Hydrothermal Synthesis of Metal Oxide Nanoparticles in Supercritical Water
Abstract
:1. Introduction

2. Hydrothermal Synthesis of Metal Oxide
2.1. Batch Reaction System
| Starting Materials | Conditions | Products | Particle size (nm) | Reference |
|---|---|---|---|---|
| Ti(OC3H7)4 | 100–400 °C, 24 h | TiO2 | 20–115 | [4] |
| Ti(OC3H7)4, KOH | 350–450 °C, 14–44 MPa, 2–25 h | K2Ti6O13 | 12.5–20.1 | [5] |
| Nb2O5, KOH | 200–400 °C, 2–72 h | K4Nb6O19, KNbO3 | 7–19 | [6] |
| Nb2O5, KOH | 400 °C, 24 MPa, 2–24 h | KNbO3 | 15.2–42.1 | [7] |
| Ti(OC3H7)4, Nb2O5, KOH | 300–400 °C, 3–25 MPa, 2–24 h | KTiNbO5 | 1000–3000 | [8,9,10] |
| Ta2O5, KOH | 400 °C, 25 MPa, 2–48 h | KTaO3 | 1000–10000 | [11] |
| Zn(NO3)2, Mn(NO3)2 SiO2, KOH | 400 °C, 29 MPa, 0.5–1.5 h | Zn2SiO4:Mn | Several µm in length (rod-like) | [12,13,14,15,16] |
| Fe(NO3)39H2O, In(NO3)35H2O | 400 °C, 30 MPa, 4 h | -(Fe1-xInx)2O3 c-(Fe1-xInx)2O3 | 30–40 | [32] |
| FeSO47H2O, LiOH | 121–388 °C, <33.5 MPa, 10 min–1 h | LiFePO4 | 1000–2000 | [33] |
| Ce(NO3)3, lignosulfate | 250 °C, 10 min | CeO2 | 5–20 | [34] |
| V2O5,Y(NO3)3, KOH | 200–380 °C, 1–10 h | YVO4 | 50–200 | [35] |
| Co(NO3)26H2O, Fe(NO3)39H2O, (Li, Na,K)OH | 390 °C, pH 12 <6 h | CoFe2O4 | 5 | [36] |
| Fe(NO3)39H2O | 394 °C, 5–30 d | Fe2O3 | 16–36 | [37] |
| Y(NO3)36H2O, Al(NO3)39H2O, Eu(NO3)36H2O, KOH | 400 °C, 30 MPa, 2 h, pH 7–11 | Y3Al5O12 (YAG):Eu | <3000 in length (rod-like) | [38] |
| Ce(NO3)36H2O, NaOH | 390 °C, pH 7–9 2 h | CeO2 | 3–8 | [39] |
| Er2O3, NaOH | 300 °C, 25 MPa2–22 h | ErOOH, Er2OCO3(OH)2 | 6000–12000 (rod-like) | [40] |
| MgCl26H2O, K4P2O7, HCl | 400–450 °C, 25–32 MPa,5–120 min | Mg3.5H2(PO4)3 | 20–500 | [41] |
| Cu(NO3)23H2O, Al(NO3)39H2O, HCOOH, NaOH | 400 °C, 30 MPa,10–30 min | CuAlO2 | 2000–5000 | [42] |
2.2. Flow Reaction System
| Starting Materials | Conditions | Products | Particle size (nm) | Reference |
|---|---|---|---|---|
| TiO2 sol, KOH | 350–420 °C, 30 MPa, 2–3 s | K2Ti6O13, TiO2 | 10 (width), 500–1000 (length) | [17,18] |
| ZrO(NO3)2, ZrO(Ac)2 | 400 °C, 30 MPa, 1.8 s | ZrO2 | 6.8–7 | [19,27,28] |
| Al(NO3)3 | 350–400 °C, 25–40 MPa, 2–64 s | γ-AlOOH | 63–473 | [20] |
| Al(NO3)3 | 400–500 °C, 25–35 MPa, 0.063–3 s | γ-AlOOH, γ-Al2O3 | 3.9–6.4 | [21] |
| Ba(OH)2, TiO2 sol | 300–420 °C, 30 MPa, 0.1–40 s | BaTiO3 | 13–48.4 | [22] |
| Ba(OH)2, TiO2 sol | 300–420 °C, 20–40 MPa, 0.7–5.1 s | Tetragonal/Cubic BaTiO3 | 10–100 | [23] |
| Ba(OH)2, TiO2 sol | 400 °C, 30 MPa, 7 ms–2 s | Tetragonal/Cubic BaTiO3 | 9–32 | [24] |
| Ca(NO3)2, Sr(NO3)2, Fe(NO3)3, TiO2 sol | 300–400 °C, 30 MPa, 10 s | Ca0.8Sr0.2Ti1xFexO3- | 20–27 | [25] |
| ZrO(NO3)2, Y(NO3)3 | 300–400 °C, 30 MPa, 0.17–0.35 s | YSZ | 4–6 | [26] |
| Zn(CH3CO2)2, H2O2 | 400 °C, 245atm, 8.9–16.3 s | ZnO | 39–320 | [43] |
| Fe(NO3)3, Co(NO3)2, NaOH | 475–675K, 25 MPa, 11–23 s | CoFe2O4 | 13–23 | [45] |
| Al(NO3)3 Y(NO3)3 Tb(NO3)3, KOH | 400 °C, 30 MPa, 2.5 s | (Y2.7Tb0.3)Al5O12 | 14–152 | [46] |
| Zn(NO3)26H2O | 390 °C, 30MPa, 22 s | ZnO | <10000 (whisker) | [47] |
| Zn(NO3)26H2O, LiOH | 390 °C, 30MPa, 0.7 s | ZnO | 16–57 | [48] |
| Zn(NO3)26H2O, LiOH | 400 °C, 30MPa, 0.03 s | ZnO(nanorod) | 38 (width), 230 (length) | [49] |
| Ba(OiPr)2, Ti(OiPr)4, EtOH | 330–380 °C, 16 MPa, 119–166 s | Cubic BaTiO3 | 15–36 | [51,52] |
| (Ba,Sr)(OiPr)2, Ti(OiPr), EtOH | 380 °C, 26 MPa, 119–166 s | Cubic BaTiO3 | <50 | [53,54] |
| Mn(NO3)26H2O, LiOH, LiNO3 | 400–420 °C, 30 MPa, 10–40 s | LiMn2O4 | <100 | [55] |
| Starting Materials | Conditions | Products | Particle Size (nm) | Reference |
|---|---|---|---|---|
| SnCl2 InCl3 | 350–380 °C, 30 MPa | Cubic/Tetragonal In2O3, SnO2, ITO | <10 | [57] |
| Al(AcAc)/Al(NO3)3, Y acetate/Y(NO3)3 | 260–385 °C, 24 MPa | Cubic Y2Al5O12 | <150 | [58] |
| Al(NO3)3, Y(NO3)3, Eu(NO3)3, KOH | 400 °C, 28 MPa | Cubic (Y,Eu)2Al5O12 | <100 | [59] |
| La(NO3)3, Ni(NO3)2, KOH | 400°C, 24 MPa | Rhombohedral Lan+1NinO3n+1 | <430 | [60,76] |
| Zr(CH3COO)4/Zr(CH3CH2O) | 300–450 °C, 10–45 MPa | Tetragonal/monoclinic ZrO2 | <10 | [67] |
| SnCl2 | 385–415 °C, 30 MPa | Tetragonal/SnO2 | <10 | [68] |
| Al(NO3)3, KOH | 400 °C, 30–40 MPa | γ-AlOOH, γ-Al2O3 | <20 | [69] |
| Fe(NO3)3, PVA | 487–648 K, 21.7–23 MPa | α-Fe2O3 | <23 | [70] |
| FeSO4, H3PO4, LiOH | 573–658 K | Orthorhombic LiFePO4 | <130 | [71] |
| TiO2 sol, Ba(OH)2 | 400 °C, 30 MPa | Tetragonal BaTiO3 | <20 | [72] |
| ZrO(NO3)2, Ba(OH)2 /Ba(NO3)2/Ba(CH3CO2)2, NaOH | 450–485 °C, 30 MPa | Cubic BaZrO3 | <100 | [73] |
| Fe(NO3)3, Ni(NO3)2, Cu(NO3)2, Zn(NO3)2, KOH | 400 °C, 30 MPa | Rhombohedral/Cubic, Tetragonal (Ni, Cu,Zn)Fe2O4 | <22 | [74] |
| Ca(NO3)2, Mg(NO3)2, (NH4)2HPO4 | 400 °C, 30 MPa | Ca10-xMgx(PO4)6(OH)2/Ca3-yMgy(HPO4)2(PO4)2-2x/3 | <80 | [75] |
| ZrO(NO3)2, Ce(NO3)3, NH4OH | Supercritical Conditions | Cubic/Tetragonal CexZr1-xO2 | 7–16 | [77] |
| Co(NO3)2, KOH, Ni(NO3)2/Ni(CH3CO2)2H2O2 | 90–310 °C, 24.1 MPa | Hexagonal, Cubic Ni(OH)2CoxNi1-x(OH)2, NiCo2O4 | <100 | [78] |
| Zn(NO3)2, KOH, hexylamine | 400 °C, 30 MPa | Hexagonal ZnO | <150×600 (rod) | [79] |
| Ce(NO3)3, Hexanoic acid | 250 °C, 25 MPa | Cubic CeO2 | <60 | [80] |
| Ce(NO3)3, Decanoic acid (MetOH) | 400 °C, 30 MPa | Cubic CeO2 | <50 | [81] |
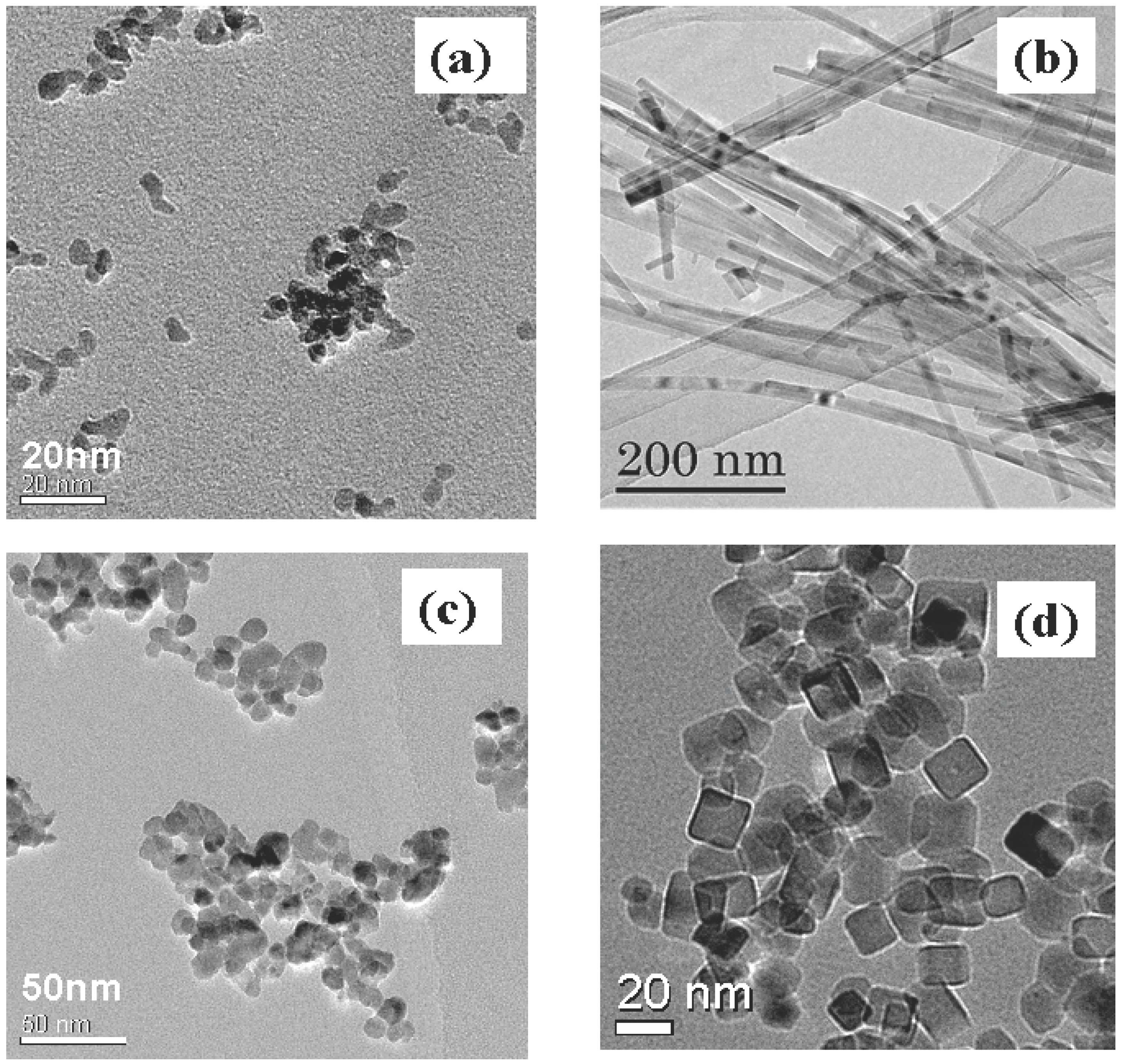
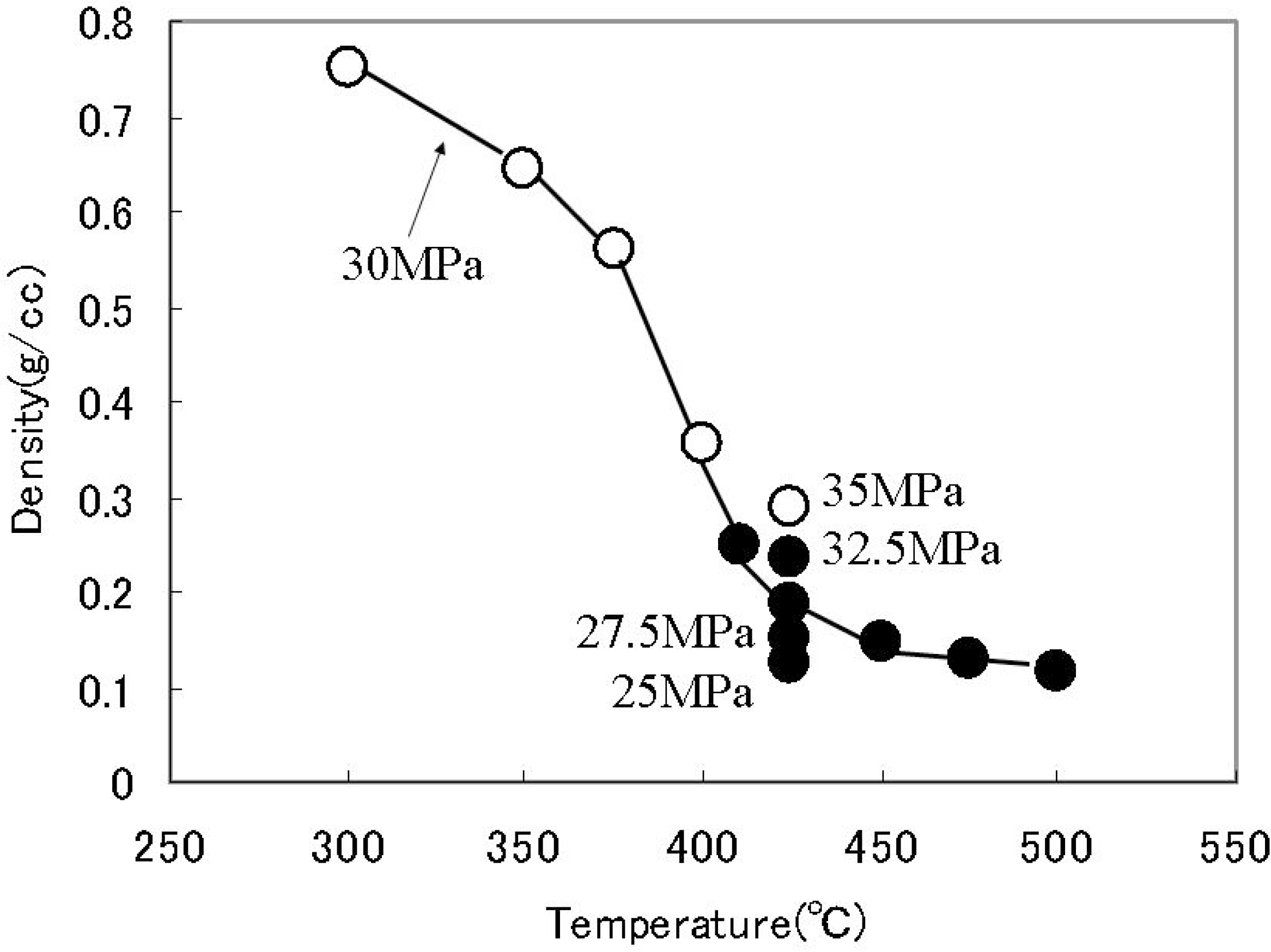

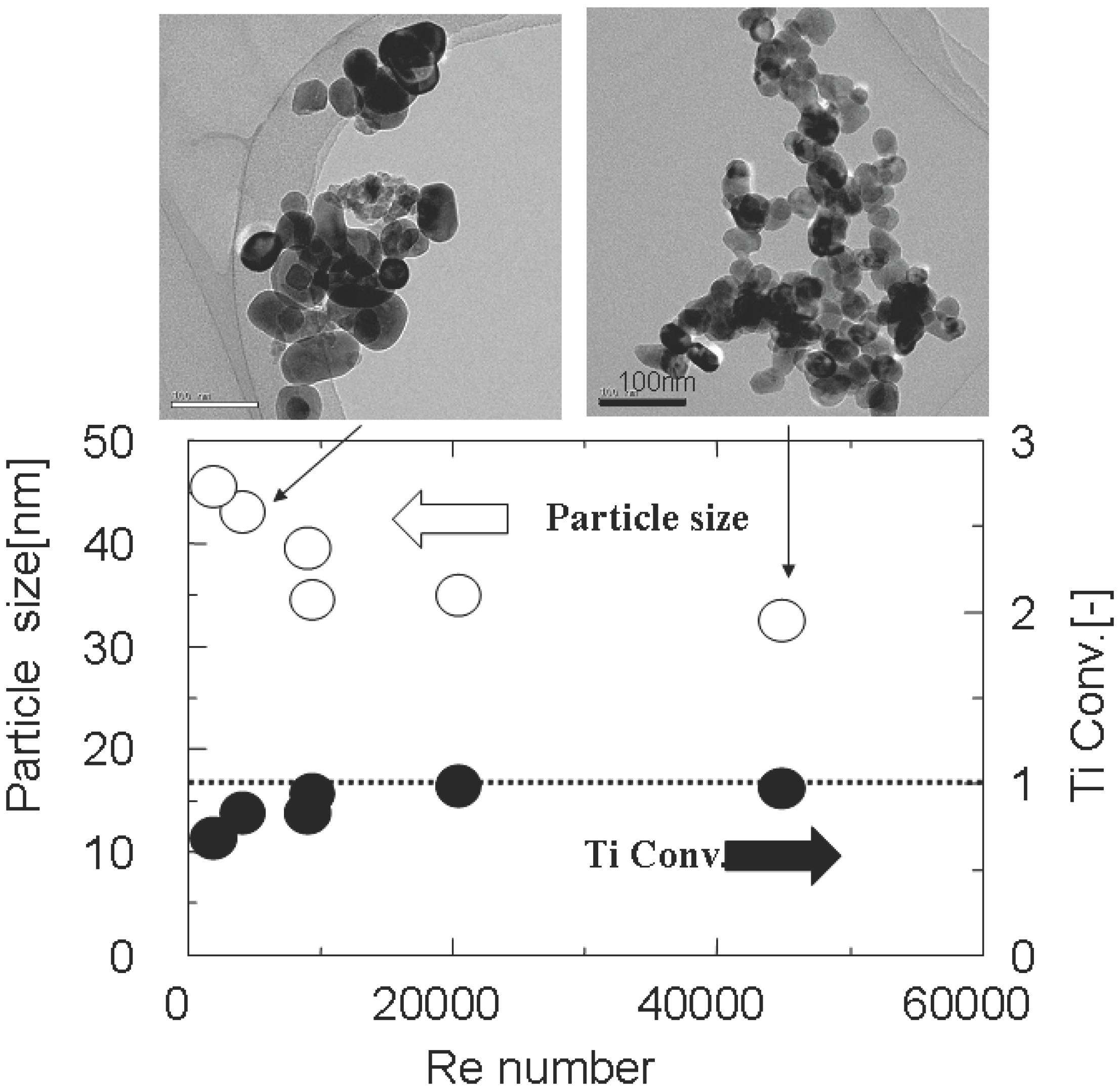
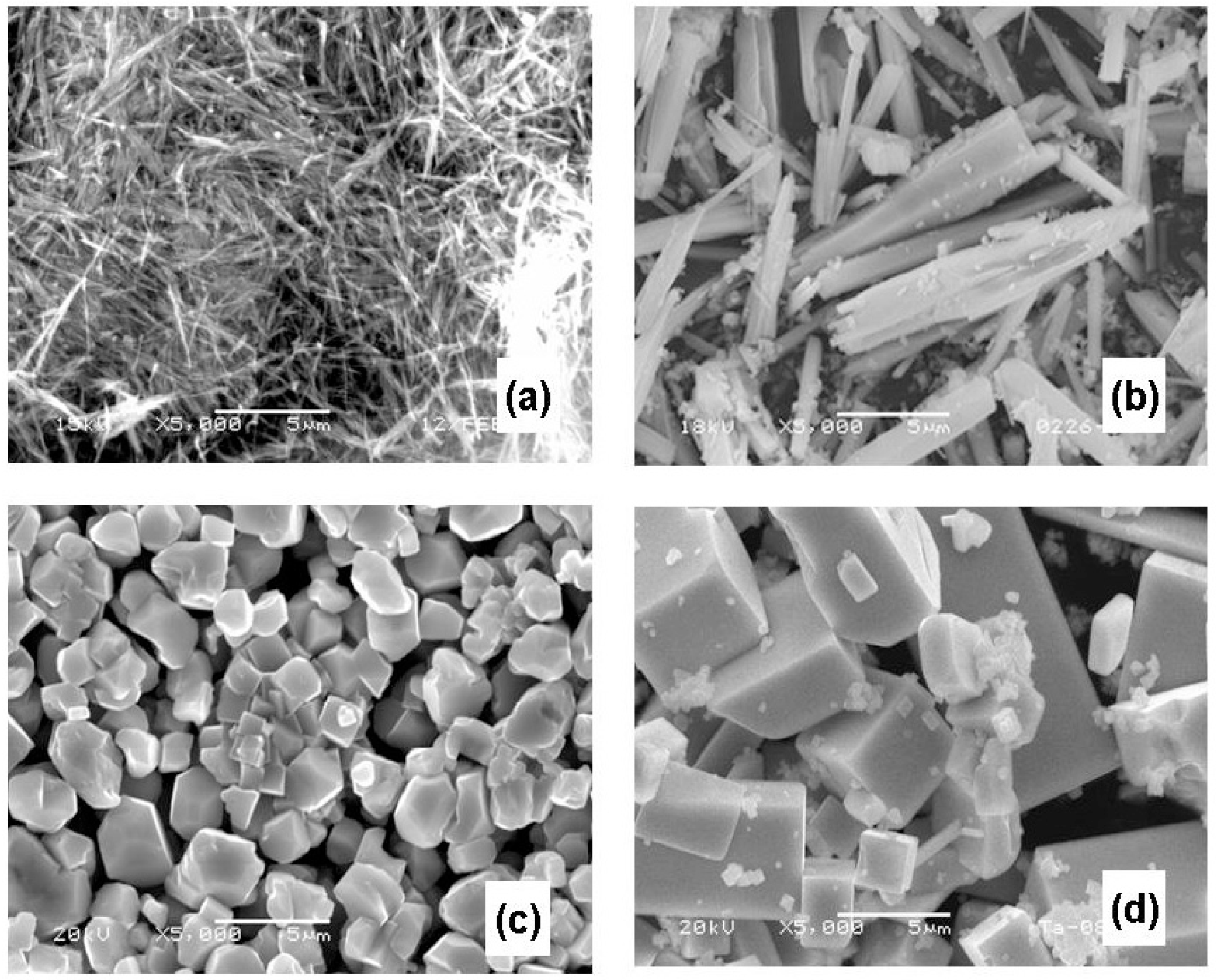
2.3. Future Issues
3. Experimental Section
3.1. Supercritical Hydrothermal Batchwise System
3.2. Supercritical Hydrothermal Flow System
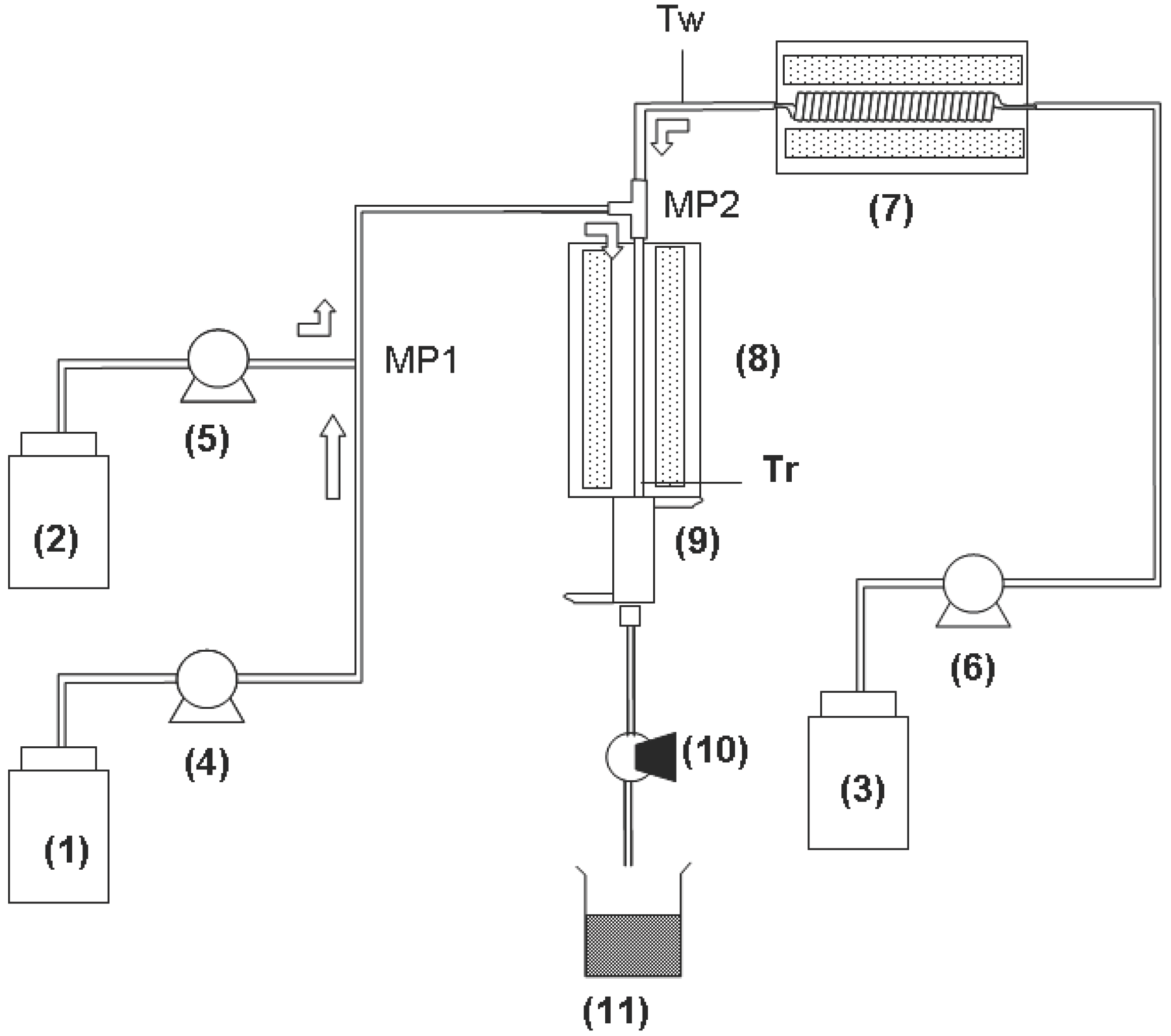
4. Conclusions
References
- Adschiri, T.; Hakuta, Y.; Arai, K. Hydrothermal synthesis of metal oxide fine particles at supercritical conditions. Ind. Eng. Chem. Res. 2000, 39, 4901–4907. [Google Scholar] [CrossRef]
- Adschiri, T.; Hakuta, Y.; Sue, K.; Arai, K. Hydrothermal synthesis of metal oxide nanoparticles at supercritical conditions. J. Nanopart. Res. 2000, 3, 227–235. [Google Scholar] [CrossRef]
- Hakuta, Y.; Hayashi, H.; Arai, K. Fine particle formation using supercritical fluids. Curr. Opin. Solid State Mat. Sci. 2003, 7, 341–351. [Google Scholar] [CrossRef]
- Hayashi, H.; Torii, K. Hydrothermal synthesis of titania photocatalyst under subcritical and supercritical water conditions. J. Mater. Chem. 2002, 12, 3671–3676. [Google Scholar] [CrossRef]
- Yahya, R.B.; Hayashi, H.; Nagase, T.; Ebina, T.; Onodera, Y.; Saitoh, N. Hydrothermal synthesis of potassium hexatitanates under subcritical and supercritical water conditions and its application in photocatalysts. Chem. Mater. 2001, 13, 842–847. [Google Scholar] [CrossRef]
- Hayashi, H.; Hakuta, Y.; Kurata, Y. Hydrothermal synthesis of potassium niobate photocatalysts under subcritical and supercritical water conditions. J. Mater. Chem. 2004, 14, 2046–2051. [Google Scholar] [CrossRef]
- Li, B.; Hakuta, Y.; Hayashi, H. Hydrothermal synthesis of KNbO3 powders in supercritical water and its nonlinear optical properties. J. Supercrit. Fluid. 2005, 35, 254–259. [Google Scholar] [CrossRef]
- Li, B.; Hakuta, Y.; Hayashi, H. Hydrothermal synthesis of crystalline rectangular titanoniobate particles. Chem. Commum. 2005, 1732–1734. [Google Scholar] [CrossRef]
- Li, B.; Hakuta, Y.; Hayashi, H. Synthesis of potassium titanoniobate in supercritical and subcritical water and investigations on its photocatalytic performance. J. Supercrit. Fluid. 2006, 39, 63–69. [Google Scholar] [CrossRef]
- Li, B.; Hakuta, Y.; Hayashi, H. The synthesis of titanoniobate compound characteristic of various particle morphologies through a novel solvothermal route. Mater. Lett. 2007, 61, 3791–3794. [Google Scholar] [CrossRef]
- Hayashi, H.; Hakuta, Y. Hydrothermal epitaxy of KTaO3 thin films under supercritical water conditions. J. Mater. Sci. 2008, 43, 2342–2347. [Google Scholar] [CrossRef]
- Takesue, M.; Shimoyama, K.; Murakami, S.; Hakuta, Y.; Hayashi, H.; Smith, R.L., Jr. Phase formation of Mn-doped zinc silicate in water at high-temperatures and high-pressures. J. Supercrit. Fluid. 2007, 43, 214–221. [Google Scholar] [CrossRef]
- Takesue, M.; Suino, A.; Hakuta, Y.; Hayashi, H.; Smith, R.L., Jr. Formation mechanism and luminescence appearance of Mn-doped zinc silicate particles synthesized in supercritical water. J. Solid State Chem. 2008, 181, 1307–1313. [Google Scholar] [CrossRef]
- Takesue, M.; Suino, A.; Shimoyama, K.; Hakuta, Y.; Hayashi, H.; Smith, R.L., Jr. Formation of α- and β-phase Mn-doped zinc silicate in supercritical water and its luminescent properties at Si/(Zn+Mn) ratios from 0.25 to 1.25. J. Cryst. Growth 2008, 310, 4185–4189. [Google Scholar] [CrossRef]
- Takesue, M.; Shimoyama, K.; Shibuki, K.; Suino, A.; Hakuta, Y.; Hayashi, H.; Ohishi, Y.; Smith, R.L., Jr. Formation of Mn-doped zinc silicate in supercritical water followed with in situ synchrotron radiation X-ray diffraction. J. Supercrit. Fluid. 2009, 49, 351–355. [Google Scholar] [CrossRef]
- Takesue, M.; Suino, A.; Hakuta, Y.; Hayashi, H.; Smith, R.L., Jr. Crystallization trigger of Mn-doped zinc silicate in supercritical water via Zn, Mn, Si sources and complex agent ethylenediamine tetraacetic acid. Mater. Chem. Phys. 2010, 121, 330–334. [Google Scholar] [CrossRef]
- Hakuta, Y.; Shimoyachi, K.; Hayashi, H.; Arai, K. Hydrothermal synthesis of potassium hexatitanate photocatalyst under supercritical water conditions. J. Ion Exch. 2003, 14, 393–396. [Google Scholar] [CrossRef]
- Hakuta, Y.; Hayashi, H.; Arai, K. Hydrothermal synthesis of photocatalyst potassium hexatitanate nanowires under supercritical water conditions. J. Mater. Sci. 2004, 39, 4977–4980. [Google Scholar] [CrossRef]
- Hakuta, Y.; Ohashi, T.; Hayashi, H.; Arai, K. Hydrothermal synthesis of zirconia nanocrystals in supercritical water. J. Mater. Res. 2004, 19, 2230–2234. [Google Scholar] [CrossRef]
- Hakuta, Y.; Ura, H.; Hayashi, H.; Arai, K. Effects of hydrothermal synthetic conditions on the particle size of γ-☐AlO(OH) in sub and supercritical water using a flow reaction system. Mater. Chem. Phys. 2005, 93, 466–472. [Google Scholar] [CrossRef]
- Noguchi, T.; Matsui, K.; Islam, N.M.; Hakuta, Y.; Hayashi, H. Rapid synthesis of γ-Al2O3 nanoparticles in supercritical water by continuous hydrothermal flow reaction system. J. Supercrit. Fluid. 2008, 46, 129–136. [Google Scholar] [CrossRef]
- Hakuta, Y.; Ura, H.; Hayashi, H.; Arai, K. Effect of water density on polymorph of BaTiO3 nanoparticles synthesized under sub and supercritical water conditions. Mater. Lett. 2005, 59, 1387–1390. [Google Scholar] [CrossRef]
- Hakuta, Y.; Ura, H.; Hayashi, H.; Arai, K. Continuous production of BaTiO3 nanoparticles by hydrothermal synthesis. Ind. Eng. Chem. Res. 2005, 44, 840–846. [Google Scholar] [CrossRef]
- Matsui, K.; Noguchi, T.; Islam, N.M.; Hakuta, Y.; Hayashi, H. Rapid synthesis of BaTiO3 nanoparticles in supercritical water by continuous hydrothermal flow reaction system. J. Cryst. Growth 2008, 310, 2584–2589. [Google Scholar] [CrossRef]
- Lu, J.; Hakuta, Y.; Hayashi, H.; Ohashi, T.; Nagase, T.; Hoshi, Y.; Sato, K.; Nishioka, M.; Inoue, T.; Hamakawa, S. Preparation of Ca0.8Sr0.2Ti1-xFexO3-□(x = 0.1–0.3) nanoparticles using a flow supercritical reaction system. J. Supercrit. Fluid. 2008, 46, 77–82. [Google Scholar] [CrossRef]
- Hayashi, H.; Ueda, A.; Suino, A.; Hiro, K.; Hakuta, Y. Hydrothermal synthesis of yttria stabilized ZrO2 nanoparticles in subcritical and supercritical water using a flow reaction system. J. Solid State Sci. 2009, 182, 2985–2990. [Google Scholar] [CrossRef]
- Imai, Y.; Terahara, A.; Hakuta, Y.; Matsui, K.; Hayashi, H.; Ueno, N. Transparent poly(bisphenol A carbonate)-based nanocomposites with high refractive index nanoparticles. Euro. Poly. J. 2009, 45, 630–638. [Google Scholar] [CrossRef]
- Sue, K.; Suzuki, M.; Arai, K.; Ohashi, T.; Ura, H.; Matsui, K.; Hakuta, Y.; Hayashi, H.; Watanabe, M.; Hiaki, T. Size-controlled synthesis of metal oxide nanoparticles with a flow-through supercritical water method. Green Chem. 2006, 8, 634–638. [Google Scholar] [CrossRef]
- Goh, G.K.L.; Lange, F.F.; Haile, S.M.; Levi, C.G. Hydrothermal syntheis of KNbO3 and NaNbO3 powders. J. Mater. Res. 2003, 18, 338–345. [Google Scholar] [CrossRef]
- Lu, C.-H.; Lo, S.-Y.; Wang, Y.-L. Glycothermal preparation of potassium niobate ceramic particles under supercritical conditions. Mater. Lett. 2002, 55, 121–125. [Google Scholar] [CrossRef]
- Goh, G.K.L.; Haile, S.M.; Levi, C.G.; Lange, F.F. Hydrothermal synthesis of perovskite and pyrochlore powders of potassium tantalate. J. Mater. Res. 2002, 17, 3168–3174. [Google Scholar] [CrossRef]
- Sorescua, M.; Diamandescua, L.; Tarabasanu, D. α-Fe2O3–In2O3 mixed oxide nanoparticles synthesized under hydrothermal supercritical conditions. J. Phys. Chem. Solids 2004, 65, 1719–1725. [Google Scholar] [CrossRef]
- Lee, J.; Teja, A.S. Characteristics of lithium iron phosphate (LiFePO4) particles synthesized in subcritical and supercritical water. J. Supercrit. Fluid. 2005, 35, 83–90. [Google Scholar] [CrossRef]
- Umetsu, M.; Man, X.; Okuda, K.; Tahereh, M.; Ohara, S.; Zhang, J.; Takami, S.; Adschiri, T. Biomass-assisted hydrothermal synthesis of ceria nanoparticle–A new application of lignin as a bio-nanopool. Chem. Lett. 2006, 35, 732–733. [Google Scholar] [CrossRef]
- Zheng, Q.X.; Li, B.; Xue, M.; Zhang, H.D.; Zhan, Y.J.; Pang, W.S.; Tao, X.T.; Jiang, M.H. Synthesis of YVO4 and rare earth-doped YVO4 ultra-fine particles in supercritical water. J. Supercrit. Fluid. 2006, 39, 63–69. [Google Scholar] [CrossRef]
- Zhao, D.; Han, E.; Wu, X.; Guan, H. Hydrothermal synthesis of ceria nanoparticles supported on carbon nanotubes in supercritical water. Mater. Lett. 2006, 60, 3544–3547. [Google Scholar] [CrossRef]
- Xu, C.B.; Teja, A.S. Supercritical water synthesis and deposition of iron oxide(alpha-Fe2O3) nanoparticles in activated carbon. J. Supercrit. Fluid. 2006, 39, 135–141. [Google Scholar] [CrossRef]
- Yoon, M.J.; In, J.H.; Lee, H.C.; Lee, C.H. Comparison of YAG:Eu phosphor synthesized by supercritical water and solid-state methods in a batch reactor. Korean J. Chem. Eng. 2006, 23, 842–846. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, X.; Guan, H.; Han, E. Study on supercritical hydrothermal synthesis of CoFe2O4 nanoparticles. J. Supercrit. Fluid. 2007, 42, 226–233. [Google Scholar] [CrossRef]
- Assaaoudi, H.; Fang, Z.; Butler, I.S.; Ryan, D.H.; Kozinski, J.A. Characterization of a new magnesium hydrogen orthophosphate salt, Mg3.5H2(PO4)3, synthesized in supercritical water. Solid State Sci. 2007, 9, 385–393. [Google Scholar] [CrossRef]
- Assaaoudi, H.; Fang, Z.; Butler, I.S.; Kozinski, J.A. Synthesis of erbium hydroxide microflowers and nanostructures in subcritical water. Nanotechnology 2008, 19, 185606. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sue, K.; Tsumatori, H.; Suzuki, M.; Tanaka, S.; Kawai-Nakamura, A.; Saitoh, K.; Aida, K.; Hiaki, T. Hydrothermal synthesis of CuAlO2 with the delafossite structure in supercritical water. J. Supercrit. Fluid. 2008, 46, 173–177. [Google Scholar] [CrossRef]
- Viswanathan, R.; Gupta, R.B. Formation of zinc oxide nanoparticles in supercritical water. J. Supercrit. Fluid. 2003, 27, 187–193. [Google Scholar] [CrossRef]
- Hao, Y.; Teja, A.S. Continuous hydrothermal crystallization of α-Fe2O3 and Co3O4 nanoparticles. J. Mater. Res. 2003, 18, 415–422. [Google Scholar] [CrossRef]
- Cote, L.J.; Teja, A.S.; Wilkinson, A.P.; Zhang, Z.J. Continuous hydrothermal synthesis of CoFe2O4 nanoparticles. Fluid Phase Equilibria 2003, 210, 307–317. [Google Scholar] [CrossRef]
- Hakuta, Y.; Haganuma, T.; Sue, K.; Adschiri, T.; Arai, K. Continuous production of phosphor YAG:Tb nanoparticles by hydrothermal synthesis in supercritical water. Mater. Res. Bull. 2003, 38, 1257–1265. [Google Scholar] [CrossRef]
- Ohara, S.; Mousavand, T.; Umetsu, M.; Takami, S.; Adschiri, T.; Kuroki, Y.; Takata, M. Hydrothermal synthesis of fine zinc oxide particles under supercriticalconditions. Solid State Ionics 2004, 172, 261–264. [Google Scholar] [CrossRef]
- Sue, K.; Kimura, K.; Murata, K.; Arai, K. Effect of cations and anions on properties of zinc oxide particles synthesized in supercritical water. J. Supercrit. Fluid. 2004, 30, 325–331. [Google Scholar] [CrossRef]
- Sue, K.; Kimura, K.; Yamamoto, M.; Arai, K. Rapid hydrothermalsynthesis of ZnO nanorods without organics. Mater. Lett. 2004, 58, 3350–3352. [Google Scholar] [CrossRef]
- Lee, H.-C.; Kim, J.-J.; In, J.-H.; Lee, C.-H. NaFeEDTA decomposition and hematite nanoparticle formation in supercritical water oxidation. Ind. Eng. Chem. Res. 2005, 44, 6615–6621. [Google Scholar] [CrossRef]
- Reverón, H.; C. Aymonier, H.; Loppinet-Serani, A.; Elissalde, C.; Maglione, M.; Cansell, F. Single-step synthesis of well-crystallized and pure barium titanate nanoparticles in supercritical fluids. Nanotechnology 2005, 16, 1137–1143. [Google Scholar] [CrossRef]
- Elissalde, C.; Reverón, H.; Aymonier, C.; Michau, D.; Cansell, F.; Maglione, M. The ferroelectric transition temperature as an intrinsic probe for sinterednanocrystalline BaTiO3 synthesized under supercritical conditions. Nanotechnology 2005, 16, 797–802. [Google Scholar] [CrossRef]
- Reverón, H.; Elissalde, C.; Aymonier, C.; Bidault, O.; Maglione, M.; Cansell, F. Supercritical fluid route for synthesizing crystalline barium strontium titanate nanoparticles. J. Nanosci. Nanotechnol. 2005, 5, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Reverón, H.; Elissade, C.; Aymonier, C.; Bousquet, C.; Maglione, M.; Cansell, F. Continuous supercritical synthesis and dielectric behavior of the whole BST solid solution. Nanotechnology 2006, 17, 3527–3532. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ham, J.Y. Synthesis of manganese oxide particles in supercritical water. Korean J. Chem. Eng. 2006, 23, 714–719. [Google Scholar] [CrossRef]
- Levy, C.; Watanabe, M.; Aizawa, Y.; Inomata, H.; Sue, K. Synthesis of nanophased metal oxides in supercritical water. Catalysts for biomass conversion. Int. J. Appl. Ceram. Technol. 2006, 3, 337–344. [Google Scholar] [CrossRef]
- Fang, Z.; Assaaoudi, H.; Guthrie, R.I.L.; Kozinski, J.A. Continuous synthesis of tin and indium oxide nanoparticles in sub- and supercritical water. J. Amer. Ceram. Soc. 2007, 90, 2367–2371. [Google Scholar] [CrossRef]
- Cabanas, A.; Li, J.; Blood, P.; Chudoba, T.; Lojkowski, W.; Poliakoff, M.; Lester, E. Synthesis of nanoparticulate yttrium aluminum garnet in supercritical water-ethanol mixtures. J. Supercrit. Fluid. 2007, 40, 284–292. [Google Scholar] [CrossRef]
- In, J.H.; Lee, H.C.; Yoon, M.J.; Lee, K.K.; Lee, J.W.; Lee, C.H. Syntesis of nano-sized YAG: Eu3+ phosphor in continuous supercritical water system. J. Supercrit. Fluid. 2007, 40, 389–396. [Google Scholar] [CrossRef]
- Weng, X.L.; Boldrin, P.; Abrahams, I.; Skinner, S.J.; Darr, J.A. Direct syntheses of mixed ion and electronic conductors La4Ni3O10 and 3Ni2O7 from nanosized coprecipitates. Chem. Mater. 2007, 19, 4382–4384. [Google Scholar] [CrossRef]
- Yoon, M.J.; Bae, Y.S.; Son, S.H.; Lee, J.W.; Lee, C.H. Comparison of YAG: Eu phosphors synthesized by supercritical water in batch and continuous reactors. Korean J. Chem. Eng. 2007, 24, 877–880. [Google Scholar] [CrossRef]
- Zhang, J.; Ohara, S.; Umetsu, M.; Naka, T.; Hatakeyama, Y.; Adschiri, T. Colloidal ceria nanocrystals: A tailor-made crystal morphology in supercritical water. Adv. Mater. 2007, 19, 203–206. [Google Scholar] [CrossRef]
- Rangappa, D.; Ohara, S.; Naka, T.; Kondo, A.; Ishii, M.; Adschiri, T. Synthesis and organic modification of CoAl2O4 nanocrystals under supercritical water conditions. J. Mater. Chem. 2007, 17, 4426–4429. [Google Scholar] [CrossRef]
- Rangappa, D.; Ohara, S.; Naka, T.; Kondo, A.; Ishii, M.; Kobayashi, T.; Adschiri, T. Transparent CoAl2O4 hybrid nano pigment by organic ligand-assisted supercritical water. J. Amer. Chem. Soc. 2007, 129, 11061–11066. [Google Scholar] [CrossRef]
- Mousavand, T.; Zhang, J.; Ohara, S.; Umetsu, M.; Naka, T.; Adschiri, T. Organic ligand-assisted supercritical hydrothermal synthesis of titanium oxide nano-crystals leading to perfect dispersed titanium oxide nanoparticle in organic phase. J. Nanopart. Res. 2007, 9, 1067–1071. [Google Scholar] [CrossRef]
- Mousavand, T.; Ohara, S.; Umetsu, M.; Zhang, J.; Takami, S.; Naka, T.; Adschiri, T. Hydrothermal synthesis and in situ surface modification of boemite nano-particles in supercritical water. J. Supercrit. Fluid. 2007, 40, 397–401. [Google Scholar] [CrossRef]
- Becker, J.; Hald, P.; Bremholm, M.; Pedersen, J.S.; Chevallier, J.; Iversen, S.B.; Iversen, B.B. Critical size of crystalline ZrO2 nanoparticles synthesized in near- and supercritical water and supercritical isopropyl alcohol. ACS Nano 2008, 2, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Assaaoudi, H.; Lin, H.B.; Wang, X.M.; Butler, I.S.; Kozinski, J.A. Synthesis of nanocrystalline SnO2 in supercritical water. J. Nanopart. Res. 2007, 9, 683–687. [Google Scholar] [CrossRef]
- Sato, T.; Sue, K.; Akiyama, Y.; Shibata, K.; Kawasaki, S.I.; Tanaka, S.; Saitoh, K.; Kawai-Nakamura, A.; Aida, K.; Hiaki, T. Effect of pH on hydrothermal synthesis of gamma-Al2O3 nanoparticles at 673 K. Chem. Lett. 2008, 37, 242–243. [Google Scholar] [CrossRef]
- Xu, C.B.; Teja, A.S. Continuous hydrothermal synthesis of iron oxide and PVA-protected iron oxide nanoparticles. J. Supercrit. Fluid. 2008, 44, 85–91. [Google Scholar] [CrossRef]
- Xu, C.B.; Lee, J.; Teja, A.S. Continuous hydrothermal synthesis of lithium iron phosphate particles in subcritical and supercritical water. J. Supercrit. Fluid. 2008, 44, 92–97. [Google Scholar] [CrossRef]
- Atashfaraz, M.; Shariaty-Niassar, M.; Ohara, S.; Minami, K.; Umetsu, M.; Naka, T.; Adschiri, T. Effect of titanium dioxide solubility on the formation of BaTiO3 nanoparticles in supercritical water. Fluid Phase Equilibria 2007, 257, 233–237. [Google Scholar] [CrossRef]
- Aimable, A.; Xin, B.; Millot, N.; Aymes, D. Continuous hydrothermal synthesis of nanometric BaZrO3 in supercritical water. J. Solid State Chem. 2008, 181, 183–189. [Google Scholar] [CrossRef]
- Sato, T.; Sue, K.; Suzuki, W.; Suzuki, M.; Matsui, K.; Hakuta, Y.; Hayashi, H.; Arai, K.; Kawasaki, S. I.; Kawai-Nakamura, A.; Hiaki, T. Rapid and continuous production of ferrite nanoparticles by hydrothermal synthesis at 673 K and 30 MPa. Ind. Eng. Chem. Res. 2008, 47, 1855–1860. [Google Scholar] [CrossRef]
- Chaudhry, A.A.; Goodall, J.; Vickers, M.; Cockcroft, J.K.; Rehman, I.; Knowles, J.C.; Darr, J.A. Synthesis and characterization of magnesium substituted calcium phosphate bioceramic nanoparticles made via continuous hydrothermal flow synthesis. J. Mater. Chem. 2008, 18, 5900–5908. [Google Scholar] [CrossRef]
- Weng, X.L.; Boldrin, P.; Abrahams, I.; Skinner, S.J.; kellici, S.; Darr, J.A. Direct syntheses of Lan +1 NinO3n+1 phases (n = 1,2,3 and infinity) from nanosized cocrystallites. J. Solid State Chem. 2008, 181, 1123–1132. [Google Scholar] [CrossRef]
- Kim, J.R.; Myeong, W.J.; Ihm, S.K. Characteristics in oxygen strage capacity of ceria-zirconia mixed oxides prepared by continuous hydrothermal synthesis in supercritical water. Appl. Catal. B-Environ. 2007, 71, 57–63. [Google Scholar] [CrossRef]
- Boldrin, P.; Hebb, A.K.; Chaudhry, A.A.; Otley, L.; Thiebaut, B.; Bishop, P.; Darr, J.A. Direct syntheses of nanosized NiCo2O4 spinel and related compounds via continuous hydrothermal synthesis methods. Ind. Eng. Chem. Res. 2007, 46, 4830–4838. [Google Scholar] [CrossRef]
- Ohara, S.; Mousavand, T.; Sasaki, T.; Umetsu, M.; Naka, T.; Adschiri, T. Continuous production of fine zinc oxide nanorods by hydrothermal synthesis in supercritical water. J. Mater. Sci. 2008, 43, 2393–2396. [Google Scholar] [CrossRef]
- Takami, S.; Ohara, S.; Adschiri, T.; Wakayama, Y.; Chikyow, T. Continuous synthesis of organic-inorganic hybridized cubic nanoassemblies of octahedral cerium oxide nanocrystals and hexanedioic acid. Dalton Trans. 2008, 40, 5442–5446. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, Y.S.; Veriansyah, B.; Kim, J.D.; Lee, Y.W. Continuous synthesis of surface-modified metal oxide nanoparticles using supercritical methanol for highly stabilized nanofluids. Chem. Mater. 2008, 20, 6301–6303. [Google Scholar] [CrossRef]
- Chudoba, T.; Lester, E.; Lojkowski, W.; Poliakoff, M.; Li, J.; Grzanka, E.; Presz, A. Synthesis of nano-sized yttrium–aluminum garnet in a continuous-flow reactor in supercritical fluids. Z. Naturforsch. Sect. B 2008, 63, 756–764. [Google Scholar]
- Wang, B.; Wilkers, G.L. New Ti-PTMO and Zr-PTMO creamer hybrid materials prepared by the sol gel method: Synthesis and characterization. J. Polym. Sci. Part A-Polym. Chem. 1991, 29, 905–909. [Google Scholar] [CrossRef]
- Wang, B.; Wilkers, G.L.; Smith, C.D.; McGrath, J.E. High refractive index hybrid creamer materials prepared from titanium tetraisopropoxide and poly(arylene ether phosphine oxide) through sol-gel processing. Polym. Commun. 1991, 32, 400–402. [Google Scholar]
- Wang, B.; Wilkers, G.L.; Hendrick, J.C.; Liptak, S.C.; McGrath, J.E. New high refractive index organic/inorganic hybrid materials from sol-gel processing. Macromolecules 1991, 24, 3449–3450. [Google Scholar] [CrossRef]
- Nussbaumer, R.J.; Caseri, W.R.; Smith, P.; Tervoort, T. Polymer-TiO2 nanocomposites: A route towards visually transparent broadband UV filters and high refractive index materials. Macromol. Mater. Eng. 2003, 288, 44–49. [Google Scholar] [CrossRef]
- Lu, C.; Cui, Z.; Guan, J.; Yang, B.; Shen, J. Research on preparation, structure and properties of TiO2/polythiourethane hybrid optical films with high refractive index. Macromol. Mater. Eng. 2003, 288, 717–723. [Google Scholar] [CrossRef]
- Xiong, M.; Zhou, S.; Wu, L.; Wang, B.; Yang, L. Sol-gel derived organic-inorganic hybrid from trialkoxysilane-capped acrylic resin and titania: effects of preparation conditions on the structure and properties. Polymer 2004, 45, 8127–8138. [Google Scholar] [CrossRef]
- Chen, W.C.; Liu, W.C.; Wu, P.T.; Chen, P.F. Synthesis and characterization of oligomeric phenylsilsesquioxane-titania hybrid optical films. Mater. Chem. Phys. 2004, 83, 71–77. [Google Scholar] [CrossRef]
- Nakayama, N.; Hayashi, T. Preparation and characterization of TiO2-ZrO2 and thiol-acrylate resin nanocompostes with high refractive indez via UV-induced crosslinking polymerization. Compos. Part A-Appl. Sci. Manuf. 2007, 38, 1996–2004. [Google Scholar] [CrossRef]
- Liu, J.G.; Nakamura, Y.; Ogura, T.; Shibasaki, Y.; Ando, S.; Ueda, M. Optically transparent sulfur-containing polyimide-TiO2 nanocomposite films with high refractive index and negative pattern formation from poly(amic acid)-TiO2 nanocomposite film. Chem. Mater. 2008, 20, 273–281. [Google Scholar] [CrossRef]
- Su, H.W.; Chen, W.C. High refractive index polyimide-nanocrystalite-titania hybrid optical materials. J. Mater. Chem. 2008, 18, 1139–1145. [Google Scholar] [CrossRef]
- Lee, S.; Shin, H.J.; Yoon, S.M.; Yi, D.K.; Choi, J.Y.; Paik, U. Refractive index engineering of transparent ZrO2-polydimethylsiloxane nanocomposites. J. Mater. Chem. 2008, 18, 1751–1755. [Google Scholar] [CrossRef]
- Lee, Y.-W. Formation of nano particles in supercritical fluids. In Proceedings of ISHR & ICSTR, Sendai, Japan, 5–9 August 2006.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hayashi, H.; Hakuta, Y. Hydrothermal Synthesis of Metal Oxide Nanoparticles in Supercritical Water. Materials 2010, 3, 3794-3817. https://doi.org/10.3390/ma3073794
Hayashi H, Hakuta Y. Hydrothermal Synthesis of Metal Oxide Nanoparticles in Supercritical Water. Materials. 2010; 3(7):3794-3817. https://doi.org/10.3390/ma3073794
Chicago/Turabian StyleHayashi, Hiromichi, and Yukiya Hakuta. 2010. "Hydrothermal Synthesis of Metal Oxide Nanoparticles in Supercritical Water" Materials 3, no. 7: 3794-3817. https://doi.org/10.3390/ma3073794




