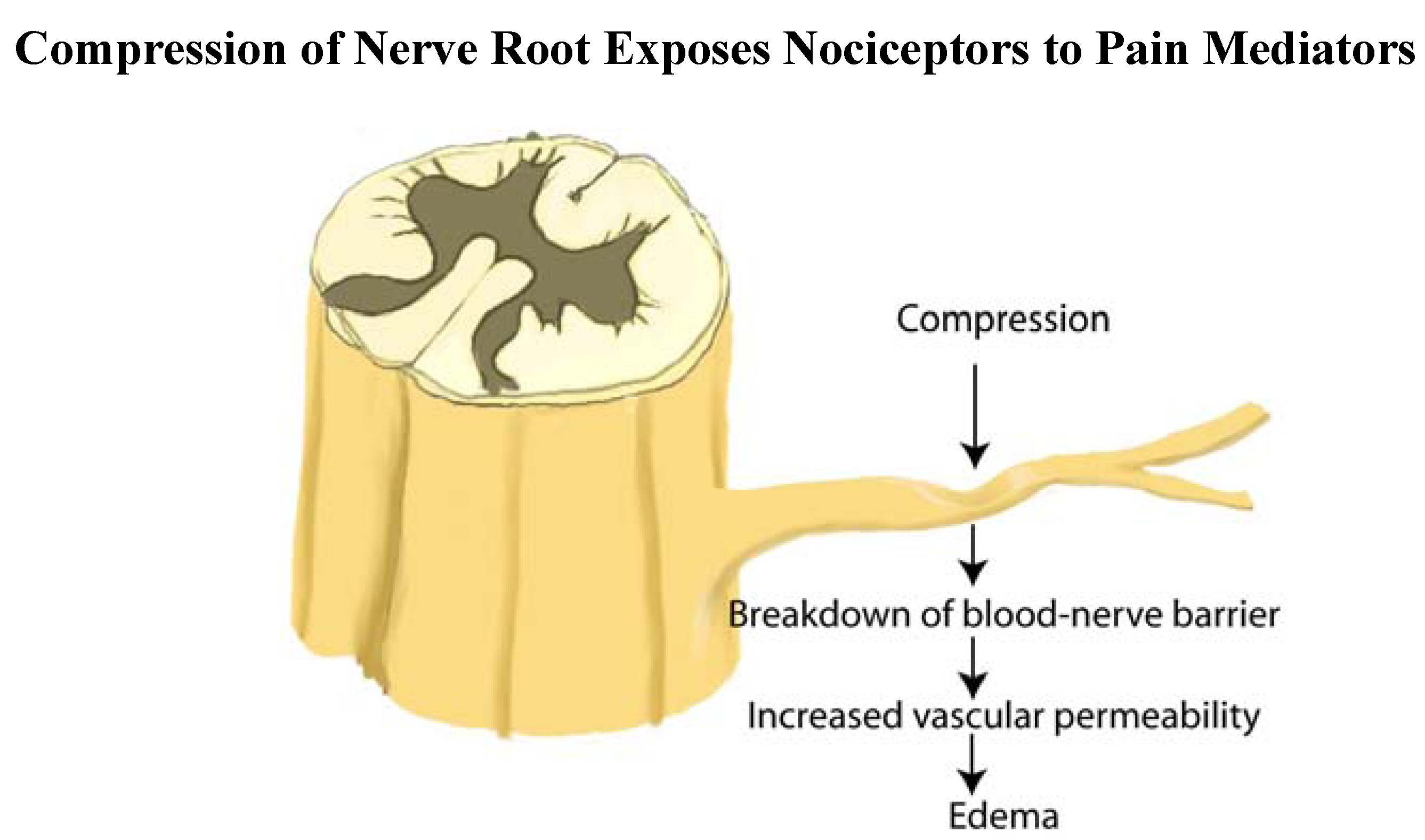
2.12. Sensitization by Fibrosis
2.13. Epidural Adhesions
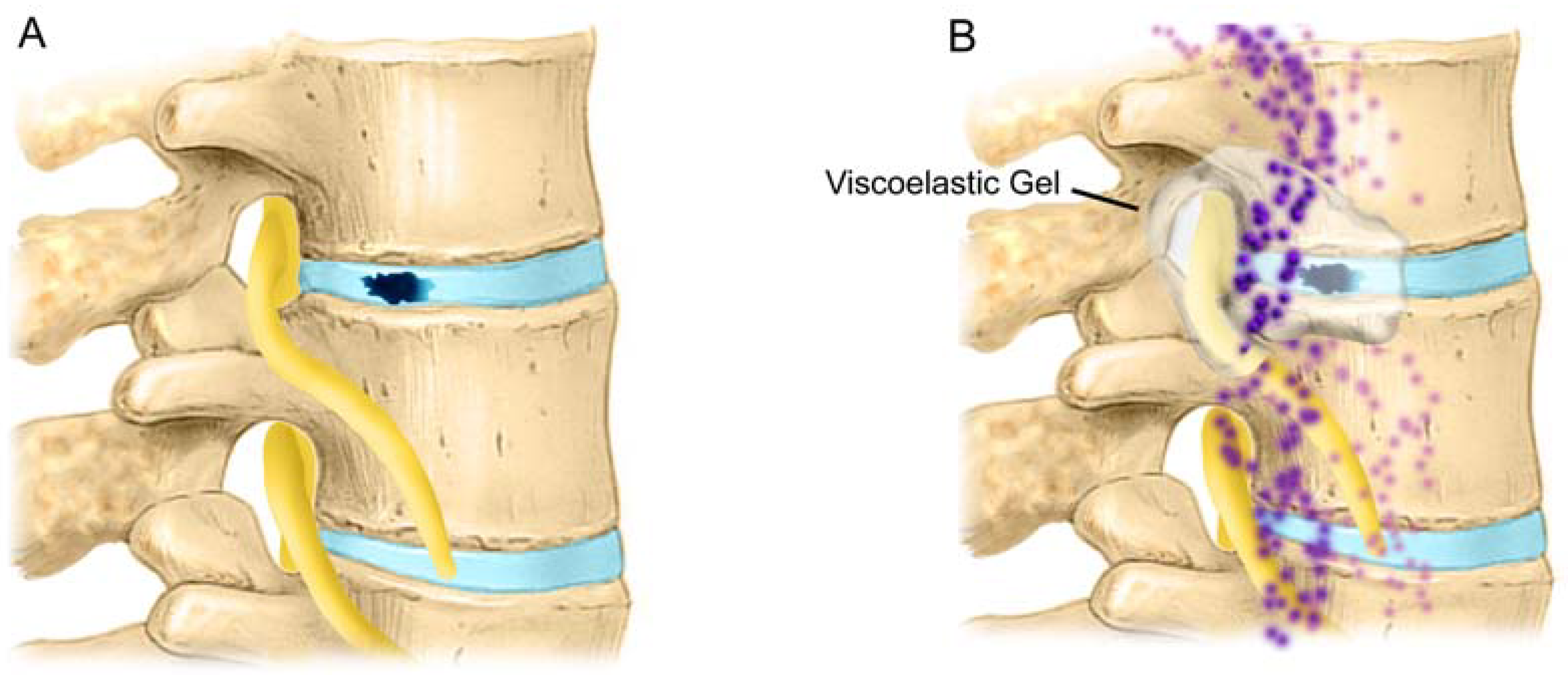
2.14. Protection of Epidural Sensory Nerves by Gels
Acknowledgements
References and Notes
- Koes, B.W. Evidence-based management of acute low back pain. Lancet 2007, 370, 1595–1596. [Google Scholar] [CrossRef] [PubMed]
- Koes, B.W.; van Tulder, M.V.; Peul, W.C. Diagnosis and treatment of sciatica. BMJ 2007, 334, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, K.; Dunn, K. Review of epidemiological studies and prevalence estimates. Spine 2008, 33, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.M.; Blood, E.A.; Frymoyer, J.W.; Herkowitz, H.; Abdu, W.A.; Woodward, R.; Longley, M.; Emery, S.E.; Lurie, J.D.; Tosteson, T.D.; Weinstein, J.N. SPORT lumbar intervertebral disk herniation and back pain: does treatment, location, or morphology matter? Spine 2008, 33, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Lurie, J.D.; Tosteson, T.D.; Skinner, J.S.; Hanscom, B.; Tosteson, A.N.A.; Herkowitz, H.; Fischgrund, J.; Cammisa, F.P.; Albert, T.; Deyo, R.A. Surgical vs. nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA 2006, 296, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Massie, J.B.; Schimizzi, A.L.; Huang, B.; Kim, C.; Garfin, S.R.; Akeson, W.H. Topical high molecular weight hyaluronan reduces radicular pain post laminectomy in a rat model. Spine J. 2005, 5, 494–502. [Google Scholar] [CrossRef] [PubMed]
- diZerega, G.S.; Cortese, S.; Rodgers, K.E.; Block, K.M.; Falcone, S.J.; Juarez, T.G.; Berg, R. A modern biomaterial for adhesion prevention. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Toyone, T.; Tanaka, T.; Kato, D.; Kaneyama, R. Low-back pain following surgery for lumbar disc herniation. A prospective study. J. Bone Joint Surg. Am. 2004, 86A, 893–896. [Google Scholar] [PubMed]
- Anderson, D.G.; Albert, T. The Molecular Basis of Intervertebral Disk Degeneration. Semin. Spine Surg. 2003, 15, 352–360. [Google Scholar] [CrossRef]
- Burke, J.G.; Watson, R.W.; McCormack, D.; Dowling, F.E.; Walsh, M.G.; Fitzpatrick, J.M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J. Bone Joint Surg. Br. 2002, 84, 196–201. [Google Scholar] [CrossRef] [PubMed]
- McCarron, R.F.; Wimpee, M.W.; Hudkins, P.G.; Laros, G.S. The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine 1987, 12, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Myers, R.R.; Kikuchi, S.; Rydevik, B. Pathophysiology of Nerve Root Pain in Disc Herniation and Spinal Stenosis. In the Lumbar Spine, 3rd ed.; Herkowitz, H.N., Dvorak, J., Bell, G.R., Nordin, M., Grob, D., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; Chapter 2; pp. 11–30. [Google Scholar]
- Kobayashi, S.; Yoshizawa, H.; Yamada, S. Pathology of lumbar nerve root compression. Part 1: Intraradicular inflammatory changes induced by mechanical compression. J. Orthop. Res. 2004, 22, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Winkelstein, B.A.; DeLeo, J.A. Nerve root injury severity differentially modulates spinal glial activation in a rat lumbar radiculopathy model: considerations for persistent pain. Brain Res. 2002, 956, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Winkelstein, B.A.; Weinstein, J.N.; DeLeo, J.A. The role of mechanical deformation in lumbar radiculopathy: an in vivo model. Spine 2002, 27, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Yabuki, S.; Aoki, Y.; Kikuchi, S. Pathomechanisms of nerve root injury caused by disc herniation: an experimental study of mechanical compression and chemical irritation. Spine 2003, 28, 435–441. [Google Scholar] [PubMed]
- Wu, G.; Ringkamp, M.; Hartke, T.V.; Murinson, B.B.; Campbell, J.N.; Griffin, J.W.; Meyer, R.A. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J. Neurosci. 2001, 21, RC140. [Google Scholar] [PubMed]
- Garfin, S.R.; Glover, M.; Booth, R.E.; Simeone, F.A.; Rothman, R.H. Laminectomy: a review of the Pennsylvania hospital experience. J. Spinal Disord. 1988, 1, 116–133. [Google Scholar] [PubMed]
- Garfin, S.R.; Rydevik, B.; Lind, B.; Massie, J. Spinal nerve root compression. Spine 1995, 20, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Schimizzi, A.L.; Massie, J.B.; Murphy, M.; Perry, A.; Kim, C.W.; Garfin, S.R.; Akeson, W.H. High molecular-weight hyaluronan inhibits macrophage proliferation and cytokine release in the early wound of a preclinical postlaminectomy rat model. Spine J. 2006, 6, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Freemont, A.J. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology 2009, 48, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Sekine, M.; Yamashita, T.; Takebayashi, T.; Sakamoto, N.; Minaki, Y.; Ishii, S. Mechanosensitive afferent units in the lumbar posterior longitudinal ligament. Spine 2001, 26, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.A. and Hernandez-Vaquero, D. Preventing peridural fibrosis with nonsteroidal anti-inflammatory drugs. Eur. Spine J. 2008, 17, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Slipman, C.W.; Shin, C.H.; Patel, R.K.; Isaac, Z.; Huston, C.W.; Lipetz, J.S.; Lenrow, D.A.; Braverman, D.L.; Vresilovic, E.J., Jr. Etiologies of failed back surgery syndrome. Pain Med. 2002, 3, 200–217. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, C.; Ingelmark, B.E.; Miller, M. The anatomical basis for low back pain. Studies on the presence of sensory nerve endings in ligamentous, capsular and intervertebral disc structures in the human lumbar spine. Acta Orthop. Scand. 1963, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kuslich, S.D.; Ulstrom, C.L.; Michael, C.J. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop. Clin. North Am. 1991, 22, 181–187. [Google Scholar] [PubMed]
- Mooney, V. Where is the pain coming from? Spine 1987, 12, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Nachemson, A.L. The lumbar spine: an orthopaedic challenge. Spine 1976, 1, 59–76. [Google Scholar] [CrossRef]
- Smyth, M.J.; Wright, V. Sciatica and the intervertebral disc; an experimental study. J. Bone Joint Surg. Am. 1958, 40, 1401–1418. [Google Scholar] [PubMed]
- Wiberg, G. Back pain in relation to the nerve supply of the intervertebral disc. Acta Orthop. Scand. 1949, 19, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.M.; Ozaktay, A.C.; Yamashita, T.; Avramov, A.; Getchell, T.V.; King, A.I. Mechanisms of low back pain: a neurophysiologic and neuroanatomic study. Clin. Orthop. Relat. Res. 1997, 335, 166–180. [Google Scholar] [PubMed]
- Edgar, M.A. The nerve supply of the lumbar intervertebral disc. J. Bone Joint Surg. Br. 2007, 89, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Malinsky, J. The ontogenetic development of nerve terminations in the intervertebral discs of man. (Histology of intervertebral discs, 11th communication). Acta Anat. 1959, 38, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.P. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008, 8, 18–44. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Takahashi, Y.; Ohtori, S.; Moriya, H.; Takahashi, K. Distribution and immunocytochemical characterization of dorsal root ganglion neurons innervating the lumbar intervertebral disc in rats: a review. Life Sci. 2004, 74, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Freemont, A.J.; Peacock, T.E.; Goupille, P.; Hoyland, J.A.; O’Brien, J.; Jayson, M.I. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997, 350, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Kayama, S.; Konno, S.; Olmarker, K.; Yabuki, S.; Kikuchi, S. Incision of the anulus fibrosus induces nerve root morphologic, vascular, and functional changes. An experimental study. Spine 1996, 21, 2539–2543. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Aoki, Y.; Ohtori, S. Resolving discogenic pain. Eur. Spine J. 2008, 17, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, H.; O'Brien, J.P.; Smith, W.T.; Trumper, M. The neuropathology of intervertebral discs removed for back pain. J. Pathol. 1980, 132, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Akeda, K.; An, H.S.; Aoki, Y.; Pichika, R.; Muehleman, C.; Kimura, T.; Masuda, K. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine 2007, 32, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Doita, M.; Kanatani, T.; Harada, T.; Mizuno, K. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine 1996, 21, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Inoue, G.; Ohtori, S.; Aoki, Y.; Ozawa, T.; Doya, H.; Saito, T.; Ito, T.; Akazawa, T.; Moriya, H.; Takahashi, K. Exposure of the nucleus pulposus to the outside of the anulus fibrosis induces nerve injury and regeneration of the afferent fibers innervating the lumbar intervertebral discs in rats. Spine 2006, 31, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Takahashi, K.; Ohtori, S.; Moriya, H. Neuropathology of discogenic low back pain: a review. Int. J. Spine Surg. 2005, 2, 1–21. [Google Scholar]
- Peng, B.; Wu, W.; Li, Z.; Guo, J.; Wang, X. Chemical radiculitis. Pain 2007, 127, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G. The molecular pathophysiology of pain: abnormal expression of sodium channel genes and its contributions to hyperexcitability of primary sensory neurons. Pain 1999, Suppl 6, S133–S140. [Google Scholar] [CrossRef]
- Waxman, S.G.; Cummins, T.R.; Dib-Hajj, S.; Fjell, J.; Black, J.A. Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain. Muscle Nerve 1999, 22, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Brisby, H. Pathology and possible mechanisms of nervous system response to disc degeneration. J. Bone Joint Surg. 2006, 88 (Suppl. 2), 68–71. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Rydevik, B. Pathophysiology of sciatica. Orthoped. Clin. N. Amer. 1991, 22, 223–234. [Google Scholar]
- Olmarker, K.; Rydevik, B.; Nordborg, C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine 1993, 18, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Blomquist, J.; Strömberg, J.; Nannmark, U.; Thomsen, P.; Rydevik, B. Inflammatogenic properties of nucleus pulposus. Spine 1995, 20, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Nordborg, C.; Larsson, K.; Rydevik, B. Ultrastructural changes in spinal nerve roots induced by autologous nucleus pulposus. Spine 1996, 21, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K. and Larsson, K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine 1998, 23, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Myers, R.R. Pathogenesis of sciatic pain: Role of herniated nucleus pulposus and deformation of spinal nerve root and dorsal root ganglion. Pain 1998, 78, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Rydevik, B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine 2001, 26, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Storkson, R.; Berge, O. Pathogenesis of sciatic pain: A study of spontaneous behavior in rats exposed to experimental disc herniation. Spine 2002, 27, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A. Aging and degeneration of the human intervertebral disc. Spine 1995, 20, 1307–1314. [Google Scholar] [PubMed]
- Brown, M.F.; Hukkanen, M.V.J.; Hukkanen, M.V.J; McCarthy, I.D.; Redfern, D.R.M.; Batten, J.J.; Crock, H.V.; Hughes, S.P.F.; Polak, J.M. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J. Bone Joint Surg. Br. 1997, 79B, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Inoue, G.; Ito, T.; Kosi, T.; Ozawa, T.; Doya, H.; Saito, T.; Moriya, H.; Takahashi, K. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back Pain and Modic Type 1 or Type 2 changes on MRI. Spine 2006, 31, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N. International Spinal Injection Society guidelines for the performance of spinal injection procedures. Part 1: Zygapophysial joint blocks. Clin. J. Pain 1997, 13, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Takahashi, K.; Takahashi, Y.; Morinaga, T.; Shimada, Y.; Moriya, H. Origin of nerves supplying the posterior portion of lumbar intervertebral discs in rats. Spine 1996, 21, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Coppes, M.H.; Marani, E.; Thomeer, R.T.; Groen, G.J. Innervation of “painful” lumbar discs. Spine 1997, 22, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Lotz, J.C.; Ulrich, J.A. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J. Bone Joint Surg. Am. 2006, 88, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Herlihy, W.F. The sinuvertebral nerve. N. Z. Med. J. 1949, 48, 214–216. [Google Scholar] [PubMed]
- Coppes, M.H.; Marani, E.; Thomeer, R.T.; Oudega, M.; Groen, G.J. Innervation of annulus fibrosis in low back pain. Lancet 1990, 336, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Gillette, R.G.; Kramis, R.C.; Roberts, W.J. Sympathetic activation of cat spinal neurons responsive to noxious stimulation of deep tissues in the low back. Pain 1994, 56, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ness, T.J.; Gebhart, G. Visceral pain: a review of experimental studies. Pain 1990, 41, 167–234. [Google Scholar] [CrossRef] [PubMed]
- Suseki, K.; Takahashi, Y.; Takahashi, K.; Chiba, T.; Tanaka, K.; Moriya, H. CGRP-immunoreactive nerve fibers projecting to lumbar facet joints through the paravertebral sympathetic trunk in rats. Neurosci. Lett. 1996, 221, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N.; Tynan, W.; Wilson, A.S. The nerve supply to the human lumbar intervertebral discs. J. Anat. 1981, 132, 39–56. [Google Scholar] [PubMed]
- Jackson, H.C., 2nd; Winkelmann, R.K.; Bickel, W.H. Nerve endings in the human lumbar spinal column and related structures. J. Bone Joint Surg. Am. 1966, 48, 1272–1281. [Google Scholar] [PubMed]
- Ohtori, S.; Inoue, G.; Koshi, T.; Ito, T.; Doya, H.; Moriya, H.; Takahashi, K. Substance P-saporin down-regulates substance P receptor immunoreactive sensory dorsal root ganglion neurons innervating the lumbar intervertebral discs in rats. Spine 2006, 31, 2987–2991. [Google Scholar] [CrossRef] [PubMed]
- Ashton, I.K.; Roberts, S.; Jaffray, D.C.; Polak, J.M.; Eisenstein, S.M. Neuropeptides in the human intervertebral disc. J. Orthop. Res. 1994, 12, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.G.; Winegarner, F.G. Causalgia. A review of twenty-eight treated cases. Am. J. Surg. 1969, 117, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Carlton, S.M.; Lekan, H.A.; Kim, S.H.; Chung, J.M. Behavioral manifestations of an experimental model for peripheral neuropathy produced by spinal nerve ligation in the primate. Pain 1994, 56, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Tahmoush, A.J. Causalgia: redefinition as a clinical pain syndrome. Pain 1981, 10, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J. Animal model of neuropathic pain: a review. Muscle Nerve 1993, 10, 1040–1048. [Google Scholar] [CrossRef]
- Meller, S.T.; Gebhart, G.F.; Maves, T.J. Neonatal capsaicin treatment prevents the development of the thermal hyperalgesia produced in a model of neuropathic pain in the rat. Pain 1992, 51, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Averill, S.; McMahon, S.B.; Clary, D.O.; Reichardt, L.F.; Priestly, J.V. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur. J. Neurosci. 1995, 1484–1494. [Google Scholar] [CrossRef]
- Palmgren, T.; Gronblad, M.; Seitsalo, S.; Ruuskanen, M.; Karaharju, E. Immunohistochemical demonstration of sensory and autonomic nerve terminals in herniated lumbar disc tissue. Spine 1996, 21, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, T.; Grönblad, M.; Virri, J.; Kääpä, E; Karaharju, E. An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine 1999, 24, 2075–2079. [Google Scholar] [CrossRef] [PubMed]
- Nachemson, A.L. Disc Pressure Measurements. Spine 1981, 6, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, T.; Cavanaugh, J.M.; Kallakuri, S.; Chen, C.; Yamashita, T. Sympathetic afferent units from lumbar intervertebral discs. J. Bone Joint Surg. Br. 2006, 88, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Grönblad, M.; Virri, J.; Tolonen, J.; Seitsalo, S.; Kääpä, E.; Kankare, J.; Myllynen, P.; Karaharju, E.O. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine 1994, 19, 2744–2751. [Google Scholar] [CrossRef] [PubMed]
- O'Donnell, J.L.; O'Donnell, A.L. Prostaglandin E2 content in herniated lumbar disc disease. Spine 1996, 21, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Saal, J.S.; Franson, R.C.; Dobrow, R.; Saal, J.A.; White, A.H.; Goldthwaite, N. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine 1990, 15, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.M.; Swift, J.Q.; Roszkowski, M.T.; Bowles, W.; Garry, M.G.; Jackson, D.L. Pharmacology of peripheral neuropeptide and inflammatory mediator release. Oral Surg. Oral Med. Oral Pathol. 1994, 78, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Echlin, F. MD. Pain responses on stimulation of the lumbar sympathetic chain under local anesthesia; a case report. J. Neurosurg. 1949, 6, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Elves, M.W.; Bucknill, T.; Sullivan, M.F. In vitro inhibition of leukocyte migration in patients with intervertebral disc lesions. Orthop. Clin. North. Am. 1975, 6, 59–65. [Google Scholar] [PubMed]
- Gertzbein, S.D.; Tile, M.; Gross, A.; Falk, R. Autoimmunity in degenerative disc disease of the lumbar spine. Orthop. Clin. North Am. 1975, 6, 67–73. [Google Scholar] [PubMed]
- Takenaka, Y.; Kahan, A.; Amor, B. Experimental autoimmune spondylodiscitis in rats. J. Rheumatol. 1986, 13, 397–400. [Google Scholar] [PubMed]
- Warner, S.J.; Libby, P. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. J. Immunol. 1989, 142, 100–109. [Google Scholar] [PubMed]
- Cohen, S.P.; Larkin, T.M.; Barna, S.A.; Palmer, W.E.; Hecht, A.C.; Stojanovic, M.P. Lumbar discography: a comprehensive review of outcome studies, diagnostic accuracy, and principles. Reg. Anesth. Pain Med. 2005, 30, 163–813. [Google Scholar] [PubMed]
- Korecki, C.L.; Costi, J.J.; Iatridis, J.C. Needle puncture injury affects intervertebral disc mechanics and biology in an organ culture model. Spine 2008, 33, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Ohtori, S.; Inoue, G.; Aoki, Y.; Moriya, H.; Takahashi, K. The degenerated lumbar intervertebral disc is innervated primarily by peptide-containing sensory nerve fibers in humans. Spine 2006, 31, 2418–2422. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Weinstein, J.N.; Chantani, K.; Spratt, K.F.; Meller, S.T.; Gebhart, G.F. Experimental lumbar radiculopathy. Behavioral and histologic changes in a model of radicular pain after spinal nerve root irritation with chromic gut ligatures in the rat. Spine 1994, 19, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Weinstein, J.N.; Spratt, K.F.; Chantani, K.; Traub, R.J.; Meller, S.T.; Gebhart, G.F. Experimental lumbar radiculopathy. Immunohistochemical and quantitative demonstrations of pain induced by lumbar nerve root irritation of the rat. Spine 1994, 19, 1780–1794. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.; Claverie, W.; Gibson, S. The pain of discography. Spine 1988, 13, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Eisenstein, S.M.; Menage, J.; Evans, E.H.; Ashton, I.K. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine 1995, 20, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, T.; Takahashi, K.; Yamagata, M.; Chiba, T.; Tanaka, K.; Takahashi, Y.; Nakamura, S.; Suseki, K.; Moriya, H. Sensory innervation to the anterior portion of lumbar intervertebral disc. Spine 1996, 21, 1848–1851. [Google Scholar] [CrossRef] [PubMed]
- Franson, R.; Saal, J.S.; Saal, J.A. Human disc phospholipase A2 is inflammatory. Spine 1992, 17, S129–S132. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Rydevik, B.; Kikuchi, S.; Olmarker, K. Local application of disc-related cytokines on spinal nerve roots. Spine 2002, 27, 1614–1617. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Pathobiology of neuropathic pain. Eur. J. Pharmacol. 2001, 429, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Seguin, C.A.; Pilliar, R.; Roughly, P.J.; Kandel, R.A. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine 2005, 30, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Tobinick, E.L.; Britschgi-Davoodifar, S. Perispinal TNF-alpha inhibition for discogenic pain. Swiss Med. Wkly. 2003, 133, 170–177. [Google Scholar] [PubMed]
- Zanella, J.M.; Burright, E.N.; Hildebrand, K.; Hobot, C.; Cox, M.; Christoferson, L.; McKay, W.F. Effect of etanercept, a tumor necrosis factor-alpha inhibitor, on neuropathic pain in the rat chronic constriction injury model. Spine 2008, 33, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Hao, S.; Hou, S.; Wu, W.; Jiang, D.; Fu, X.; Yang, Y. Possible pathogenesis of painful intervertebral disc degeneration. Spine 2006, 31, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Saxler, G.; Brankamp, J.; von Knoch, M.; Löer, F.; Hilken, G.; Hanesch, U. The density of nociceptive SP- and CGRP-immunopositive nerve fibers in the dura mater lumbalis of rats is enhanced after laminectomy, even after application of autologous fat grafts. Eur. Spine J. 2008, 17, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Kosharskyy, B.; Rozen, D. Lumbar discogenic pain. Disk degeneration and minimally invasive interventional therapies. Anasthesiol. Intensivmed Notfallmed Schmerzther 2007, 42, 262–267. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Revel, M.; Loty, B. A quantitative model of post-laminectomy scar formation. Spine 1995, 20, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Doita, M.; Kanatani, T.; Ozaki, T.; Matsui, N.; Kurosaka, M.; Yoshiya, S. Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine 2001, 26, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, M.D.; DeLeo, J.A. The Role of Cytokines in the Initiation and Maintenance of Chronic Pain. Drug News Perspect. 2002, 15, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. The biology of interleukin 1 and comparison to tumor necrosis factor. Immunol. Lett. 1987, 16, 227–231. [Google Scholar]
- Rotshenker, S.; Aamar, S.; Barak, V. Interleukin-1 activity in lesioned peripheral nerve. J. Neuroimmunol. 1992, 39, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Greenwald, D.; Hulmes, J.D.; Chang, M.; Pan, Y.C.E.; Mathixon, J.; Ulevitch, R.; Cermai, A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature 1985, 316, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.J.; Higgs, E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Carlsson, C.A.; Thulin, C.A. Regeneration of feline dorsal roots. Cell. Mol. Life Sci. 1967, 23, 125–126. [Google Scholar] [CrossRef]
- Dayer, J.M.; Graham, R.; Russell, G.; Krance, S.M. Collagenase production by rhematoid synovial cells: stimulation by a human lymphocyte factor. Science 1977, 195, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Essick, C.R. Formation of macrophages by the cells lining the subarachnoid cavity in response to the stimulus of particulate matter. Carnegie Contr. Embry 1920, 42, 379–389. [Google Scholar]
- Lagunoff, D. The mechanism of histamine release from mast cells. Biochem. Pharmacol. 1972, 21, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Haro, H.; Kato, T.; Komori, H.; Osada, M.; Shinomiya, K. Vascular endothelial growth factor (VEGF)-induced angiogenesis in herniated disc resorption. J. Orthop. Res. 2002, 20, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Suguro, T.; Okazima, Y.; Motegi, M.; Okada, Y.; Kakiuchi, T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine 1996, 21, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.S.; Kikuchi, S.; Shubayev, V.; Myers, R.R. 2000 Volvo Award winner in basic science studies: Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine 2000, 25, 2975–2980. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, M.; Kikuchi, S.; Myers, R.R. Experimental spinal stenosis: relationship between degree of cauda equina compression, neuropathology, and pain. Spine 2004, 29, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, A.; Kikuchi, S.; Konno, S.; Olmarker, K. Inflammatory cytokines released from the facet joint tissue in degenerative lumbar spinal disorders. Spine 2004, 29, 2091–2095. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Ohtori, S.; Miyagi, M.; Ishikawa, T.; Inoue, G.; Doya, H.; Koshi, T.; Ito, T.; Yamashita, M.; Yamauchi, K.; et al. Up-regulation of p55 TNF alpha-receptor in dorsal root ganglia neurons following lumbar facet joint injury in rats. Eur. Spine J. 2007, 16, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Onda, A.; Hamba, M.; Yabuki, S.; Kikuchi, S. Exogenous tumor necrosis factor-alpha induces abnormal discharges in rat dorsal horn neurons. Spine 2002, 27, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Weiler, C.; Nerlich, A.; Bachmeier, B.E.; Boos, N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs; a study in surgical specimen and autopsy controls. Spine 2004, 30, 44–54. [Google Scholar]
- Nerlich, A.G.; Weiler, C.; Zipperer, J.; Narozny, M.; Boos, N. Immunolocalization of phagocytic cells in normal and degenerated intervertebral discs. Spine 2002, 27, 2484–2490. [Google Scholar] [CrossRef] [PubMed]
- Weiler, C.; Nerlich, A.G.; Zipperer, J.; Bachmeier, B.E.; Boos, N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur. Spine J. 2002, 11, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, H.; Sato, T.; Sasaki, H.; Tanaka, Y. Discogenic pain in acute nonspecific low-back pain. Eur. Spine J. 2005, 14, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Moneta, G.B.; Videman, T.; Kaivanto, K.; Aprill, C.; Spivey, M.; Vanharanta, H.; Sachs, B.L.; Guyer, R.D.; Hochsculer, S.H.; Raschbaum, R.F.; Mooney, V. Reported pain during lumbar discography as a function of annular ruptures and disc degeneration. A re-analysis of 833 discograms. Spine 1994, 19, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.B.; Rydevik, B.; Takahashi, K.; Larsson, K.; Olmarker, K. Incision of the intervertebral disc induces disintegration and increases permeability of the dorsal root ganglion capsule. Spine 2005, 30, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Kayama, S.; Olmarker, K.; Larsson, K.; Sjögren-Jansson, E.; Lindahl, A.; Rydevik, B. Cultured, autologous nucleus pulposus cells induce functional changes in spinal nerve roots. Spine 1998, 23, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, T.; Cavanaugh, J.M.; Ozaktay, A.C.; Kallakuri, S.; Chen, C. Effect of nucleus pulposus on the neural activity of dorsal root ganglion. Spine 2001, 26, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, S.; Kawaguchi, Y.; Nordborg, C.; Kikuchi, S.; Rydevik, B.; Olmarker, K. Effects of lidocaine on nucleus pulposus-induced nerve root injury. A neurophysiologic and histologic study of the pig cauda equina. Spine 1998, 23, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, S.; Kikuchi, S.; Olmarker, K.; Myers, R.R. Acute effects of nucleus pulposus on blood flow and endoneurial fluid pressure in rat dorsal root ganglia. Spine 1998, 23, 2517–2523. [Google Scholar] [CrossRef] [PubMed]
- Jou, I.M.; Tai, T.W.; Tsai, C.L.; Tsai, T.M.; Yung, W.S.; Jung, Y.C. Spinal somatosensory evoked potential to evaluate neurophysiologic changes associated with postlaminotomy fibrosis: an experimental study. Spine 2007, 32, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Emmez, H.; Kardes, O.; Dogulu, F.; Kurt, G.; Memis, L.; Baykaner, M.K. Role of antifibrotic cytokine interfern-y in the prevention of postlaminectomy peridural fibrosis in rats. Neurosurgery 2008, 62, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.R.; Berman, B. γ interferon is the lymphokine and β interferon is the monokine responsible for inhibition of fibroblast collagen production and late but not early fibroblast proliferation. J. Exp. Med. 1985, 162, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Gillery, P.; Serpier, H.; Polette, M.; Bellon, G.; Clavel, C.; Wegrowski, Y.; Birembaut, P.; Kalis, B.; Cariou, R.; Maquart, F.X. Gamma-interferon inhibits extracellular matrix synthesis and remodeling in collagen lattice cultures of normal and scleroderma skin fibroblasts. Eur. J. Cell. Biol. 1992, 57, 244–253. [Google Scholar] [PubMed]
- Goldring, M.B.; Sandell, L.J.; Stephenson, M.L.; Krane, S.M. Immune interferon suppresses levels of procollagen mRNA and type III collagen synthesis in cultured human articular and costal chondrocytes. J. Biol. Chem. 1986, 261, 9049–9055. [Google Scholar] [PubMed]
- Granstein, R.D.; Flotte, T.J.; Amento, E.P. Interferons and collagen production. J. Invest. Dermatol. 1990, 95, S75–S80. [Google Scholar] [CrossRef]
- Jaffe, H.A.; Gao, Z.; Mori, Y.; Varga, J. Selective inhibition of collagen gene expression in fibroblasts by an interferon-gamma transgene. Exp. Lung Res. 1999, 25, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Melin, M.; Hartmann, D.J.; Magloire, H.; Falcoff, E.; Auriaylt, C.; Grimaud, J.A. Human recombinant y-interferon stimulates proliferation and inhibits collagen and fibronectin production by human dental pulp fibroblasts. Cell. Mol. Biol. 1989, 35, 97–110. [Google Scholar] [PubMed]
- Nguyen, K.D.; Hoang, A.T.; Lee, D. Transcriptional control of human Tenon's capsule fibroblast collagen synthesis in vitro by y-interferon. Invest. Ophthalmol. Vis. Sci. 1994, 35, 3064–3070. [Google Scholar] [PubMed]
- Sime, P.J.; O'Reilly, K.M. Fibrosis of the lung and other tissues: New concepts in pathogenesis and treatment. Clin. Immunol. 2001, 99, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Tredget, E.E.; Wang, R.; Shen, Q.; Scott, P.G.; Ghahary, A. Transforming growth factor-beta mRNA and protein in hypertrophic scar tissues and fibroblasts: antagonism by IFN-alpha and IFN-gamma in vitro and in vivo. J. Interferon Cytokine Res. 2000, 20, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ziesche, R.; Block, L.H. Mechanisms of antifibrotic action of IFN y-1b in pulmonary fibrosis. Wien. Klin. Wochenschr. 2000, 112, 785–790. [Google Scholar] [PubMed]
- Fransen, P. A Prospective Randomized Controlled Study to Evaluate the Use of a Synthetic Fibrosis Inhibitor in the Reduction of Low Back Pain Following Lumbar Microdiscectomy. Spine J. 2008, 8, S56–S57. [Google Scholar] [CrossRef]
- Fransen, P. Safety of carboxymethylcellulose/polyethylene oxide for the prevention of adhesions in lumbar disc herniation – consecutive case series review. Ann. Surg. Innov. Res. 2008, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Atlas, S.J.; Keller, R.B.; Chang, Y.; Deyo, R.A.; Singer, D.E. Surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: five-year outcomes from the Maine Lumbar Spine Study. Spine 2001, 26, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- North, R.B.; Ewend, M.G.; Lawton, M.T.; Kidd, D.H.; Piantadosi, S. Failed back surgery syndrome: 5-year follow-up after spinal cord stimulator implantation. Neurosurgery 1991, 28, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang, J.P.; Finkenstaedt, M.; Vogelsang, M.; Markakis, E. Recurrent pain after lumbar discectomy: the diagnostic value of peridural scar on MRI. Eur. Spine J. 1999, 8, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Coskun, E.; Süzer, T.; Topuz, O.; Zencir, M.; Pakdemirli, E.; Tahta, K. Relationships between epidural fibrosis, pain, disability, and psychological factors after lumbar disc surgery. Eur. Spine J. 2000, 9, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ido, K.; Urushidani, H. Fibrous adhesive entrapment of lumbosacral nerve roots as a cause of sciatica. Spinal Cord 2001, 39, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Simons, J. Reduction of radiculopathy using MediShield anti-adhesion gel in spinal surgery. In the Congress of Neurological Surgeons, San Francisco, CA, USA, October 2004.
- Liu, L.S.; Berg, R.A. Adhesion barriers of carboxymethylcellulose and polyethylene oxide composite gels. J. Biomed. Mater. Res. Appl. Biomater. 2002, 63, 326–332. [Google Scholar] [CrossRef]
- Madsen, F.; Eberth, K.; Smart, J.D. A rheological examination of the mucoadhesive/mucus interaction: the effect of mucoadhesive type and concentration. J. Control Release 1998, 50, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Akeson, W.H.; Massie, J.B.; Huang, B.; Giurea, A.; Sah, R.; Garfin, S.R.; Kim, C.W. Topical high-molecular-weight hyaluronan and a roofing barrier sheet equally inhibit postlaminectomy fibrosis. Spine J. 2005, 5, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Haro, H.; Komori, H.; Shinomiya, K. Evaluation of hyaluronic acid sheet for the prevention of postlaminectomy adhesions. Spine J. 2005, 5, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Songer, M.N.; Ghosh, L.; Spencer, D.L. Effects of sodium hyaluronate on peridural fibrosis after lumbar laminotomy and discectomy. Spine 1990, 15, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Songer, M.N.; Rauschning, W.; Carson, E.W.; Pandit, S.M. Analysis of peridural scar formation and its prevention after lumbar laminotomy and discectomy in canines. Spine 1995, 20, 571–80. [Google Scholar] [CrossRef]
- Tatsui, C.E.; Martinez, G.; Li, X.; Pattany, P.; Levi, A.D. Evaluation of Duragen in preventing peridural fibrosis. J. Neurosurg. Spine 2006, 4, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Falcone, S.J.; Palmeri, D.M.; Berg, R.A. Biomedical Applications of Hyaluronic Acid. In Polysaccharides for Drug Delivery and Pharmaceutical Applications; Marchessault, R.H., Ravenelle, F., Zhu, X.X., Eds.; American Chemical Society: Washington, DC, USA, 2006; Volume 934, pp. 155–174. [Google Scholar]
- Falcone, S.J.; Palmeri, D.M.; Berg, R.A. Rheological and cohesive properties of hyaluronic acid. J. Biomed. Mater. Res. 2006, 76A, 721–728. [Google Scholar] [CrossRef]
- Assietti, R.; Mora, A.; Brayda-Bruno, M. Use of carboxymethylcellulose/polyethylene oxide gel in microdiscectomy with interlaminectomy: a case series comparison with long-term follow-up. Spine 2008, 33, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.D.; Wang, J.C.; Roberston, D.P.; Brodke, D.S.; BenDebba, M.; Block, K.M.; diZerega, G.S. Reduction in leg pain and lower-extremity weakness with Oxiplex/SP Gel for 1 year after laminectomy, laminotomy, and discectomy. Neurosurg. Focus 2004, 17, 1–6. [Google Scholar] [CrossRef]
- Kim, K.D.; Wang, J.C.; Robertson, D.P.; Brodke, D.S.; Olson, E.M.; Duberg, A.C.; BenDebba, M.; Block, K.M.; diZerega, G.S. Reduction of radiculopathy and pain with Oxiplex/SP Gel after laminectomy, laminotomy, and discectomy: A pilot clinical study. Spine 2003, 28, 1080–1088. [Google Scholar] [PubMed]
- Rhyne, A.L.; Blumenthol, S.L.; Frank, E.H.; Hsu, K.Y.; Kim, K.D.; Youssef, J.A.; Wang, J.C.; Arnold, P.; BenDebba, M.; Block, K.M.; et al. Oxiplex reduces leg pain, back pain and associated symptoms 6 months following single-level lower lumbar laminectomy for removal of a herniated disc. Spine 2009. submitted . [Google Scholar]
- Zuki, Z. The use of anti-adhesion gel (carboxymethylcellulose+polyethylene oxide) after spinal decompression surgery. Malaysia Orthopedic Association: Selangor, Malaysia, 2006. [Google Scholar]
- Mortazavi, S.A.; Smart, J.D. An investigation of some factors influencing the in vitro assessment of mucoadhesion. Itnl. J. Pharm. 1995, 116, 223–230. [Google Scholar]
- Rossi, S.; Bonferoni, M.C.; Lippoli, G.; Bertoni, M.; Ferrari, F.; Caramella, C.; Conte, U. Influence of mucin type on polymer-mucin rheological interactions. Biomaterials 1995, 16, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Atha, D.H.; Ingham, K.C. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J. Biol. Chem. 1981, 256, 12108–12117. [Google Scholar] [PubMed]
- Gombotz, W.R.; Wang, G.H.; Horbett, T.A.; Hoffman, A.S. Protein adsorption to poly(ethylene oxide) surfaces. J. Biomed. Mater. Res. 1991, 25, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.G. (Ed.) Protein Purification Process Engineering; Marcel Decker: New York, NY, USA, 1993; pp. 115–208.
- Lee, J.H.; Kopecek, J.; Andrade, J.D. Protein-resistant surfaces prepared by PEO-containing block copolymer surfactants. J. Biomed. Mater. Res. 1989, 23, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Paleg, L.G.; Stewart, G.R.; Bradbeer, J.W. Proline and Glycine Betaine Influence Protein Solvation. Plant Physiol. 1984, 75, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Kurt, G.; Cemil, B.; Celik, B.; Durdag, E.; Erdem, O.; Ceviker, N. Comparison of Oxiplex and Gore-Tex effectivity in an experimental peridural fibrosis model. Neurocirugía 2009, 20, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.E.; Robertson, J.T.; Espinoza, T.; Oppelt, W.; Cortese, S.; diZerega, G.S.; Berg, R.A. Reduction of epidural fibrosis in lumbar surgery with Oxiplex adhesion barriers of carboxymethylcellulose and polyethylene oxide. Spine J. 2003, 3, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Mulleman, D.; Mammou, S.; Griffoul, I.; Watier, H.; Goupille, P. Pathophysiology of disk-related sciatica. I. Evidence supporting chemical component. Joint Bone Spine 2006, 73, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Mulleman, D.; Mammou, S.; Griffoul, I.; Watier, H.; Goupille, P. Pathophysiology of disk-related low back pain and sciatica. II. Evidence supporting treatment with TNF-alpha antagonists. Joint Bone Spine 2006, 73, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Spangfort, E.V. The lumbar disc herniation. A computer-aided analysis of 2,504 operations. Acta Orthop. Scand. 1972, 142 (Suppl.), 1–95. [Google Scholar] [CrossRef]
- Atlas, S.J.; Keller, R.B.; Wu, Y.A.; Deyo, R.A.; Singer, D.E. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the Maine lumbar spine study. Spine 2005, 30, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, J.; Gauchat, M.H.; Valach, L. The outcome of surgery for lumbar disc herniation. I. A 4-17 years' follow-up with emphasis on somatic aspects. Spine 1988, 12, 1418–1422. [Google Scholar] [CrossRef]
- Asch, H.L.; Lewis, P.J.; Moreland, D.B.; Egnatchik, J.G.; Yu, Y.J.; Clabeaux, D.E.; Hyland, A.H. Prospective multiple outcomes study of outpatient lumbar microdiscectomy: should 75 to 80% success rates be the norm? J. Neurosurg. 2002, 96 (Suppl. 1), 34–44. [Google Scholar] [PubMed]
- Loupasis, G.A.; Stamos, K.; Katonis, P.G.; Sapkas, G.; Korres, D.S.; Hartofilakidis, G. Seven- to 20-year outcome of lumbar discectomy. Spine 1999, 24, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Silvers, H.R.; Lewis, P.J.; Asch, H.L.; Clabeaux, D. Lumbar microdiscectomy in the elderly patient. Br. J. Neurosurg. 1997, 11, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.J.; Weir, B.K.; Broad, R.W.; Grace, M.G. Long-term prospective study of lumbosacral discectomy. J. Neurosurg. 1987, 67, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Weber, H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine 1983, 8, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, T.; Karppinen, J.; Paimela, L.; Malmivaara, A.; Lindgren, K.A.; Bowman, C.; Hammond, A.; Kirkham, B.; Järvinen, S.; Niinimäki, J.; et al. The treatment of disc-herniation-induced sciatica with infliximab: one-year follow-up results of FIRST II, a randomized controlled trial. Spine 2006, 31, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Silvers, H.R.; Lewis, P.J.; Clabeaux, D.; Asch, H.L. Lumbar disc excisions in patients under the age of 21 years. Spine 1994, 19, 2387–2391. [Google Scholar] [CrossRef] [PubMed]
- Osterman, H.; Seitsalo, S.; Karppinen, J.; Malmivaara, A. Effectiveness of microdiscectomy for lumbar disc herniation A randomized controlled trial with 2 years of follow-up. Spine 2006, 31, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Peul, W.C.; Brand, R.; Thomeer, R.T.; Koes, B.W. Influence of gender and other prognostic factors on outcome of sciatica. Pain 2008, 138, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Peul, W.C.; van Houwelingen, H.C.; van den Hout, W.B.; Brand, R.; Eekhof, J.A.H.; Tans, J.T.J.; Thomeer, R.T.W.M.; Koes, B.W. for the Leiden–The Hague Spine Intervention Prognostic Study Group. Surgery versus prolonged conservative treatment for sciatica. New Engl. J. Med. 2007, 356, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.J.; O'neill, C.W.; Lotz, J.C. Glucosamine HCl alters production of inflammatory mediators by rat intervertebral disc cells in vitro. Spine J. 2007, 7, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; An, H.S.; Takahashi, K.; Miyamoto, K.; Lenz, M.E.; Moriya, H.; Masuda, K. Axonal growth potential of lumbar dorsal root ganglion neurons in an organ culture system: response of nerve growth factor-sensitive neurons to neuronal injury and an inflammatory cytokine. Spine 2007, 32, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Omoigui, S. The biochemical origin of pain--proposing a new law of pain: the origin of all pain is inflammation and the inflammatory response. Part 1 of 3--a unifying law of pain. Med. Hypotheses 2007, 69, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Yaksh, T.L.; Hua, X.Y.; Kalcheva, I.; Nozaki-Taguchi, N.; Marsala, M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc. Natl. Acad. Sci. USA 1999, 96, 7680–7686. [Google Scholar] [CrossRef] [PubMed]
| Author | No. of Subjects | Conclusion |
|---|---|---|
| Spangfort, E.V. | 2504 | 30% continued to have lumbar back pain; 23% continued to have sciatica following discectomy [181]. |
| Pearson, A.M.; et al. | 775 | 72% with lumbar back pain after discectomy; sciatica improved more following surgery than lumbar back pain; lumbar back pain improvement only moderately correlated with sciatica improvement [4]. |
| Atlas, S.J.; et al. | 507 | Residual lumbar back pain 1.9; residual sciatica 1.5, 10 years after disc surgery [182]. |
| Dvorak, J.; et al. | 382 | 66% continued to have significant lumbar back pain; 45% continued to have sciatica 2-5 years after discectomy [183]. |
| Asch, H.L.; et al. | 212 | Lumbar back pain relief: 77%; sciatica relief: 80% [184]. |
| Loupasis, G.A.; et al. | 109 | Good-excellent relief of lumbar back pain: 80%; good-excellent relief of sciatica: 86% [185] |
| Silvers, H.R.; et al. | 104 | Relief of lumbar back pain: 76%; relief of sciatica: 89% following discectomy [186]. |
| Lewis, P.J.; et al. | 100 | 13% had greater lumbar back pain than sciatica at baseline [187]. |
| Weber, H. | 45 | 17% of 45 subjects no lumbar back pain; 41% of 36 subjects no sciatica, 4 years after discectomy [188]. |
| Toyone, T.; et al. | 40 | Lumbar disc herniation might be a possible cause of lumbar back pain; potential sensory pathways described for discogenic pain due to disc herniation [8]. |
| Korhonen, T.; et al. | 21 | Lumbar back pain relief 51%; sciatica relief 68% following novel drug therapy [189]. |
| Silvers, H.R.; et al. | 15 | Relief of lumbar back pain: 77%; relief of sciatica: 85% following discectomy [190]. |
| Osterman; et al. | 58 | Leg pain baseline VAS score 9/100, back pain VAS score 7/100. Only leg pain superior at 6 weeks [191]. |
| Peul; et al. | 283 | Baseline VAS score; leg pain 17.7/100, back pain 11.3/100. 81% disappearance of symptoms at 8 weeks [192,193]. |
| Mediator | Description / Complete Name | Activity | Source / Location | Reference |
|---|---|---|---|---|
| Cytokines/Growth Factors (Signaling Substances) | ||||
| COX | Cyclooxygenase |
|
| Walsh [194] |
| FGF2 or FGFb | Fibroblast growth factor, basic |
|
| Weiler [126] |
| GAP-43 | Growth-associated protein 43 |
|
| Aoki [195] |
| GM-CSF | Granulocyte macrophage colony stimulation factor |
|
| Weiler [126] |
| IGF-1 | Insulin-like growth factor |
|
| Weiler [126] |
| IL-1 | Interleukin-1 |
|
| Ohtori [58] |
| Walsh [194] | |||
| IL-1β | Interleukin-1β |
|
| Abe [40] |
| Brisby [47] | |||
| Omoigui [196] | |||
| IL-6 | Interleukin-6 |
|
| Ohtori [58] |
| Brisby [47] | |||
| Omoigui [196] | |||
| IL-8 | Interleukin-8 |
|
| Brisby [47] |
| IL-10 | Interleukin-10 |
|
| Weiler [126] |
| Omoigui [196] | ||||
| NGF | Nerve growth factor |
|
| Abe [40] |
| NO | Nitric oxide |
|
| Walsh [194] |
| PGE2 | Prostaglandin E2 [23, 24] |
|
| Weiler [126] |
| Walsh [194] | |||
| PLA2 | Phospholipase A2 |
| Weiler [126] | |
| TGF-β | Transforming growth factor- β |
|
| Weiler [126] |
| TNF-α | Tumor necrosis factor-α |
|
| Abe [40] |
| Aoki [195] | |||
|
| Sakuma 2007 [124], Ohtori [58] | ||
|
| Weiler [126] | ||
| Neuropeptides (Ligands) | ||||
| VEGF | Vascular endothelial growth factor |
|
| Haro [119] |
| CGRP | Calcitonin-gene related peptide |
|
| Aoki [195] |
|
| Ohtori [58] | ||
| (PK) C | Protein kinase C |
| Yaksh [197] | |
| Proteoglycans |
|
| Abe [40] | |
| Neurotransmitters (Receptors) | ||||
| AMPA | Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
|
| Yaksh [197] |
| Glutamate | Glutamate |
|
| Yaksh [197] |
| NK-1 | Neurokinin-1 receptor |
|
| Ohtori [58] |
| NMDA | n-methyl-D-aspartate |
|
| Yaksh [197] |
| P (sP) | Substance P |
|
| Ohtori [70] |
| p75NGFR | p75NGFR |
|
| Abe [40] |
| TrkA | Transmembrane tyrosine kinase (“TrackA”) |
|
| Abe [40] |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
DiZerega, G.S.; Traylor, M.M.; Alphonso, L.S.; Falcone, S.J. Use of Temporary Implantable Biomaterials to Reduce Leg Pain and Back Pain in Patients with Sciatica and Lumbar Disc Herniation. Materials 2010, 3, 3331-3368. https://doi.org/10.3390/ma3053331
DiZerega GS, Traylor MM, Alphonso LS, Falcone SJ. Use of Temporary Implantable Biomaterials to Reduce Leg Pain and Back Pain in Patients with Sciatica and Lumbar Disc Herniation. Materials. 2010; 3(5):3331-3368. https://doi.org/10.3390/ma3053331
Chicago/Turabian StyleDiZerega, Gere S., Melissa M. Traylor, Lisa S. Alphonso, and Samuel J. Falcone. 2010. "Use of Temporary Implantable Biomaterials to Reduce Leg Pain and Back Pain in Patients with Sciatica and Lumbar Disc Herniation" Materials 3, no. 5: 3331-3368. https://doi.org/10.3390/ma3053331