Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering
Abstract
:1. Introduction
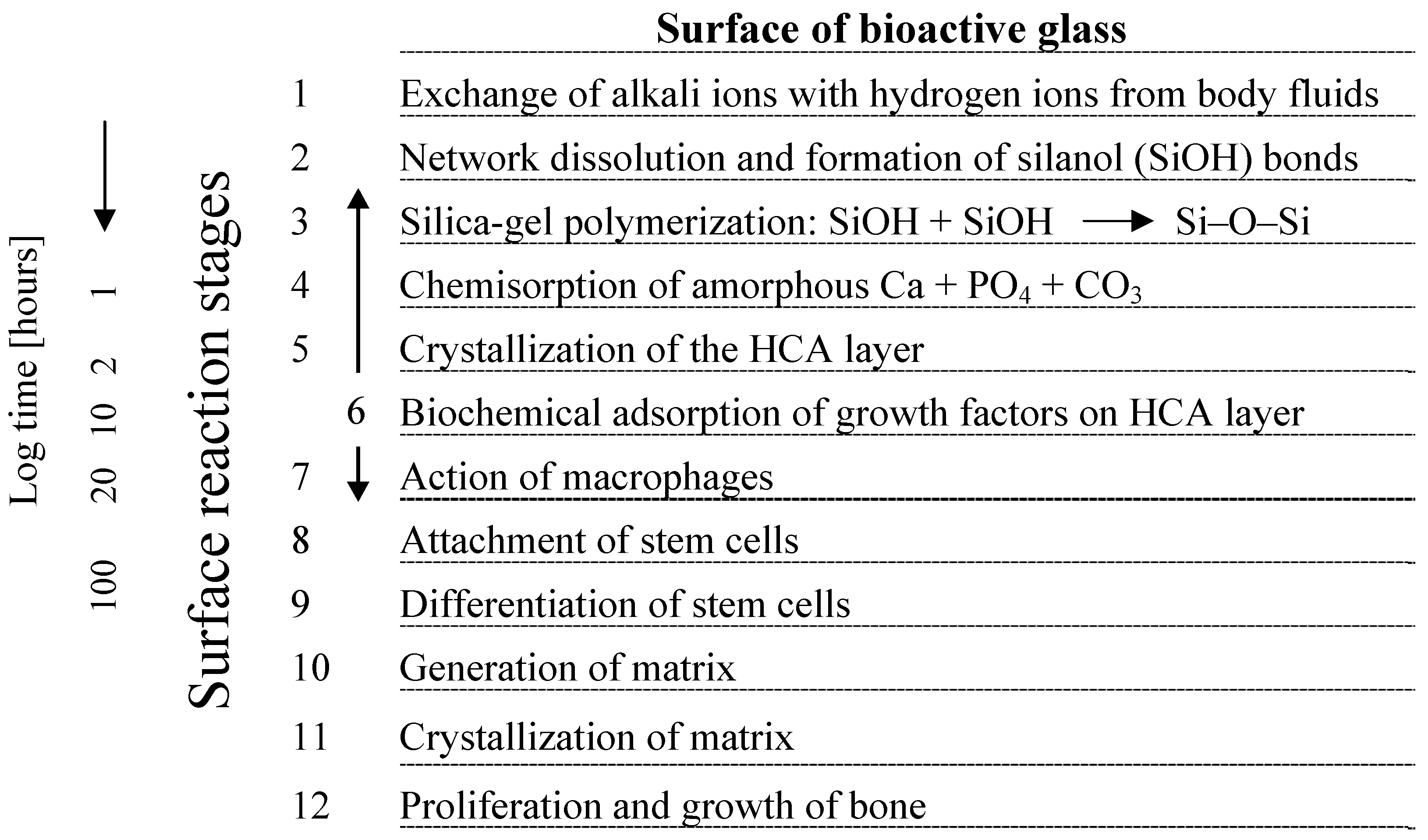
2. Basic Scaffold Requirements
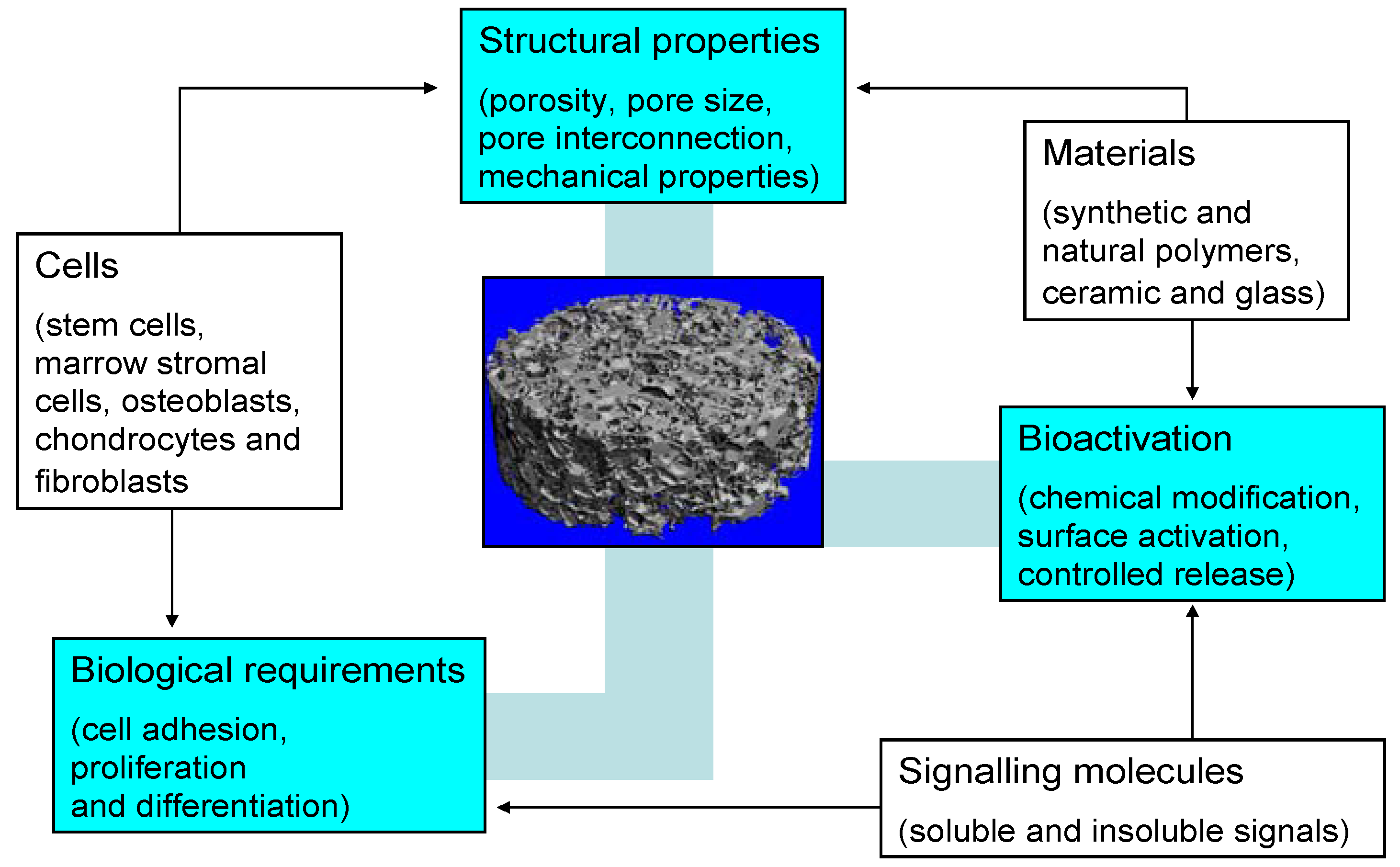
3. Silicate-Based Bioactive Glass Tissue Engineering Scaffolds
3.1. Bioactive Glass Based Glass-Ceramic Scaffolds
| Glass composition/system | Particle size of starting glass powder | Fabrication technique | Study |
|---|---|---|---|
| 45S5 | < 5 μm | Polymer foam replication | [95] |
| SiO2-CaO-CaF2-Na2O-K2O-P2O5-MgO | < 32 μm | Polymer foam replication | [13] |
| SiO2-P2O5-CaO-MgO-Na2O-K2O | < 30 µm | Polymer foam replication | [15,94] |
| SiO2-P2O5-CaO-MgO-Na2O-K2O | < 30 µm | Polymer foam replication | [108] |
| 45S5 | 10–20 µm | Polymer foam replication | [118] |
| SiO2-Na2O-CaO-MgO | < 100 μm | Starch consolidation | [14] |
| SiO2-P2O5-B2O3-CaO-MgO-K2O-Na2O | 75 μm§ | Compaction and sintering of melt-spun fibers | [113] |
| SiO2-CaO-Na2O-K2O-P2O5-MgO-CaF2 | < 106 µm | Polymer porogen bake-out | [102] |
| 45S5 | 20–50 μm | Polymer foam replication | [97] |
| SiO2-Na2O-K2O-MgO-CaO-P2O5 | 255–325 μm | Slip casting | [107] |
| SiO2-Na2O-K2O-MgO-CaO-P2O5 | < 5–10 μm | Polymer foam replication | [105] |
| SiO2-Na2O-K2O-MgO-CaO-P2O5 | < 5 µm | Freeze casting | [99] |
| SiO2-CaO-K2O | < 106 µm | Polymer porogen burn-off | [106] |
| SiO2-TiO2-B2O3-P2O5-CaO-MgO-K2O-Na2O | 75 μm§ | Compaction and sintering of melt-spun fibers | [30] |
| 45S5 | 45–90 μm | Polymer porogen bake-out | [119] |
| 45S5 | < 5 µm | Polymer foam replication | [103] |
| SiO2-Na2O-K2O-MgO-CaO-P2O5; 45S5 | 25–40 μm§ | Densification and sintering of melt-spun fibers | [114] |
| 45S5 | ≈ 5 μm | Polymer foam replication | [43] |
| 45S5 | 5–10 μm | Polymer foam replication | [109] |
| 45S5 | ≈ 10 μm | Polymer foam replication | [110] |
| SiO2-P2O5-CaO-MgO-Na2O-K2O | n.a. | Polymer burn-off, foam replication | [104] |
| 45S5 | < 5 µm | Polymer foam replication | [120] |
| SiO2-Na2O-CaO-P2O5-B2O3-TiO2 | n.a. | Solution combustion | [52] |
| SiO2-Na2O-CaO-P2O5-B2O3-TiO2 | n.a. | Solution combustion | [121] |
| SiO2-CaO-P2O5-Al2O3 | 8–30 μm§ | Manual free-forming of melt- spun fibers | [122] |
| SiO2-CaO-Na2O-P2O5-K2O-MgO-B2O3 | n.a. | Polymer foam replication | [123] |
| SiO2-CaO-Na2O-K2O-MgO-P2O5-B2O3 | 75 μm§ | Densification and sintering of melt-spun fibers | [124] |
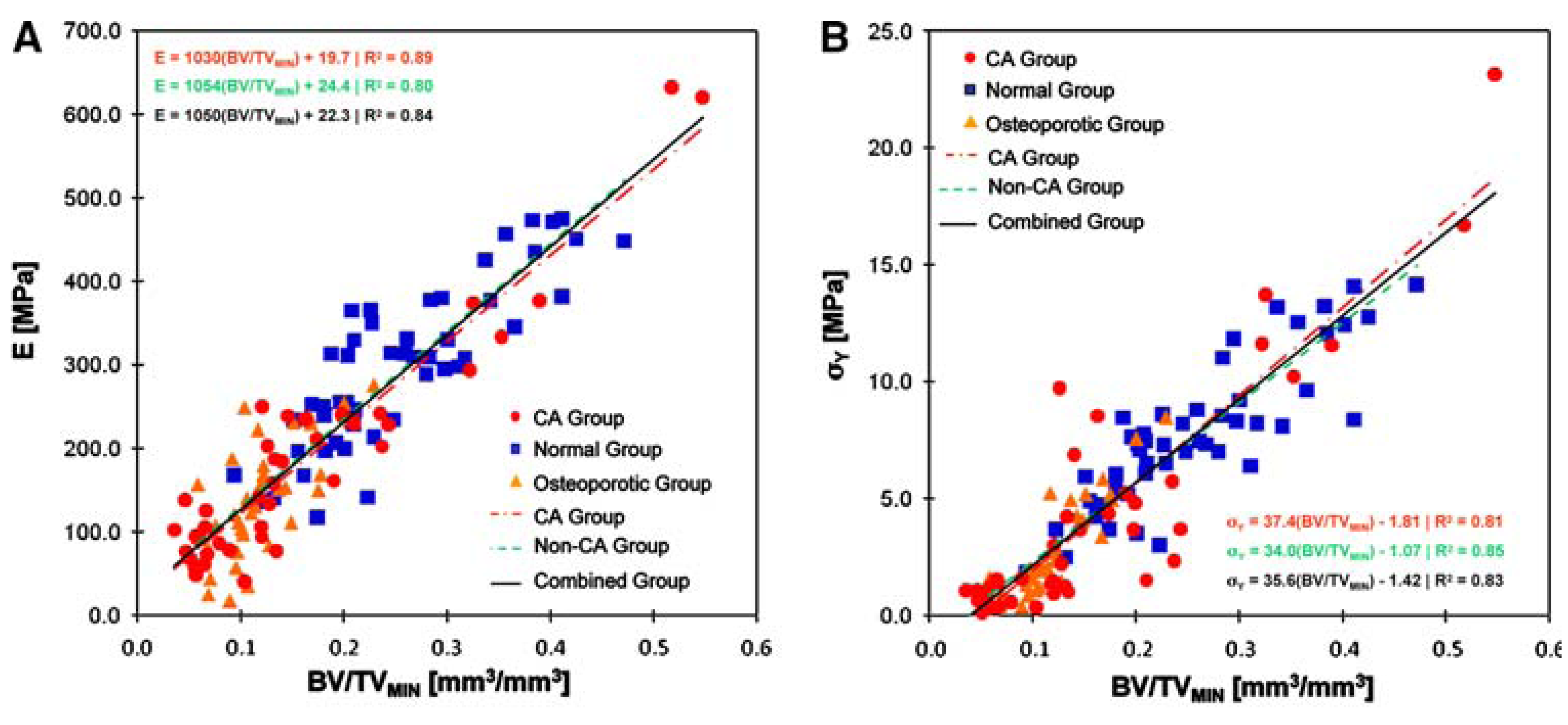
| Material property | Trabecular bone | Cortical bone | Bioglass® 45S5 |
|---|---|---|---|
| Compressive strength [MPa] | 0.1–16 [125,126] | 130–200 [37,125] | 500 [37] |
| Tensile strength [MPa] | n.a. | 50–151 [37] | 42 [70] |
| Compressive modulus [GPa] | 0.12–1.1 [127,128] | 11.5–17 [74] | n.a. |
| Young’s modulus [GPa] | 0.05–0.5 [37,129] | 7–30 [ 6,37,129] | 35 [70] |
| Fracture toughness [MPa·m1/2] | n.a. | 2–12 [37,70] | 0.7–1.1 [130,131] |
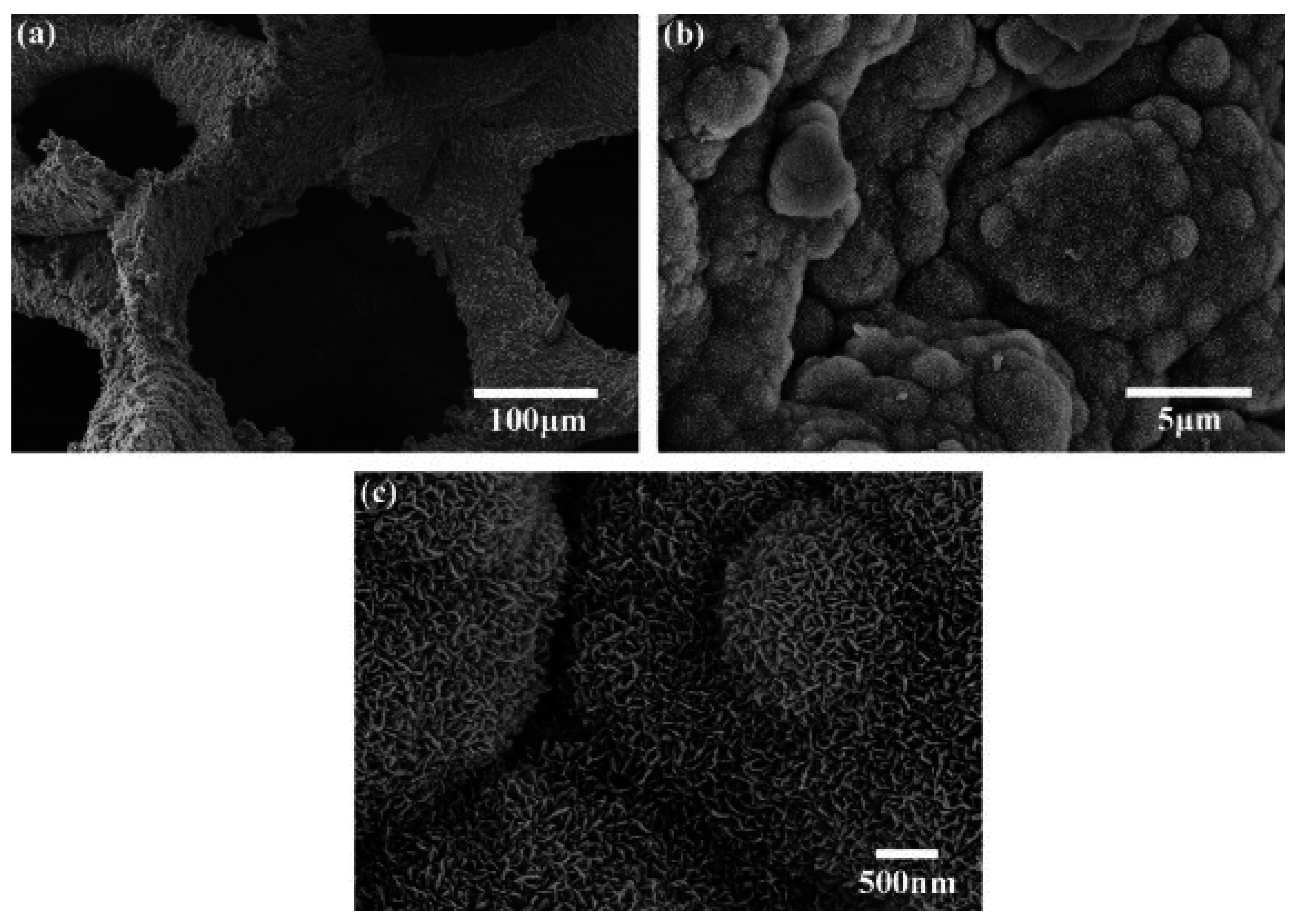

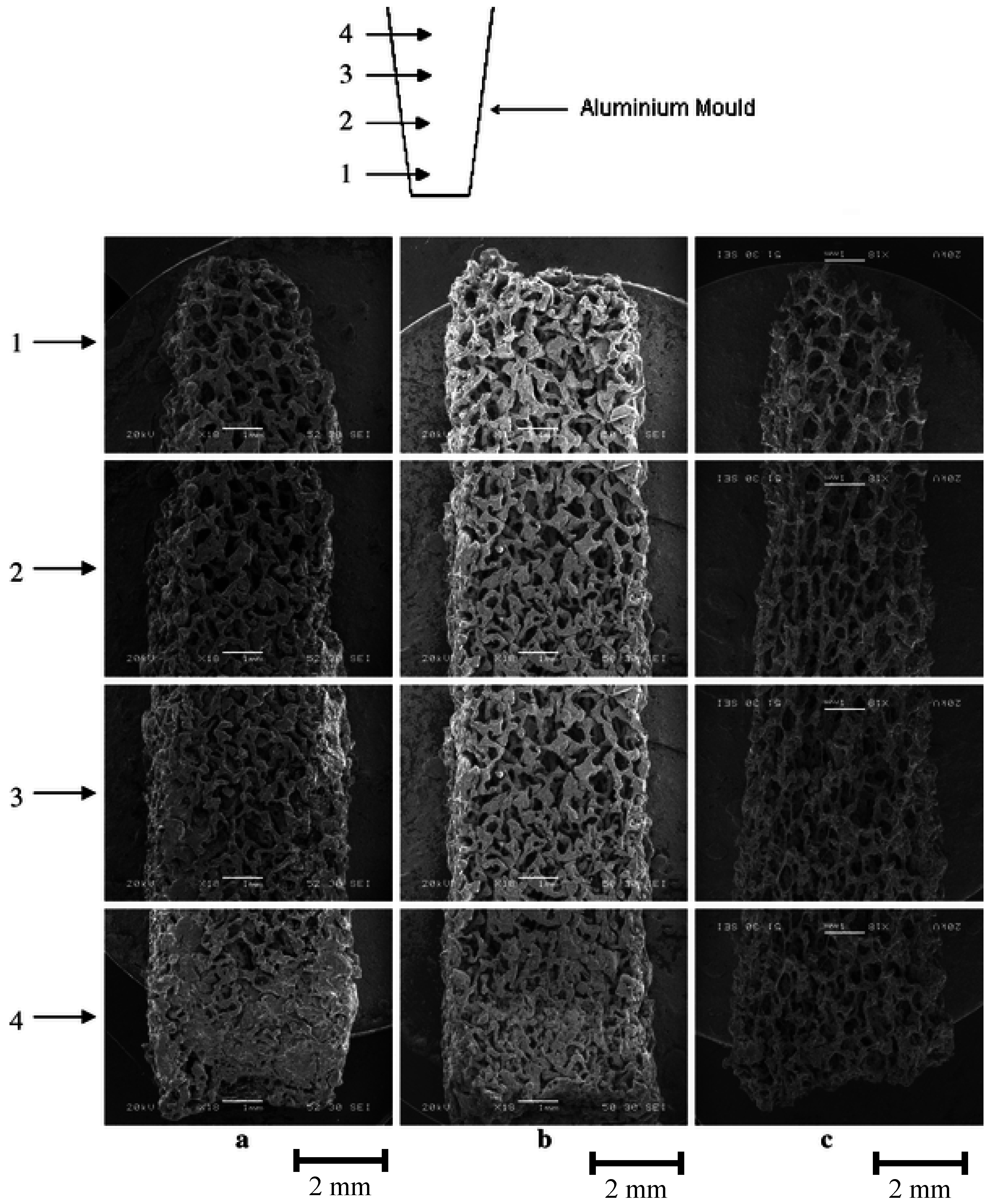


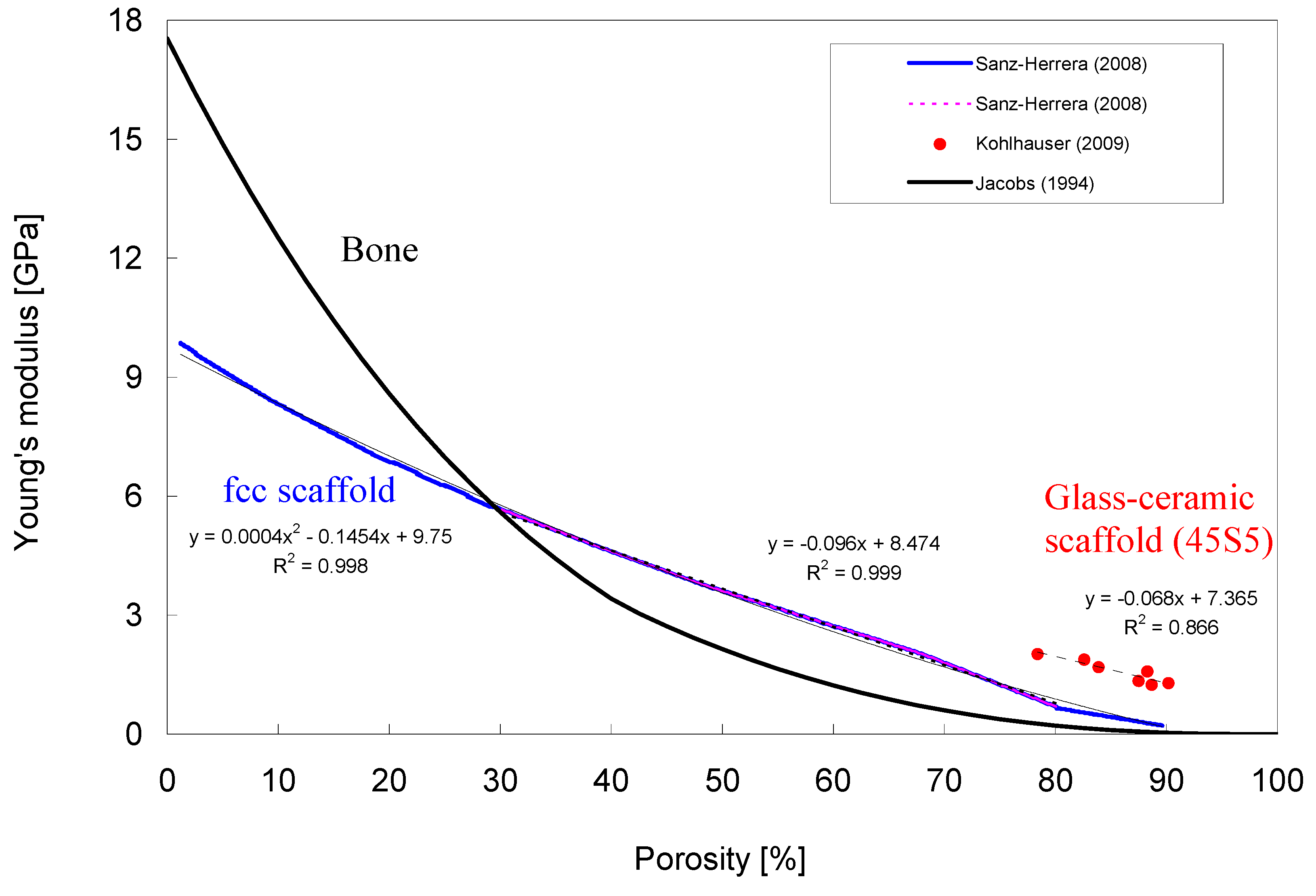
3.2. Bioactive Glass containing Composite Scaffolds
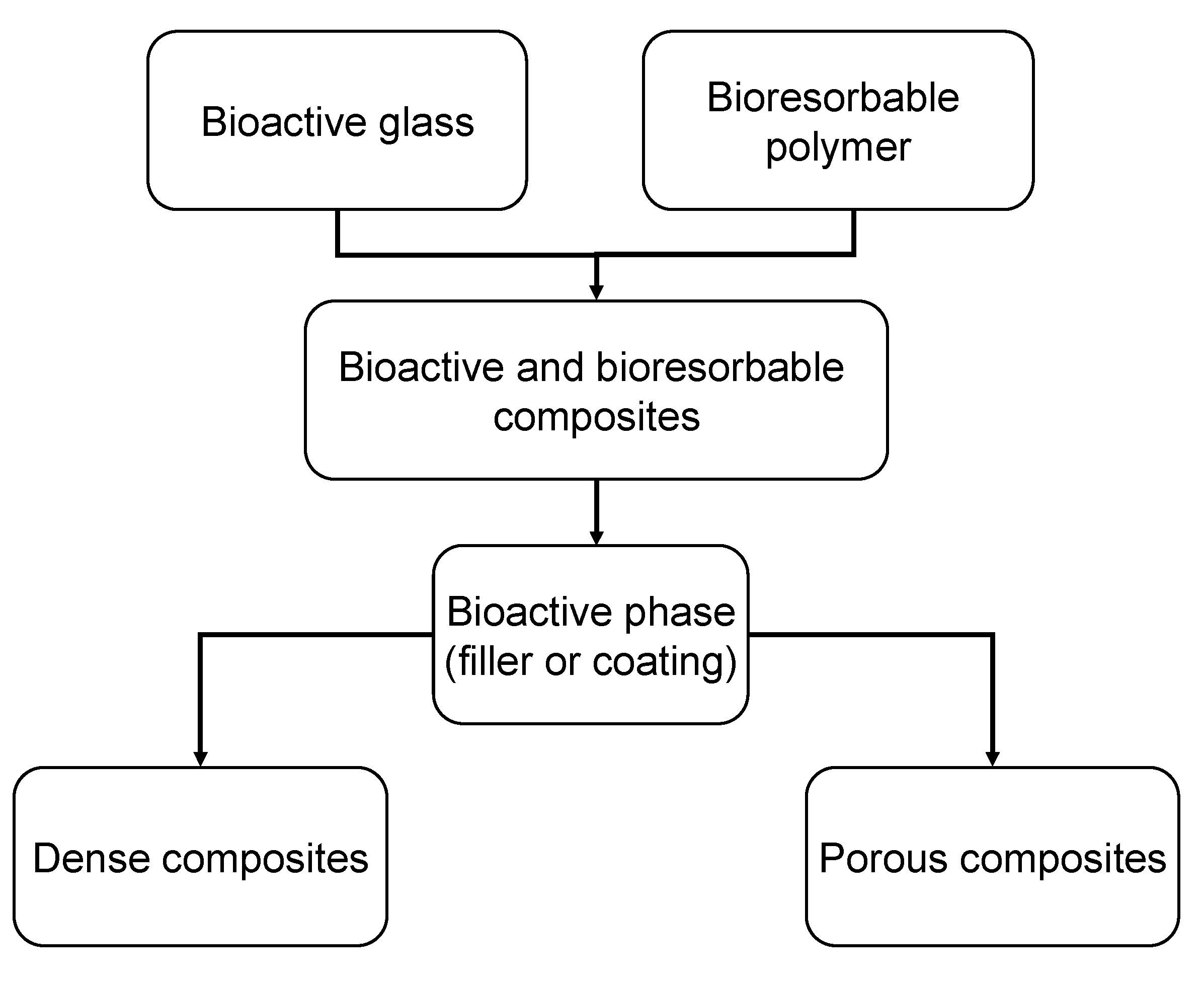
| Bioactive glass | wt % | Particle size | Matrix | Fabrication technique/process | Ref. |
| 45S5 m-BG | 5, 29, 40 | < 40 μm | PDLLA | Co-extrusion+compaction; TIPS | [159] |
| 45S5 m-BG | 4.8, 28.6 | 5–20 μm | PDLLA | TIPS | [160] |
| 45S5 m-BG | 10 | < 5 μm | P(3HB) | ST/PL | [47] |
| 45S5 n-BG | 10 | 30 nm | P(3HB) | ST/PL | [47] |
| S53P4 m-BG | 20, 50 | 90–315 µm | P(CL/DLLA) | ST/PL | [161] |
| S53P4 m-BG | 30 | < 45 μm | P(CL/DLLA) | ST/PL | [162] |
| 45S5 m-BG | 10, 30 | < 40 μm | PLGA | Microsphere emulsification | [163] |
| 45S5 m-BG | 10 | 4 μm | PDLG | TIPS | [164] |
| 45S5 m-BG | 25, 50 | 50–63 µm | PLA | Freeze extraction technique | [165] |
| 45S5 m-BG | 5, 40 | > 90 μm | PDLLA | Solvent casting | [166] |
| 45S5 m-BG | 10, 25, 50 | < 5 μm | PDLLA | TIPS | [167] |
| 45S5 m-BG | 10, 25, 50 | < 5 μm | PLGA | TIPS | [167] |
| 45S5 m-BG | 5, 10, 40 | < 5 μm | PDLLA | TIPS | [168] |
| 45S5 m-BG | 5, 40 | < 5 μm | PDLLA | TIPS | [169] |
| 45S5 m-BG | 10, 25, 50 | < 5 μm | PLGA | TIPS | [42] |
| 45S5 m-BG | 25 | < 40 μm | PLGA | Solvent casting | [50] |
| 45S5 m-BG | 20 | < 10 μm | P(3HB) | Solvent casting | [170] |
| 45S5 m-BG | 20 | < 5 μm | P(3HB) | Solvent casting | [171] |
| 45S5 n-BG | 10, 20 | 29 nm | P(3HB) | Solvent casting | [46] |
| 45S5 m-BG | 10, 20, 30 | < 5 μm | P(3HB) | Solvent casting | [48] |
| 45S5 n-BG | 10, 20, 30 | 30–50 nm | P(3HB) | Solvent casting | [48] |
| 45S5 m-BG | 5, 30 | 5 μm | PDLLA | TIPS | [172] |
| 45S5 m-BG | 5, 30 | 5 μm | PDLLA | TIPS | [173] |
| 45S5 m-BG | 5, 40 | < 5 μm | PDLLA | TIPS | [174] |
| SiO2-3CaO-P2O5-MgO | 10, 30, 50 | 10 μm | PLA | TIPS | [175] |
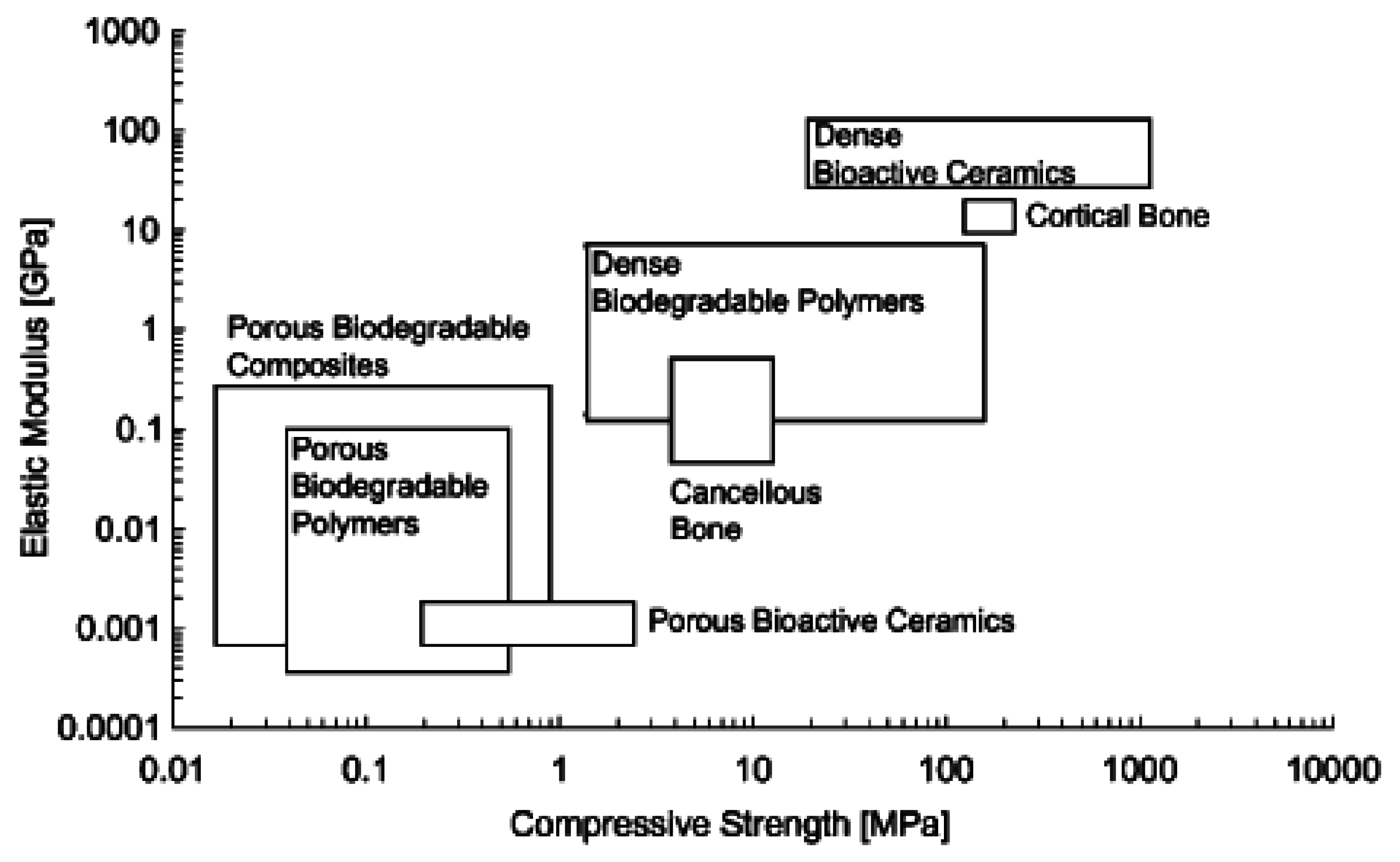
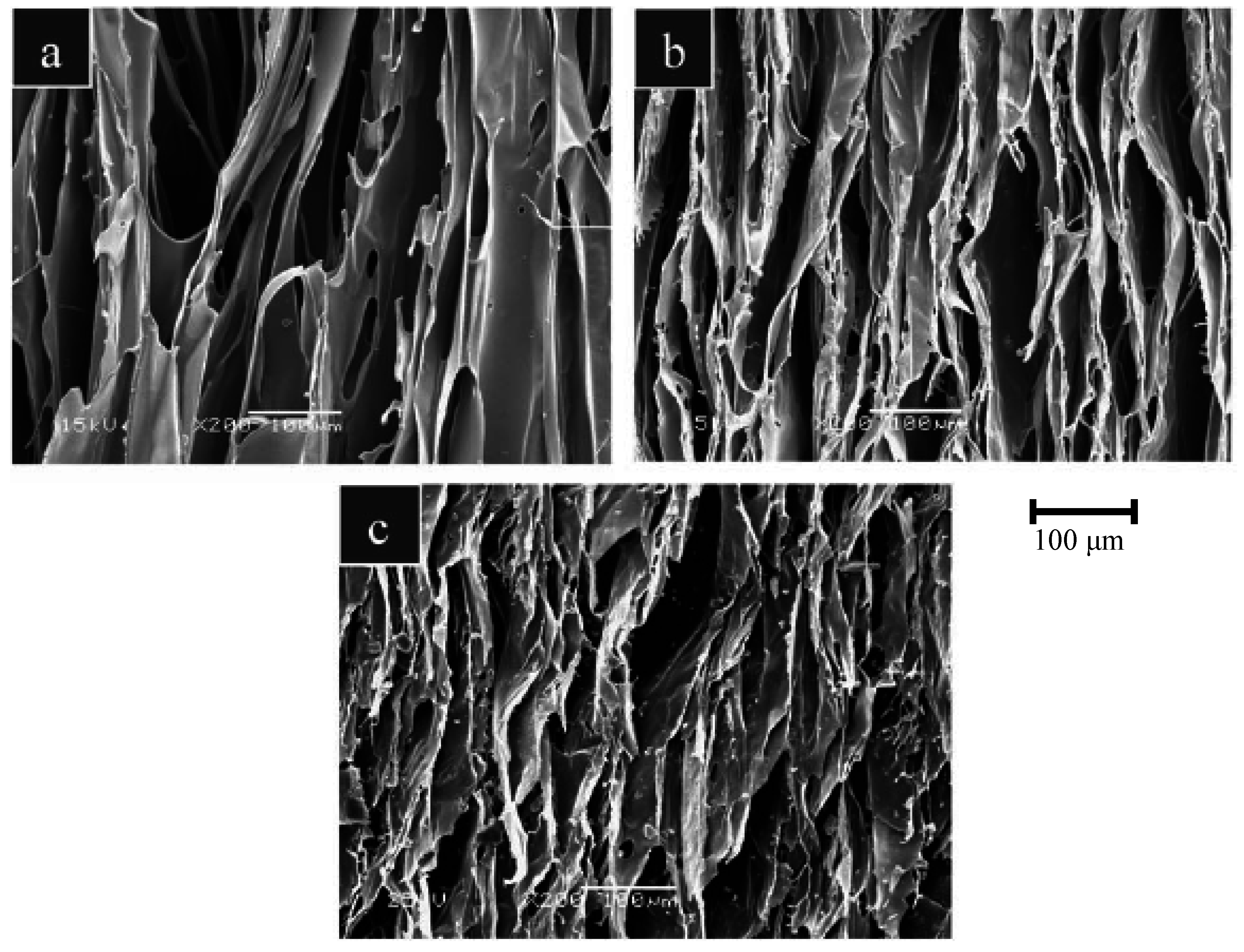
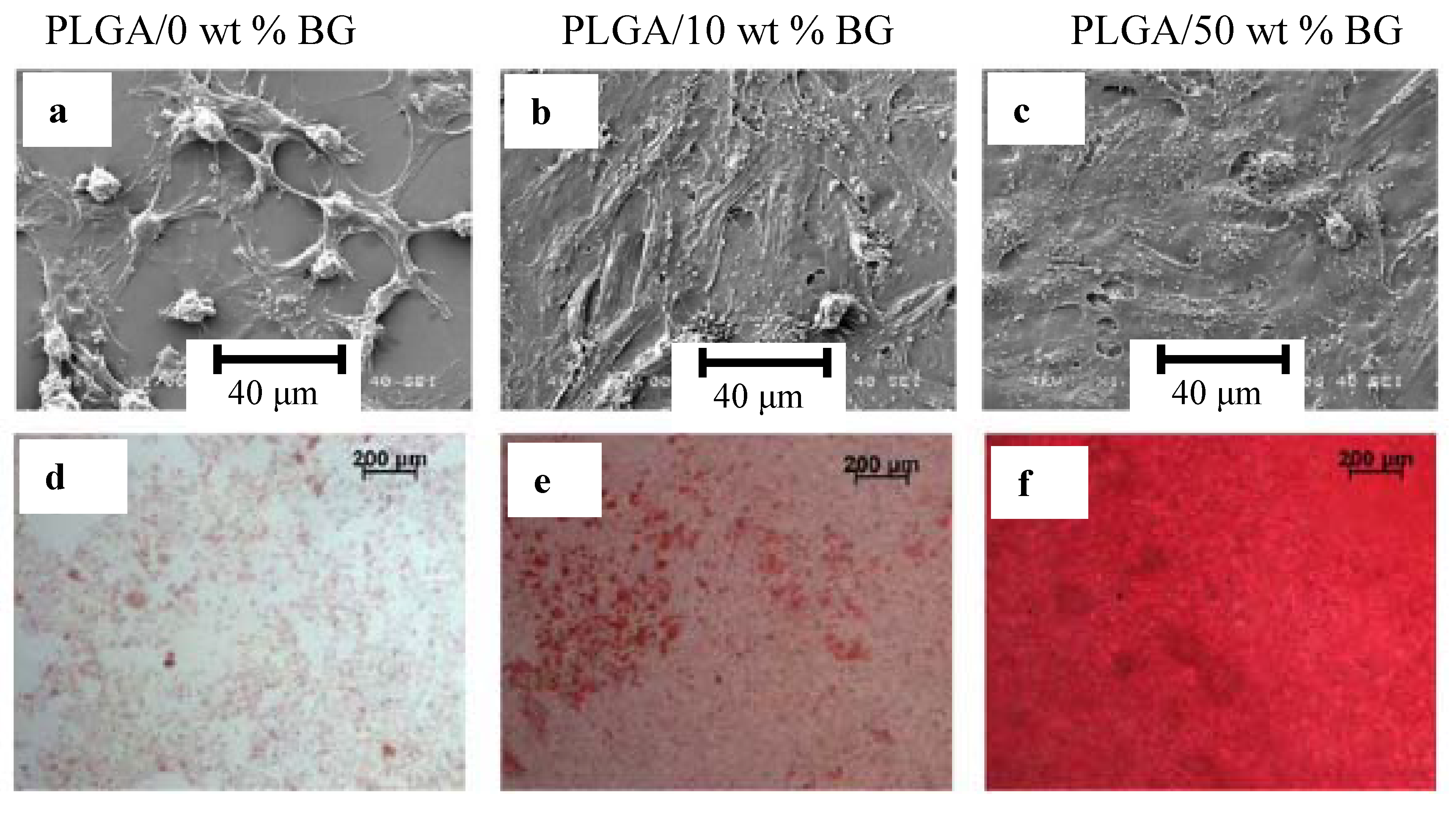
4. Ion Release from Silicate Scaffolds: Effects on Osteogenesis and Angiogenesis
4.1. Ion Dissolution from Bioactive Glasses: Genetic Control of Osteoblast Cell Cycle and Osteogenesis
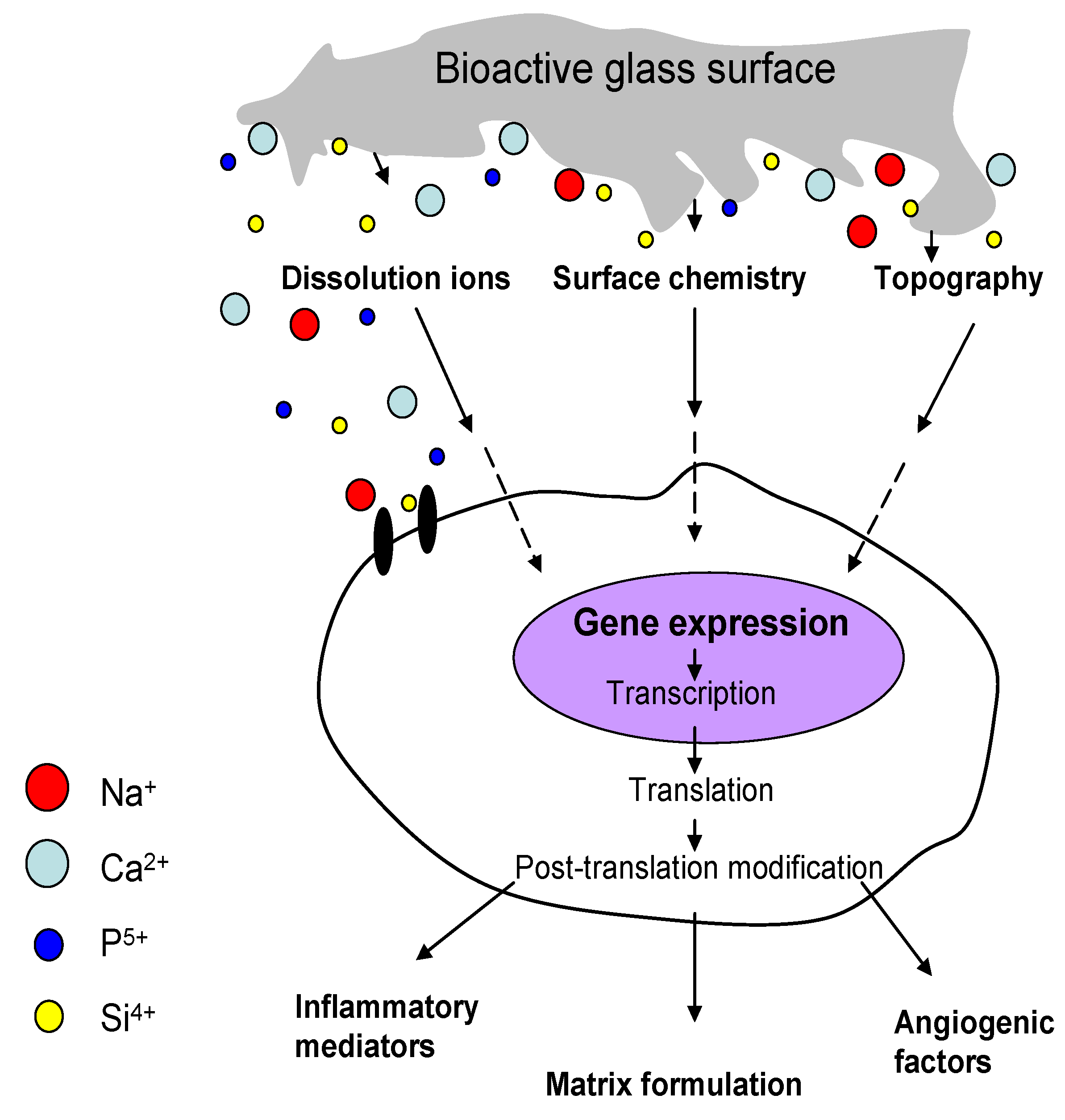
4.2. The Role of Angiogenesis in Bone Regeneration
4.3. Effect of Bioactive Glass on Angiogenesis
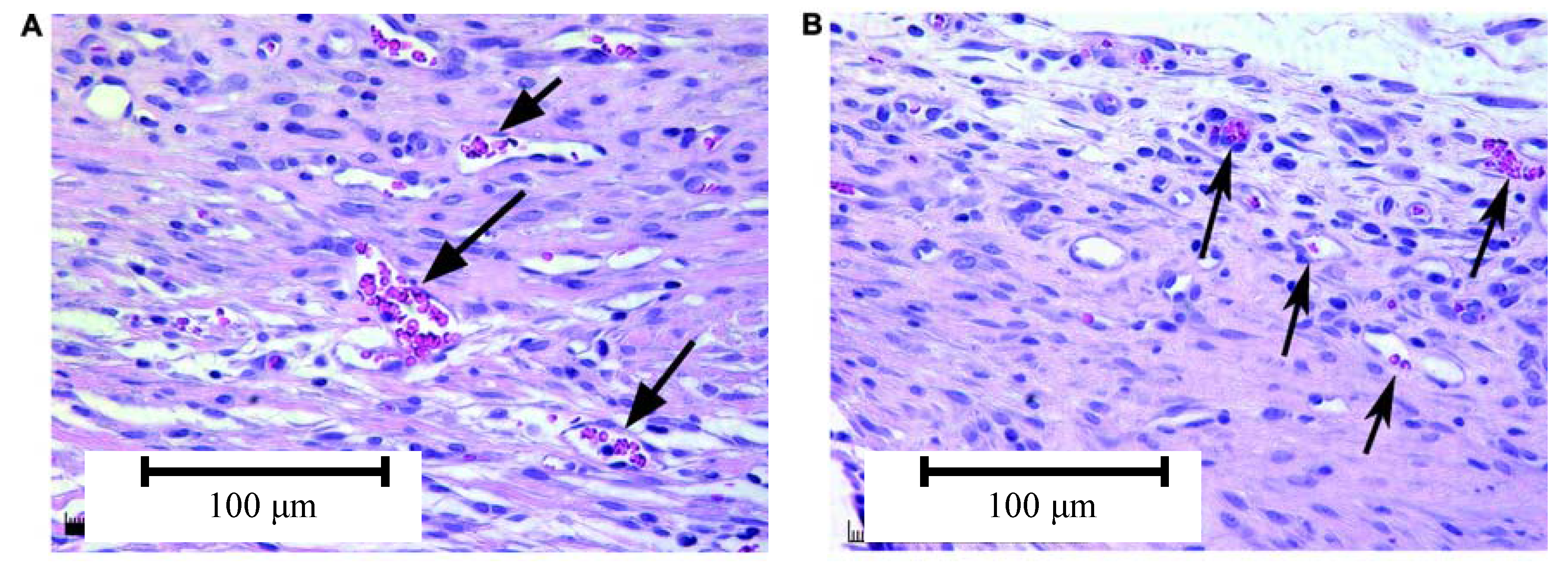
5. Conclusions and Future Work
Acknowledgements
References and Notes
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. Benefit and risk in tissue engineering. Mater. Today 2004, 7, 24–29. [Google Scholar] [CrossRef]
- Guarino, V.; Causa, F.; Ambrosio, L. Bioactive scaffolds for bone and ligament tissue. Expert Rev. Med. Devices 2007, 4, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass® 45S5 dissolution. J. Biomed. Mater. Res. 2001, 55, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Jones, J.R. New trends in bioactive scaffolds: The importance of nanostructure. J. Eur. Ceram. Soc. 2009, 29, 1275–1281. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: from concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hench, L.L.; Andersson, Ö. Bioactive glasses. In An Introduction to Bioceramics, 1st ed.; Hench, L.L., Wilson, J., Eds.; World Scientific Publishing: Singapore, 1993; Volume 1, pp. 41–62. [Google Scholar]
- Vitale-Brovarone, C.; Miola, M.; Balagna, C.; Verné, E. 3D-glass-ceramic scaffolds with antibacterial properties for bone grafting. Chem. Eng. J. 2008, 137, 129–136. [Google Scholar] [CrossRef]
- Vitale-Brovarone, C.; Verne, E.; Bosetti, M.; Appendino, P.; Cannas, M. Microstructural and in vitro characterization of SiO2-Na2O-CaO-MgO glass-ceramic bioactive scaffolds for bone substitutes. J. Mater. Sci. Mater. Med. 2005, 16, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Vitale-Brovarone, C.; Verne, E.; Robiglio, L.; Appendino, P.; Bassi, F.; Martinasso, G.; Muzio, G.; Canuto, R. Development of glass-ceramic scaffolds for bone tissue engineering: characterisation, proliferation of human osteoblasts and nodule formation. Acta Biomater. 2007, 3, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, E.; Fredholm, Y.C.; Jell, G.; Lotfibakhshaiesh, N.; O’Donnell, M. D.; Hill, R.G.; Stevens, M.M. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials 2010, 31, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.B.; Zhao, X.L.; Zhang, X.; Zhang, K. B.; Li, L.C.; Li, Z.Y.; Lam, W.M.; Lu, W.W.; Wang, D.P.; Huang, W.H.; Lin, K.L.; Chang, J. Strontium borate glass: Potential biomaterial for bone regeneration. J. Roy. Soc. Interface. in press.
- O’Donnell, M.D.; Hill, R.G. Influence of strontium and the importance of glass chemistry and structure when designing bioactive glasses for bone regeneration. Acta Biomater. 2010, 6, 2382–2385. [Google Scholar]
- Hsi, C.S.; Cheng, H.Z.; Hsu, H.J.; Chen, Y.S.; Wang, M.C. Crystallization kinetics and magnetic properties of iron oxide contained 25Li2O-8MnO2-20CaO-2P2O5-45SiO2 glasses. J. Eur. Ceram. Soc. 2007, 27, 3171–3176. [Google Scholar] [CrossRef]
- Balamurugan, A.; Balossier, G.; Laurent-Maquin, D.; Pina, S.; Rebelo, A.H.S.; Faure, J.; Ferreira, J.M.F. An in vitro biological and anti-bacterial study on a sol-gel derived silver-incorporated bioglass system. Dental Mater. 2008, 24, 1343–1351. [Google Scholar] [CrossRef]
- Bellantone, M.; Williams, H.D.; Hench, L.L. Broad-spectrum bactericidal activity of Ag2O-doped bioactive glass. Antimicrob. Agents Chemother. 2002, 46, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Nazhat, S.N.; Boccaccini, A.R. Development and characterisation of silver-doped bioactive glass-coated sutures for tissue engineering and wound healing applications. Biomaterials 2004, 25, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Delben, J.R.; Pimentel, O.M.; Coelho, M.B.; Candelorio, P.D.; Furini, L.N.; dos Santos, F.A.; de Vicente, F.S.; Delben, A. Synthesis and thermal properties of nanoparticles of bioactive glasses containing silver. J. Therm. Anal. Calorim. 2009, 97, 433–436. [Google Scholar]
- Liu, X.; Huang, W.; Fu, H.; Yao, A.; Wang, D.; Pan, H.; Lu, W. Bioactive borosilicate glass scaffolds: improvement on the strength of glass-based scaffolds for tissue engineering. J. Mater. Sci. Mater. Med. 2009, 20, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, W.; Fu, H.; Yao, A.; Wang, D.; Pan, H.; Lu, W.; Jiang, X.; Zhang, X. Bioactive borosilicate glass scaffolds: in vitro degradation and bioactivity behaviors. J. Mater. Sci. Mater. Med. 2009, 20, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Munukka, E.; Lepparanta, O.; Korkeamaki, M.; Vaahtio, M.; Peltola, T.; Zhang, D.; Hupa, L.; Ylanen, H.; Salonen, J.I.; Viljanen, M.K.; Eerola, E. Bactericidal effects of bioactive glasses on clinically important aerobic bacteria. J. Mater. Sci. Mater. Med. 2008, 19, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Gorriti, M.F.; Porto López, J.M.; Boccaccini, A.R.; Audisio, C.; Gorustovich, A.A. In vitro study of the antibacterial activity of bioactive glass-ceramic scaffolds. Adv. Eng. Mater. 2009, 11, B67–B70. [Google Scholar] [CrossRef]
- Cannillo, V.; Sola, A. Potassium-based composition for a bioactive glass. Ceram. Int. 2009, 35, 3389–3393. [Google Scholar] [CrossRef]
- Aina, V.; Malavasi, G.; Fiorio Pla, A.; Munaron, L.; Morterra, C. Zinc-containing bioactive glasses: Surface reactivity and behaviour towards endothelial cells. Acta Biomater. 2009, 5, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Haimi, S.; Gorianc, G.; Moimas, L.; Lindroos, B.; Huhtala, H.; Räty, S.; Kuokkanen, H.; Sándor, G.K.; Schmid, C.; Miettinen, S.; Suuronen, R. Characterization of zinc-releasing three-dimensional bioactive glass scaffolds and their effect on human adipose stem cell proliferation and osteogenic differentiation. Acta Biomater. 2009, 5, 3122–3131. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Valappil, S.P.; Roy, I.; Boccaccini, A.R. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules 2006, 7, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Meng, Y.; Li, Y.; Zhao, N. Investigation on bio-mineralization of melt and sol-gel derived bioactive glasses. Appl. Surf. Sci. 2008, 255, 562–564. [Google Scholar] [CrossRef]
- Jones, J.R. Bioactive ceramics and glasses. In Tissue Engineering Using Ceramics and Polymers, 1st ed.; Boccaccini, A.R., Gough, J.E., Eds.; Woodhead Publishing Limited CRC Press: Cambridge, UK, 2007; Volume 1, pp. 52–71. [Google Scholar]
- Gupta, R.; Kumar, A. Bioactive materials for biomedical applications using sol-gel technology. Biomed. Mater. 2008, 3, 034005. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Pigott, G.H.; Schoen, F.J.; Hench, L.L. Toxicology and biocompatibility of bioglasses. J. Biomed. Mater. Res. 1981, 15, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Xynos, I.D.; Polak, J.M. Bioactive glasses for in situ tissue regeneration. J. Biomater. Sci.-Polym. Ed. 2004, 15, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar]
- Garcia, A.J.; Ducheyne, P.; Boettiger, D. Effect of surface reaction stage on fibronectin-mediated adhesion of osteoblast-like cells to bioactive glass. J. Biomed. Mater. Res. 1998, 40, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ylanen, H.O.; Helminen, T.; Helminen, A.; Rantakokko, J.; Karlsson, K. H.; Aro, H.T. Porous bioactive glass matrix in reconstruction of articular osteochondral defects. Ann. Chir. Gynaecol. 1999, 88, 237–245. [Google Scholar] [PubMed]
- Xynos, I.D.; Hukkanen, M.V.J.; Batten, J.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Bioglass® 45S5 stimulates osteoblast turnover and enhances bone formation in vitro: Implications and applications for bone tissue engineering. Calcif. Tissue Int. 2000, 67, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Maquet, V. Bioresorbable and bioactive polymer/Bioglass® composites with tailored pore structure for tissue engineering applications. Compos. Sci. Technol. 2003, 63, 2417–2429. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Brunner, T.J.; Grass, R.N.; Stark, W.J. Glass and bioglass nanopowders by flame synthesis. Chem. Commun. 2006, 1384–1386. [Google Scholar] [CrossRef]
- Vollenweider, M.; Brunner, T.J.; Knecht, S.; Grass, R.N.; Zehnder, M.; Imfeld, T.; Stark, W.J. Remineralization of human dentin using ultrafine bioactive glass particles. Acta Biomater. 2007, 3, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Ansari, T.; Mohn, D.; Valappil, S.P.; Brunner, T.J.; Stark, W.J.; Roy, I.; Knowles, J.C.; Sibbons, P.D.; Jones, E.V.; Boccaccini, A.R.; Salih, V. Effect of nanoparticulate bioactive glass particles on bioactivity and cytocompatibility of poly(3-hydroxybutyrate) composites. J. Roy. Soc. Interface 2010, 7, 453–465. [Google Scholar] [CrossRef]
- Misra, S.K.; Ansari, T.I.; Valappil, S.P.; Mohn, D.; Philip, S.E.; Stark, W.J.; Roy, I.; Knowles, J.C.; Salih, V.; Boccaccini, A.R. Poly(3-hydroxybutyrate) multifunctional composite scaffolds for tissue engineering applications. Biomaterials 2010, 31, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Mohn, D.; Brunner, T.J.; Stark, W.J.; Philip, S.E.; Roy, I.; Salih, V.; Knowles, J.C.; Boccaccini, A.R. Comparison of nanoscale and microscale bioactive glass on the properties of P(3HB)/Bioglass composites. Biomaterials 2008, 29, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Hong, Z.; Zhuang, X.; Chen, X.; Cui, Y.; Liu, Y.; Jing, X. Surface modification of bioactive glass nanoparticles and the mechanical and biological properties of poly(L-lactide) composites. Acta Biomater. 2008, 4, 1005–1015. [Google Scholar] [CrossRef]
- Lu, H.H.; El-Amin, S.F.; Scott, K.D.; Laurencin, C.T. Three-dimensional, bioactive, biodegradable, polymer-bioactive glass composite scaffolds with improved mechanical properties support collagen synthesis and mineralization of human osteoblast-like cells in vitro. J. Biomed. Mater. Res. Part A 2003, 64, 465–474. [Google Scholar] [CrossRef]
- Hench, L.L. Genetic design of bioactive glass. J. Eur. Ceram. Soc. 2009, 29, 1257–1265. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Nandi, S.K.; Kundu, B.; Datta, S.; De, D.K.; Roy, S.K.; Basu, D. In vivo response of porous hydroxyapatite and beta-tricalcium phosphate prepared by aqueous solution combustion method and comparison with bioglass scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 217–227. [Google Scholar] [CrossRef]
- Gorustovich, A.; Roether, J.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: In-vitro and in-vivo evidence. A review. Tissue Eng. Part B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef]
- Day, R.M. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng. 2005, 11, 768–777. [Google Scholar] [CrossRef]
- Day, R.M.; Maquet, V.; Boccaccini, A.R.; Jerome, R.; Forbes, A. In vitro and in vivo analysis of macroporous biodegradable poly(D,L-lactide-co-glycolide) scaffolds containing bioactive glass. J. Biomed. Mater. Res. Part A 2005, 75, 778–787. [Google Scholar] [CrossRef]
- Leu, A.; Leach, J.K. Proangiogenic potential of a collagen/bioactive glass substrate. Pharm. Res. 2008, 25, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Mortera, R.; Onida, B.; Fiorilli, S.; Cauda, V.; Brovarone, C.V.; Baino, F.; Vernè, E.; Garrone, E. Synthesis and characterization of MCM-41 spheres inside bioactive glass-ceramic scaffold. Chem. Eng. J. 2008, 137, 54–61. [Google Scholar] [CrossRef]
- Cauda, V.; Fiorilli, S.; Onida, B.; Vernè, E.; Vitale Brovarone, C.; Viterbo, D.; Croce, G.; Milanesio, M.; Garrone, E. SBA-15 ordered mesoporous silica inside a bioactive glass-ceramic scaffold for local drug delivery. J. Mater. Sci. Mater. Med. 2008, 19, 3303–3310. [Google Scholar] [CrossRef] [PubMed]
- El-Ghannam, A.R. Advanced bioceramic composite for bone tissue engineering: design principles and structure-bioactivity relationship. J. Biomed. Mater. Res. Part A 2004, 69, 490–501. [Google Scholar] [CrossRef]
- Francis, L.; Meng, D.; Knowles, J.C.; Roy, I.; Boccaccini, A.R. Multi-functional P(3HB) microsphere/45S5 Bioglass®-based composite scaffolds for bone tissue engineering. Acta Biomater. 2010, 6, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.J.; Wolke, J.G.; Jansen, J.A. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Juan, Z.; Min, W.; Jae Min, C.; Athanasios, M. The incorporation of 70s bioactive glass to the osteogenic differentiation of murine embryonic stem cells in 3D bioreactors. J. Tissue Eng. Regen. Med. 2009, 3, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Le Geros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; Legeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kaskel, S. Comparison of the in vitro bioactivity and drug release property of mesoporous bioactive glasses (MBGs) and bioactive glasses (BGs) scaffolds. Micropor. Mesopor. Mater. 2009, 118, 176–182. [Google Scholar] [CrossRef]
- Ostomel, T.A.; Shi, Q.H.; Tsung, C.K.; Liang, H.J.; Stucky, G.D. Spherical bioactive glass with enhanced rates of hydroxyapatite deposition and hemostatic activity. Small 2006, 2, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Bretcanu, O.; Spriano, S.; Vitale, C. B.; Verne, E. Synthesis and characterization of coprecipitation-derived ferrimagnetic glass-ceramic. J. Mater. Sci. 2006, 41, 1029–1037. [Google Scholar] [CrossRef]
- Li, D.G.; Zhou, D.L.; Lin, Y.; Pan, T.H.; Chen, G.S.; Yin, Q.D. Synthesis and characterization of magnetic bioactive glass-ceramics containing Mg ferrite for hyperthermia. Mater. Sci. Eng. C 2010, 30, 148–153. [Google Scholar] [CrossRef]
- Kawashita, M.; Iwahashi, Y.; Kokubo, T.; Yao, T.; Hamada, S.; Shinjo, T. Preparation of class-ceramics containing ferrimagnetic zinc-iron ferrite for the hyperthermal treatment of cancer. J. Ceram. Soc. Jpn. 2004, 112, 373–379. [Google Scholar] [CrossRef]
- Shah, S.A.; Hashmi, M.U.; Alam, S.; Shamim, A. Magnetic and bioactivity evaluation of ferrimagnetic ZnFe2O4 containing glass ceramics for the hyperthermia treatment of cancer. J. Magn. Magn. Mater. 2010, 322, 375–381. [Google Scholar] [CrossRef]
- Thompson, I.D.; Hench, L.L. Mechanical properties of bioactive glasses, glass-ceramics and composites. Proc. Inst. Mech. Eng. Part H-J. Eng. Med. 1998, 212, 127–136. [Google Scholar] [CrossRef]
- Kokubo, T.; Ito, S.; Shigematsu, M.; Sakka, S.; Yamamuro, T. Mechanical-properties of a new type of apatite-containing glass-ceramic for prosthetic application. J. Mater. Sci. 1985, 20, 2001–2004. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamamuro, T.; Higashi, S.; Kokubo, T.; Itoo, S. A new glass-ceramic for bone replacement-evaluation of its bonding to bone. J. Biomed. Mater. Res. 1985, 19, 685–698. [Google Scholar] [CrossRef]
- Wu, C. Methods of improving mechanical and biomedical properties of Ca-Si-based ceramics and scaffolds. Expert Rev. Med. Devices 2009, 6, 237–241. [Google Scholar] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Eng. 2002, 8, 1–11. [Google Scholar]
- Yun, H.S.; Kim, S.E.; Hyeon, Y.T. Design and preparation of bioactive glasses with hierarchical pore networks. Chem. Commun. 2007, 2139–2141. [Google Scholar] [CrossRef]
- Yunos, D.M.; Bretcanu, O.; Boccaccini, A.R. Polymer-bioceramic composites for tissue engineering scaffolds. J. Mater. Sci. 2008, 43, 4433–4442. [Google Scholar] [CrossRef]
- De Diego, M.A.; Coleman, N.J.; Hench, L.L. Tensile properties of bioactive fibers for tissue engineering applications. J. Biomed. Mater. Res. 2000, 53, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Taboas, J.M.; Maddox, R.D.; Krebsbach, P.H.; Hollister, S.J. Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer-ceramic scaffolds. Biomaterials 2003, 24, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Mano, J.F.; Reis, R.L. Nature-inspired calcium phosphate coatings: present status and novel advances in the science of mimicry. Curr. Opin. Solid State Mater. Sci. 2003, 7, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Boccaccini, A.R.; Blaker, J.J. Bioactive composite materials for tissue engineering scaffolds. Expert Rev. Med. Devices 2005, 2, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.C.; Dalby, M.J.; Oreffo, R.O.C.; McCloy, D.; Affrosman, S. The interaction of human bone marrow cells with nanotopographical features in three dimensional constructs. J. Biomed. Mater. Res. Part A 2006, 79A, 431–439. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ahn, E.S. Nanostructured biomaterials for tissue engineering bone. In Tissue Engineering II; Springer: Berlin, Germany, 2007; Volume 103, pp. 275–308. [Google Scholar]
- Hench, L.L.; Wilson, J.; Greenspan, D.C. Bioglass®: A short history and bibliography. J. Aust. Ceram. Soc. 2004, 40, 1–42. [Google Scholar]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L. Ceramics for medical applications: A picture for the next 20 years. J. Eur. Ceram. Soc. 2009, 29, 1245–1255. [Google Scholar] [CrossRef]
- Arcos, D.; Izquierdo-Barba, I.; Vallet-Regi, M. Promising trends of bioceramics in the biomaterials field. J. Mater. Sci. Mater. Med. 2009, 20, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Gerhardt, L.C. Carbon nanotube composite scaffolds and coatings for tissue engineering applications. In Key Engineering Materials: Advanced Bioceramics in Nanomedicine and Tissue Engineering; Trans Tech Publications: Zurich, Swizerland, 2010; Volume 441, pp. 31–52. [Google Scholar]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Tabata, Y. Biomaterial technology for tissue engineering applications. J. Roy. Soc. Interface 2009, 6, S311–S324. [Google Scholar] [CrossRef]
- Smith, I.O.; Ren, F.; Baumann, M.J.; Case, E.D. Confocal laser scanning microscopy as a tool for imaging cancellous bone. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 79, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Vitale-Brovarone, C.; Baino, F.; Verné, E. High strength bioactive glass-ceramic scaffolds for bone regeneration. J. Mater. Sci. Mater. Med. 2009, 20, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, I.; Sanz-Herrera, J.A.; García-Aznar, J.M.; Doblaré, M.; Yunos, D.M.; Boccaccini, A.R. Permeability evaluation of 45S5 Bioglass®-based scaffolds for bone tissue engineering. J. Biomech. 2009, 42, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R. Ceramics. In Biomaterials, artificial organs and tissue engineering, 1st ed.; Hench, L.L., Jones, J.R., Eds.; Woodhead Publishing Limited CRC Press: Cambridge, UK, 2005; Volume 1, pp. 26–36. [Google Scholar]
- Boccaccini, A.R.; Chen, Q.; Lefebvre, L.; Gremillard, L.; Chevalier, J. Sintering, crystallisation and biodegradation behaviour of Bioglass-derived glass-ceramics. Faraday Discuss. 2007, 136, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.D. Biocomposites. In Biomaterials, Artificial Organs and Tissue Engineering, 1st ed.; Hench, L.L., Jones, J.R., Eds.; Woodhead Publishing Limited CRC Press: Cambridge, UK, 2005; Volume 1, pp. 48–58. [Google Scholar]
- Fu, Q.; Rahaman, M.N.; Bal, B.S.; Brown, R.F. Preparation and in vitro evaluation of bioactive glass (13-93) scaffolds with oriented microstructures for repair and regeneration of load-bearing bones. J. Biomed. Mater. Res. Part A 2010, 93A, 1380–1390. [Google Scholar]
- Kanczler, J.M.; Oreffo, R.O. Osteogenesis and angiogenesis: the potential for engineering bone. Eur. Cells Mater. 2008, 15, 100–114. [Google Scholar]
- Deville, S.; Saiz, E.; Nalla, R.K.; Tomsia, A.P. Freezing as a path to build complex composites. Science 2006, 311, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Verné, E.; Vitale-Brovarone, C. 3-D high-strength glass-ceramic scaffolds containing fluoroapatite for load-bearing bone portions replacement. Mater. Sci. Eng. C 2009, 29, 2055–2062. [Google Scholar] [CrossRef]
- Bretcanu, O.; Samaille, C.; Boccaccini, A.R. Simple methods to fabricate Bioglass®-derived glass-ceramic scaffolds exhibiting porosity gradient. J. Mater. Sci. 2008, 43, 4127–4134. [Google Scholar] [CrossRef]
- Vitale-Brovarone, C.; Baino, F.; Verne, E. Feasibility and tailoring of bioactive glass-ceramic scaffolds with gradient of porosity for bone grafting. J. Biomater. Appl. 2009. [Google Scholar]
- Fu, Q.; Rahaman, M.N.; Bal, B.S.; Brown, R.F.; Day, D.E. Mechanical and in vitro performance of 13-93 bioactive glass scaffolds prepared by a polymer foam replication technique. Acta Biomater. 2008, 4, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Brovarone, C.V.; Verne, E.; Appendino, P. Macroporous bioactive glass-ceramic scaffolds for tissue engineering. J. Mater. Sci. Mater. Med. 2006, 17, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Rahaman, M.N.; Bal, B.S.; Huang, W.; Day, D.E. Preparation and bioactive characteristics of a porous 13-93 glass, and fabrication into the articulating surface of a proximal tibia. J. Biomed. Mater. Res. Part A 2007, 82, 222–229. [Google Scholar] [CrossRef]
- Renghini, C.; Komlev, V.; Fiori, F.; Verne, E.; Baino, F.; Vitale-Brovarone, C. Micro-CT studies on 3-D bioactive glass-ceramic scaffolds for bone regeneration. Acta Biomater. 2009, 5, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Z.; Rezwan, K.; Armitage, D.; Nazhat, S.N.; Boccaccini, A.R. The surface functionalization of 45S5 Bioglass®-based glass-ceramic scaffolds and its impact on bioactivity. J. Mater. Sci. Mater. Med. 2006, 17, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Z.; Rezwan, K.; Francon, V.; Armitage, D.; Nazhat, S.N.; Jones, F.H.; Boccaccini, A.R. Surface functionalization of Bioglass®-derived porous scaffolds. Acta Biomater. 2007, 3, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Goetz, H.; Pazen, S.; Al-Nawas, B.; Wagner, W.; Duschner, H. Pore characteristics of bone substitute materials assessed by microcomputed tomography. Clin. Oral. Implants Res. 2009, 20, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kohlhauser, C.; Hellmich, C.; Vitale-Brovarone, C.; Boccaccini, A.R.; Rota, A.; Eberhardsteiner, J. Ultrasonic characterisation of porous biomaterials across different frequencies. Strain 2009, 45, 34–44. [Google Scholar] [CrossRef]
- Moimas, L.; Biasotto, M.; Di Lenarda, R.; Olivo, A.; Schmid, C. Rabbit pilot study on the resorbability of three-dimensional bioactive glass fibre scaffolds. Acta Biomater. 2006, 2, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.F.; Day, D.E.; Day, T.E.; Jung, S.; Rahaman, M.N.; Fu, Q. Growth and differentiation of osteoblastic cells on 13-93 bioactive glass fibers and scaffolds. Acta Biomater. 2008, 4, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Vitale-Brovarone, C.; Di Nunzio, S.; Bretcanu, O.; Verne, E. Macroporous glass-ceramic materials with bioactive properties. J. Mater. Sci. Mater. Med. 2004, 15, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Vitale-Brovarone, C.; Verne, E.; Robiglio, L.; Martinasso, G.; Canuto, R.A.; Muzio, G. Biocompatible glass-ceramic materials for bone substitution. J. Mater. Sci. Mater. Med. 2008, 19, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Vitale-Brovarone, C.; Baino, F.; Bretcanu, O.; Verné, E. Foam-like scaffolds for bone tissue engineering based on a novel couple of silicate-phosphate specular glasses: synthesis and properties. J. Mater. Sci. Mater. Med. 2009, 20, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Z.; Efthymiou, A.; Salih, V.; Boccaccini, A.R. Bioglass-derived glass-ceramic scaffolds: study of cell proliferation and scaffold degradation in vitro. J. Biomed. Mater. Res. Part A 2008, 84, 1049–1060. [Google Scholar] [CrossRef]
- Deb, S.; Mandegaran, R.; Di Silvio, L. A porous scaffold for bone tissue engineering/45S5 Bioglass® derived porous scaffolds for co-culturing osteoblasts and endothelial cells. J. Mater. Sci. Mater. Med. 2010, 21, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Vargas, G.E.; Mesones, R.V.; Bretcanu, O.; López, J.M.P.; Boccaccini, A.R.; Gorustovich, A. Biocompatibility and bone mineralization potential of 45S5 Bioglass®-derived glass-ceramic scaffolds in chick embryos. Acta Biomater. 2009, 5, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Kundu, B.; Datta, S.; De, D.K.; Basu, D. The repair of segmental bone defects with porous bioglass: An experimental study in goat. Res. Vet. Sci. 2009, 86, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, J.; Takita, H.; Ojima, Y.; Kobayashi, M.; Kohgo, T.; Kuboki, Y. Geometric effect of matrix upon cell differentiation: BMP-induced osteogenesis using a new bioglass with a feasible structure. J. Biochem. 2001, 129, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Mantsos, T.; Chatzistavrou, X.; Roether, J.A.; Hupa, L.; Arstila, H.; Boccaccini, A.R. Non-crystalline composite tissue engineering scaffolds using boron-containing bioactive glass and poly(D,L-lactic acid) coatings. Biomed. Mater. 2009, 4, 55002. [Google Scholar] [CrossRef]
- San Miguel, B.; Kriauciunas, R.; Tosatti, S.; Ehrbar, M.; Ghayor, C.; Textor, M.; Weber, F.E. Enhanced osteoblastic activity and bone regeneration using surface-modified porous bioactive glass scaffolds. J. Biomed. Mater. Res. Part A. in press.
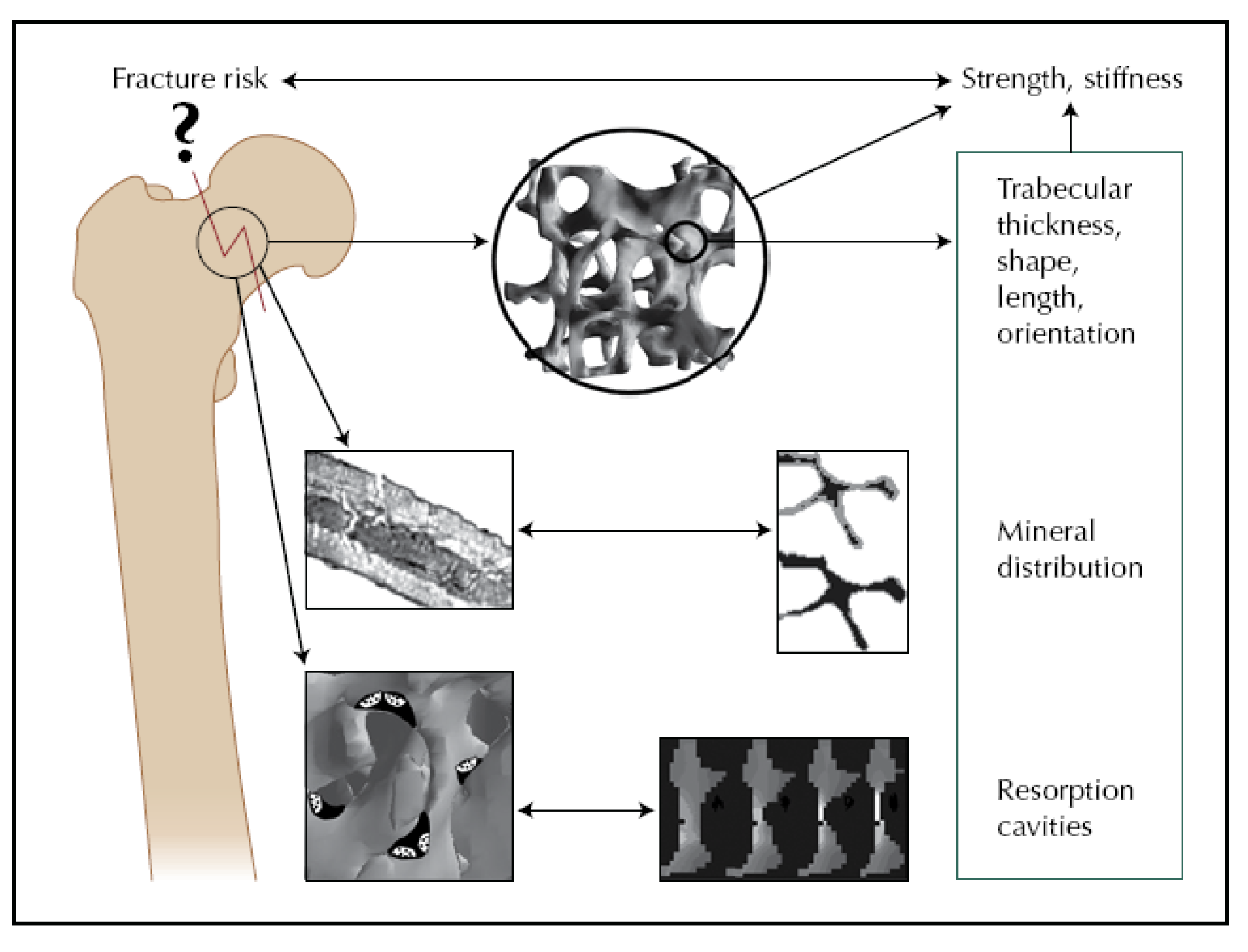
- Hernandez, C.J.; Beaupre, G.S.; Keller, T.S.; Carter, D.R. The influence of bone volume fraction and ash fraction on bone strength and modulus. Bone 2001, 29, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Keaveny, T.M.; Morgan, E.F.; Niebur, G.L.; Yeh, O.C. Biomechanics of trabecular bone. Annu. Rev. Biomed. Eng. 2001, 3, 307–333. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.S.; Ma, H.L.; Liu, C.L.; Huang, C.H.; Cheng, C.K.; Wei, H.W. Difference in femoral head and neck material properties between osteoarthritis and osteoporosis. Clin. Biomech. 2008, 23 (Suppl. 1), S39–S47. [Google Scholar] [CrossRef]
- Nazarian, A.; von Stechow, D.; Zurakowski, D.; Muller, R.; Snyder, B.D. Bone volume fraction explains the variation in strength and stiffness of cancellous bone affected by metastatic cancer and osteoporosis. Calcif. Tissue Int. 2008, 83, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Wilson, J. Introduction. In An introduction to bioceramics, 1st ed.; Hench, L.L., Wilson, J., Eds.; World Scientific Publishing: Singapore, 1993; Volume 1, pp. 1–24. [Google Scholar]
- Clupper, D.C.; Gough, J.E.; Embanga, P.M.; Notingher, I.; Hench, L.L.; Hall, M.M. Bioactive evaluation of 45S5 bioactive glass fibres and preliminary study of human osteoblast attachment. J. Mater. Sci. Mater. Med. 2004, 15, 803–838. [Google Scholar] [CrossRef] [PubMed]
- Clupper, D.C.; Hench, L.L.; Mecholsky, J.J. Strength and toughness of tape cast bioactive glass 45S5 following heat treatment. J. Eur. Ceram. Soc. 2004, 24, 2929–2934. [Google Scholar] [CrossRef]
- Frost, H.M. Could some biomechanical effects of growth hormone help to explain its effects on bone formation and resorption? Bone 1998, 23, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Chen, P.Y.; Lin, A. Y.M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- O’Flaherty, E.J. Physiologically based models for bone-seeking elements: I. Rat skeletal and bone growth. Toxicol. Appl. Pharmacol. 1991, 111, 299–312. [Google Scholar] [CrossRef]
- O’Flaherty, E.J. Physiologically based models for bone-seeking elements: III. Human skeletal and bone growth. Toxicol. Appl. Pharmacol. 1991, 111, 332–341. [Google Scholar] [CrossRef]
- Woźniak, P.; El Haj, A.J. Bone regeneration and repair using tissue engineering. In Tissue Engineering Using Ceramics and Polymers, 1st ed.; Boccaccini, A.R., Gough, J.E., Eds.; Woodhead Publishing Limited CRC Press: Cambridge, UK, 2007; Volume 1, pp. 294–318. [Google Scholar]
- Gibson, L.J. Biomechanics of cellular solids. J. Biomech. 2005, 38, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.R. Numerical simulation of bone adaptation to mechanical loading. Ph.D. Dissertation, Stanford University, California, 1994. [Google Scholar]
- Diego, R.B.; Estelles, J.M.; Sanz, J.A.; Garcia-Aznar, J.M.; Sanchez, M.S. Polymer scaffolds with interconnected spherical pores and controlled architecture for tissue engineering: fabrication, mechanical properties, and finite element modeling. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Herrera, J.A.; Garcia-Aznar, J.M.; Doblare, M. A mathematical model for bone tissue regeneration inside a specific type of scaffold. Biomech. Model. Mechanobiol. 2008, 7, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Suquet, P.M. Elements of homogenization for inelastic solid mechanics. In Homogenization Techniques for Composite Media; Sanchez-Palencia, E., Zaoui, A., Eds.; Springer: Berlin, Germany, 1987; Volume 272, pp. 193–278. [Google Scholar]
- Sanz-Herrera, J.A.; Doblaré, M.; García-Aznar, J.M. Scaffold microarchitecture determines internal bone directional growth structure: A numerical study. J. Biomech. in press.
- Peters, M.C.; Mooney, D.J. Synthetic extracellular matrices for cell transplantation. In Porous Materials for Tissue Engineering; Transtec Publications: Zurich-Uetikon, Swizerland, 1997; Volume 250, pp. 43–52. [Google Scholar]
- Li, S.; De Wijn, J.R.; Li, J.; Layrolle, P.; De Groot, K. Macroporous biphasic calcium phosphate scaffold with high permeability/porosity ratio. Tissue Eng. 2003, 9, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Botchwey, E.A.; Dupree, M.A.; Pollack, S.R.; Levine, E.M.; Laurencin, C.T. Tissue engineered bone: Measurement of nutrient transport in three-dimensional matrices. J. Biomed. Mater. Res. Part A 2003, 67, 357–367. [Google Scholar] [CrossRef]
- Sanz-Herrera, J.A.; Kasper, C.; van Griensven, M.; Garcia-Aznar, J.M.; Ochoa, I.; Doblare, M. Mechanical and flow characterization of Sponceram carriers: Evaluation by homogenization theory and experimental validation. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Haddock, S.M.; Debes, J.C.; Nauman, E.A.; Fong, K.E.; Arramon, Y.P.; Keaveny, T.M. Structure-function relationships for coralline hydroxyapatite bone substitute. J. Biomed. Mater. Res. 1999, 47, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.; Will, J.; Kohler, A.; Hopfner, U.; Aigner, J.; Wintermantel, E. Ceramic TiO2-foams: Characterisation of a potential scaffold. J. Eur. Ceram. Soc. 2004, 24, 661–668. [Google Scholar] [CrossRef]
- Kohles, S.S.; Roberts, J.B.; Upton, M.L.; Wilson, C.G.; Bonassar, L.J.; Schlichting, A.L. Direct perfusion measurements of cancellous bone anisotropic permeability. J. Biomech. 2001, 34, 1197–1202. [Google Scholar] [CrossRef]
- Nauman, E.A.; Fong, K.E.; Keaveny, T.M. Dependence of intertrabecular permeability on flow direction and anatomic site. Ann. Biomed. Eng. 1999, 27, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Müller, R. Bone microarchitecture assessment: current and future trends. Osteoporos. Int. 2003, 14 Supplementary 5, S89–S95. [Google Scholar] [CrossRef]
- Müller, R.; Bosch, T.; Jarak, D.; Stauber, M.; Nazarian, A.; Tantillo, M.; Boyd, S. Micro-mechanical evaluation of bone microstructures under load. In Developments in X-Ray Tomography III; Bonse, U., Ed.; SPIE: Bellingham, DC, USA, 2002; Volume 4503, pp. 189–200. [Google Scholar]
- Beaupied, H.; Lespessailles, E.; Benhamou, C.L. Evaluation of macrostructural bone biomechanics. Joint Bone Spine 2007, 74, 233–239. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, J.C.; Weinans, H. Effects of microarchitecture on bone strength. Curr. Osteoporos. Rep. 2007, 5, 56–61. [Google Scholar] [PubMed]
- Navarro, M.; Aparicio, C.; Charles-Harris, M.; Ginebra, M.P.; Engel, E.; Planell, J.A. Development of a biodegradable composite scaffold for bone tissue engineering: Physicochemical, topographical, mechanical, degradation, and biological properties. In Ordered Polymeric Nanostructures at Surfaces; Springer-Verlag: Berlin, Germany, 2006; Volume 200, pp. 209–231. [Google Scholar]
- Rea, S.M.; Best, S.M.; Bonfield, W. Bioactivity of ceramic-polymer composites with varied composition and surface topography. J. Mater. Sci. Mater. Med. 2004, 15, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Bismarck, A.; Boccaccini, A.R.; Young, A.M.; Nazhat, S.N. Premature degradation of poly(α-hydroxyesters) during thermal processing of Bioglass®-containing composites. Acta Biomater. 2010, 6, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Maquet, V.; Jerome, R.; Boccaccini, A. R.; Nazhat, S.N. Mechanical properties of highly porous PDLLA/Bioglass® composite foams as scaffolds for bone tissue engineering. Acta Biomater. 2005, 1, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Meretoja, V.V.; Helminen, A.O.; Korventausta, J.J.; Haapa-aho, V.; Seppala, J.V.; Narhi, T.O. Crosslinked poly(ε-caprolactone/D,L-lactide)/bioactive glass composite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2006, 77, 261–268. [Google Scholar] [CrossRef]
- Meretoja, V.V.; Malin, M.; Seppala, J.V.; Narhi, T.O. Osteoblast response to continuous phase macroporous scaffolds under static and dynamic culture conditions. J. Biomed. Mater. Res. Part A 2009, 89, 317–325. [Google Scholar] [CrossRef]
- Yao, J.; Radin, S.; Leboy, P.S.; Ducheyne, P. The effect of bioactive glass content on synthesis and bioactivity of composite poly (lactic-co-glycolic acid)/bioactive glass substrate for tissue engineering. Biomaterials 2005, 26, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Keshaw, H.; Georgiou, G.; Blaker, J.J.; Forbes, A.; Knowles, J.C.; Day, R.M. Assessment of polymer/bioactive glass-composite microporous spheres for tissue regeneration applications. Tissue Eng. Part A 2009, 15, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, A.M.; Saad, E.A.; El-Hady, B.M.A.; Farag, M.M. Synthesis of silicate glass/poly(L-lactide) composite scaffolds by freeze-extraction technique: characterization and in vitro bioactivity evaluation. Ceram. Int. 2010, 36, 995–1009. [Google Scholar]
- Tsigkou, O.; Hench, L.L.; Boccaccini, A.R.; Polak, J.M.; Stevens, M.M. Enhanced differentiation and mineralization of human fetal osteoblasts on PDLLA containing Bioglass® composite films in the absence of osteogenic supplements. J. Biomed. Mater. Res. Part A 2007, 80A, 837–851. [Google Scholar] [CrossRef]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jerome, R. Porous poly(α-hydroxyacid)/Bioglass composite scaffolds for bone tissue engineering. I: Preparation and in vitro characterisation. Biomaterials 2004, 25, 4185–4194. [Google Scholar]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jerome, R. Preparation, characterization, and in vitro degradation of bioresorbable and bioactive composites based on Bioglass-filled polylactide foams. J. Biomed. Mater. Res. Part A 2003, 66, 335–346. [Google Scholar] [CrossRef]
- Verrier, S.; Blaker, J.J.; Maquet, V.; Hench, L.L.; Boccaccini, A.R. PDLLA/Bioglass® composites for soft-tissue and hard-tissue engineering: an in vitro cell biology assessment. Biomaterials 2004, 25, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Ohashi, F.; Valappil, S.P.; Knowles, J.C.; Roy, I.; Silva, S.R.; Salih, V.; Boccaccini, A.R. Characterization of carbon nanotube (MWCNT) containing P(3HB)/bioactive glass composites for tissue engineering applications. Acta Biomater. 2010, 6, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Philip, S.E.; Chrzanowski, W.; Nazhat, S.N.; Roy, I.; Knowles, J.C.; Salih, V.; Boccaccini, A.R. Incorporation of vitamin E in poly(3-hydroxybutyrate)/Bioglass composite films: effect on surface properties and cell attachment. J. Roy. Soc. Interface 2009, 6, 401–409. [Google Scholar] [CrossRef]
- Helen, W.; Gough, J.E. Cell viability, proliferation and extracellular matrix production of human annulus fibrosus cells cultured within PDLLA/Bioglass® composite foam scaffolds in vitro. Acta Biomater. 2008, 4, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Helen, W.; Merry, C.L.R.; Blaker, J.J.; Gough, J.E. Three-dimensional culture of annulus fibrosus cells within PDLLA/Bioglass® composite foam scaffolds: Assessment of cell attachment, proliferation and extracellular matrix production. Biomaterials 2007, 28, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.B.; Webb, D.; Blaker, J.; Boccaccini, A.R.; Maquet, V.; Cooper, C.; Oreffo, R.O.C. Evaluation of human bone marrow stromal cell growth on biodegradable polymer/Bioglass® composites. Biochem. Biophys. Res. Commun. 2006, 342, 1098–1107. [Google Scholar] [CrossRef]
- Barroca, N.; Daniel-da-Silva, A.L.; Vilarinho, P.M.; Fernandes, M.H.V. Tailoring the morphology of high molecular weight PLLA scaffolds through bioglass additions. Acta Biomater. in press.
- Matthews, F.L.; Rawlings, R.D. Composite Materials: Engineering and Science, 1st ed.; Woodhead Publishing Limited CRC Press: Cambridge, UK, 1994; p. 470. [Google Scholar]
- Ishai, O.; Cohen, L.J. Elastic properties of filled and porous epoxy composites. Int. J. Mech. Sci. 1967, 9, 539–546. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Lu, H.H.; Tang, A.; Oh, S.C.; Spalazzi, J.P.; Dionisio, K. Compositional effects on the formation of a calcium phosphate layer and the response of osteoblast-like cells on polymer-bioactive glass composites. Biomaterials 2005, 26, 6323–6334. [Google Scholar] [CrossRef]
- Gao, Y.; Chang, J. Surface modification of bioactive glasses and preparation of PDLLA/bioactive glass composite films. J. Biomater. Appl. 2009, 24, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Gubler, M.; Brunner, T.J.; Zehnder, M.; Waltimo, T.; Sener, B.; Stark, W.J. Do bioactive glasses convey a disinfecting mechanism beyond a mere increase in pH? Int. Endod. J. 2008, 41, 670–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waltimo, T.; Brunner, T.J.; Vollenweider, M.; Stark, W.J.; Zehnder, M. Antimicrobial effect of nanometric bioactive glass 45S5. J. Dent. Res. 2007, 86, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Waltimo, T.; Mohn, D.; Paque, F.; Brunner, T.J.; Stark, W.J.; Imfeld, T.; Schatzle, M.; Zehnder, M. Fine-tuning of bioactive glass for root canal disinfection. J. Dent. Res. 2009, 88, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Ravarian, R.; Moztarzadeh, F.; Hashjin, M.S.; Rabiee, S.M.; Khoshakhlagh, P.; Tahriri, M. Synthesis, characterization and bioactivity investigation of bioglass/hydroxyapatite composite. Ceram. Int. 2010, 36, 291–297. [Google Scholar] [CrossRef]
- Mansur, H.S.; Costa, H.S. Nanostructured poly(vinyl alcohol)/bioactive glass and poly(vinyl alcohol)/chitosan/bioactive glass hybrid scaffolds for biomedical applications. Chem. Eng. J. 2008, 137, 72–83. [Google Scholar] [CrossRef]
- Mishra, R.; Basu, B.; Kumar, A. Physical and cytocompatibility properties of bioactive glass-polyvinyl alcohol-sodium alginate biocomposite foams prepared via sol-gel processing for trabecular bone regeneration. J. Mater. Sci. Mater. Med. 2009, 20, 2493–2500. [Google Scholar] [CrossRef]
- Hong, Z.; Reis, R.L.; Mano, J.F. Preparation and in vitro characterization of scaffolds of poly(l-lactic acid) containing bioactive glass ceramic nanoparticles. Acta Biomater. 2008, 4, 1297–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peter, M.; Sudheesh Kumar, P.T.; Binulal, N.S.; Nair, S.V.; Tamura, H.; Jayakumar, R. Development of novel α-chitin/nanobioactive glass ceramic composite scaffolds for tissue engineering applications. Carbohyd. Polym. 2009, 78, 926–931. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Ali, A.F.; Farag, M.M. Development, characterization, and in vitro bioactivity studies of sol-gel bioactive glass/poly(l-lactide) nanocomposite scaffolds. Mater. Sci. Eng. C 2010, 30, 120–131. [Google Scholar] [CrossRef]
- Xie, E.; Hu, Y.; Chen, X.; Bai, X.; Li, D.; Ren, L.; Zhang, Z. In vivo bone regeneration using a novel porous bioactive composite. Appl. Surf. Sci. 2008, 255, 545–547. [Google Scholar] [CrossRef]
- Hench, L.L.; Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Polak, J.M.; Zhong, J.P.; Liu, X.Y.; Chang, J. Gene activating glasses. J. Inorg. Mater. 2002, 17, 897–909. [Google Scholar]
- Bielby, R.C.; Christodoulou, I.S.; Pryce, R.S.; Radford, W.J.; Hench, L.L.; Polak, J.M. Time- and concentration-dependent effects of dissolution products of 58S sol-gel bioactive glass on proliferation and differentiation of murine and human osteoblasts. Tissue Eng. Part A 2004, 10, 1018–1026. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Bielby, R.C.; Boccaccini, A.R.; Polak, J.M.; Buttery, L.D. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. Part A 2004, 10, 1518–1525. [Google Scholar] [CrossRef]
- Bielby, R.C.; Pryce, R.S.; Hench, L.L.; Polak, J.M. Enhanced derivation of osteogenic cells from murine embryonic stem cells after treatment with ionic dissolution products of 58S bioactive sol-gel glass. Tissue Eng. Part A 2005, 11, 479–488. [Google Scholar] [CrossRef]
- Sun, J.Y.; Yang, Y.S.; Zhong, J.P.; Greenspan, D.C. The effect of the ionic products of Bioglass® dissolution on human osteoblasts growth cycle in vitro. J. Tissue Eng. Regen. Med. 2007, 1, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Jell, G.; Notingher, I.; Tsigkou, O.; Notingher, P.; Polak, J.M.; Hench, L.L.; Stevens, M.M. Bioactive glass-induced osteoblast differentiation: a noninvasive spectroscopic study. J. Biomed. Mater. Res. Part A 2008, 86, 31–40. [Google Scholar] [CrossRef]
- Jell, G.; Stevens, M.M. Gene activation by bioactive glasses. J. Mater. Sci. Mater. Med. 2006, 17, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Silicon-substituted calcium phosphates - a critical view. Biomaterials 2009, 30, 6403–6406. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.I.; Reis, R.L. Vascularization in Bone Tissue Engineering: Physiology, Current Strategies, Major Hurdles and Future Challenges. Macromol. Biosci. 2010, 10, 12–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, F.; Bertram, H.; Berger, I.; Lorenz, H.; Wall, O.; Eckhardt, C.; Simank, H.G.; Richter, W. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J. Bone Miner. Res. 2005, 20, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Usas, A.; Olshanski, A.; Ho, A.M.; Gearhart, B.; Cooper, G.M.; Huard, J. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J. Bone Miner. Res. 2005, 20, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M.; Boccaccini, A.R.; Shurey, S.; Roether, J.A.; Forbes, A.; Hench, L.L.; Gabe, S.M. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials 2004, 25, 5857–5866. [Google Scholar] [CrossRef] [PubMed]
- Keshaw, H.; Forbes, A.; Day, R.M. Release of angiogenic growth factors from cells encapsulated in alginate beads with bioactive glass. Biomaterials 2005, 26, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Moosvi, S.R.; Day, R.M. Bioactive glass modulation of intestinal epithelial cell restitution. Acta Biomater. 2009, 5, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.K.; Kaigler, D.; Wang, Z.; Krebsbach, P.H.; Mooney, D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials 2006, 27, 3249–3255. [Google Scholar] [CrossRef] [PubMed]
- Jarrahy, R.; Huang, W.; Rudkin, G.H.; Lee, J.M.; Ishida, K.; Berry, M.D.; Sukkarieh, M.; Wu, B.M.; Yamaguchi, D.T.; Miller, T.A. Osteogenic differentiation is inhibited and angiogenic expression is enhanced in MC3T3-E1 cells cultured on three-dimensional scaffolds. Am. J. Physiol. Cell Physiol. 2005, 289, C408–414. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.L.; Andrade, S.P.; Domingues, R.Z. In vivo performance of a sol-gel glass-coated collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 79B, 122–128. [Google Scholar] [CrossRef]
- Ross, E.A.; Batich, C.D.; Clapp, W.L.; Sallustio, J.E.; Lee, N.C. Tissue adhesion to bioactive glass-coated silicone tubing in a rat model of peritoneal dialysis catheters and catheter tunnels. Kidney Int. 2003, 63, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Lee, J.E.; Park, H.J.; Oum, B.S. Effect of synthetic bone glass particulate on the fibrovascularization of porous polyethylene orbital implants. Ophthalmic Plast. Reconstr. Surg. 2006, 22, 121–125. [Google Scholar] [CrossRef]
- Leu, A.; Stieger, S.M.; Dayton, P.; Ferrara, K.W.; Leach, J.K. Angiogenic response to bioactive glass promotes bone healing in an irradiated calvarial defect. Tissue Eng. Part A 2009, 15, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Chapanian, R.; Amsden, B.G. Combined and sequential delivery of bioactive VEGF165 and HGF from poly(trimethylene carbonate) based photo-cross-linked elastomers. J. Contr. Rel. 2010, 143, 53–63. [Google Scholar] [CrossRef]
- Chiu, L.L.Y.; Radisic, M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials 2010, 31, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.K.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Briganti, E.; Spiller, D.; Mirtelli, C.; Kull, S.; Counoupas, C.; Losi, P.; Senesi, S.; Di Stefano, R.; Soldani, G. A composite fibrin-based scaffold for controlled delivery of bioactive pro-angiogenetic growth factors. J. Contr. Rel. 2010, 142, 14–21. [Google Scholar] [CrossRef]
- Kaigler, D.; Wang, Z.; Horger, K.; Mooney, D.J.; Krebsbach, P.H. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J. Bone Miner. Res. 2006, 21, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, Å.; Oscarsson, S.; Mazzocchi, M.; Krajewski, A.; Ravaglioli, A. Protein adsorption onto two bioactive glass-ceramics. Biomaterials 2003, 24, 147–155. [Google Scholar] [CrossRef]
- Goller, G. The effect of bond coat on mechanical properties of plasma sprayed bioglass-titanium coatings. Ceram. Int. 2004, 30, 351–355. [Google Scholar] [CrossRef]
- Guo, H.B.; Miao, X.; Chen, Y.; Cheang, P.; Khor, K.A. Characterization of hydroxyapatite- and bioglass-316L fibre composites prepared by spark plasma sintering. Mater. Lett. 2004, 58, 304–307. [Google Scholar] [CrossRef]
- Verné, E.; Ferraris, S.; Vitale-Brovarone, C.; Spriano, S.; Bianchi, C.L.; Naldoni, A.; Morra, M.; Cassinelli, C. Alkaline phosphatase grafting on bioactive glasses and glass ceramics. Acta Biomater. 2010, 6, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Annala, T.; Kellomäki, M. Sterilization of biodegradable polymers. In Degradable Polymers for Skeletal Implants, 1st ed.; Wuisman, P.I.J.M., Smit, T.H., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2009; pp. 123–138. [Google Scholar]
- Cordewener, F.W.; van Geffen, M.F.; Joziasse, C.A.P.; Schmitz, J.P.; Bos, R.R.M.; Rozema, F.R.; Pennings, A.J. Cytotoxicity of poly(96L/4D-lactide): The influence of degradation and sterilization. Biomaterials 2000, 21, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Janorkar, A.V.; Metters, A.T.; Hirt, D.E. Degradation of Poly(L-Lactide) films under ultraviolet-induced photografting and sterilization conditions. J. Appl. Polym. Sci. 2007, 106, 1042–1047. [Google Scholar] [CrossRef]
- Jukes, J.M.; van Blitterswijk, C.A.; de Boer, J. Skeletal tissue engineering using embryonic stem cells. J. Tissue Eng. Regen. Med. 2010, 4, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.H.; Hong, S.J.; Jeong, I.; Yu, H.S.; Jegal, S.H.; Kim, H.W. Development of robotic dispensed bioactive scaffolds and human adipose-derived stem cell culturing for bone tissue engineering. Tissue Eng. Part C Methods 2009, September 1. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, X.J.; Li, J.S. Preparation and biocompatibility evaluation of apatite/wollastonite-derived porous bioactive glass ceramic scaffolds. Biomed. Mater. 2009, 4, 45007. [Google Scholar] [CrossRef]
- Harrison, B.S.; Atala, A. Carbon nanotube applications for tissue engineering. Biomaterials 2007, 28, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Chicatun, F.; Cho, J.; Bretcanu, O.; Roether, J.A.; Novak, S.; Chen, Q. Carbon nanotube coatings on bioglass-based tissue engnineering scaffolds. Adv. Funct. Mater. 2007, 17, 2815–2822. [Google Scholar] [CrossRef]
- Misra, S.K.; Boccaccini, A.R. Biodegradable and bioactive polymer/ceramic composite scaffolds. In Tissue Engineering Using Ceramics and Polymers, 1st ed.; Boccaccini, A.R., Gough, J.E., Eds.; Woodhead Publishing Limited CRC Press: Cambridge, UK, 2007; Volume 1, pp. 72–92. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gerhardt, L.-C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867-3910. https://doi.org/10.3390/ma3073867
Gerhardt L-C, Boccaccini AR. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials. 2010; 3(7):3867-3910. https://doi.org/10.3390/ma3073867
Chicago/Turabian StyleGerhardt, Lutz-Christian, and Aldo R. Boccaccini. 2010. "Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering" Materials 3, no. 7: 3867-3910. https://doi.org/10.3390/ma3073867





