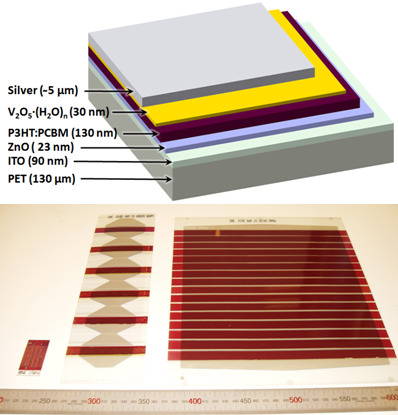
Roll-to-Roll Processing of Inverted Polymer Solar Cells using Hydrated Vanadium(V)Oxide as a PEDOT:PSS Replacement
Abstract
:1. Introduction
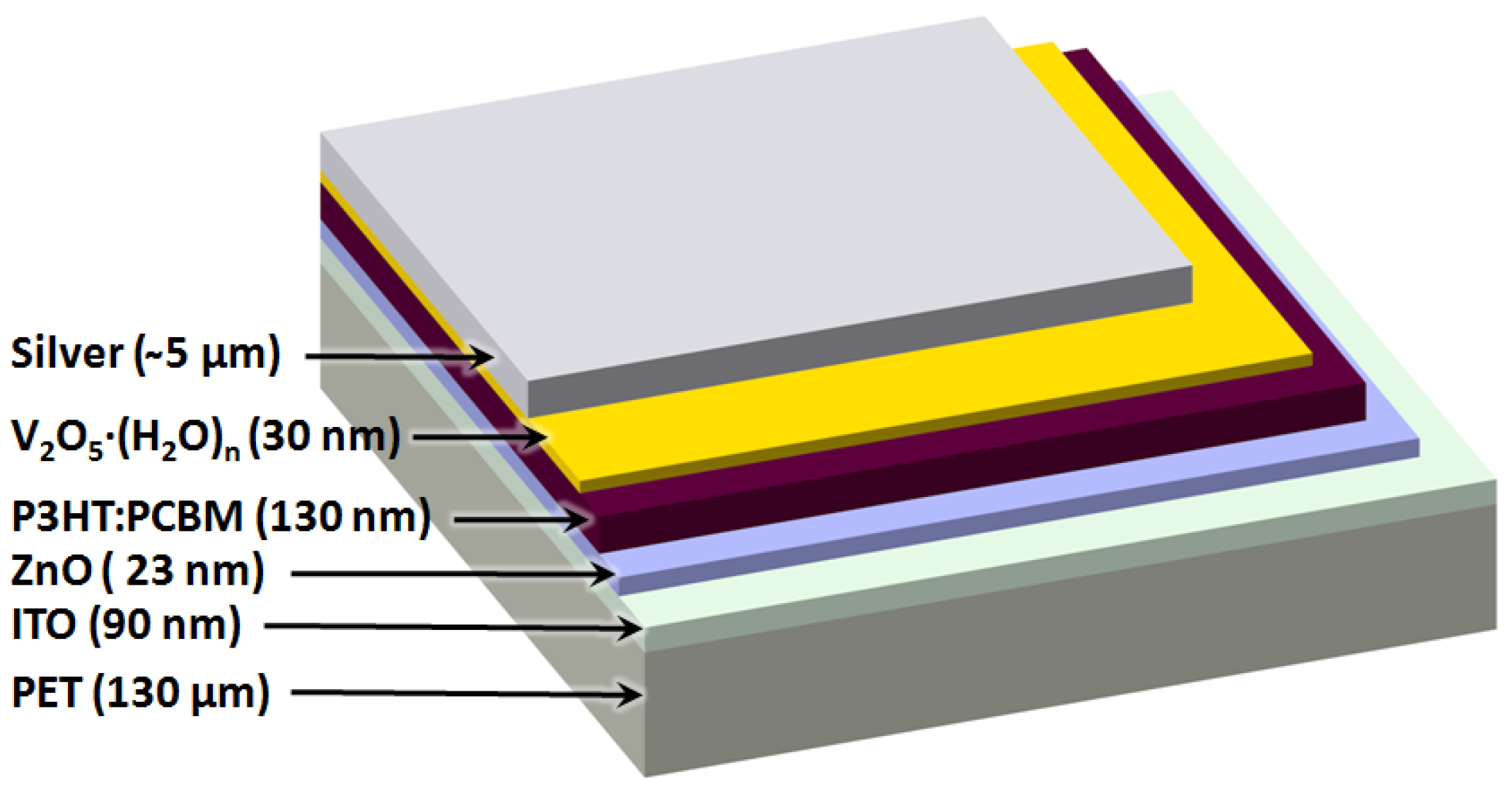
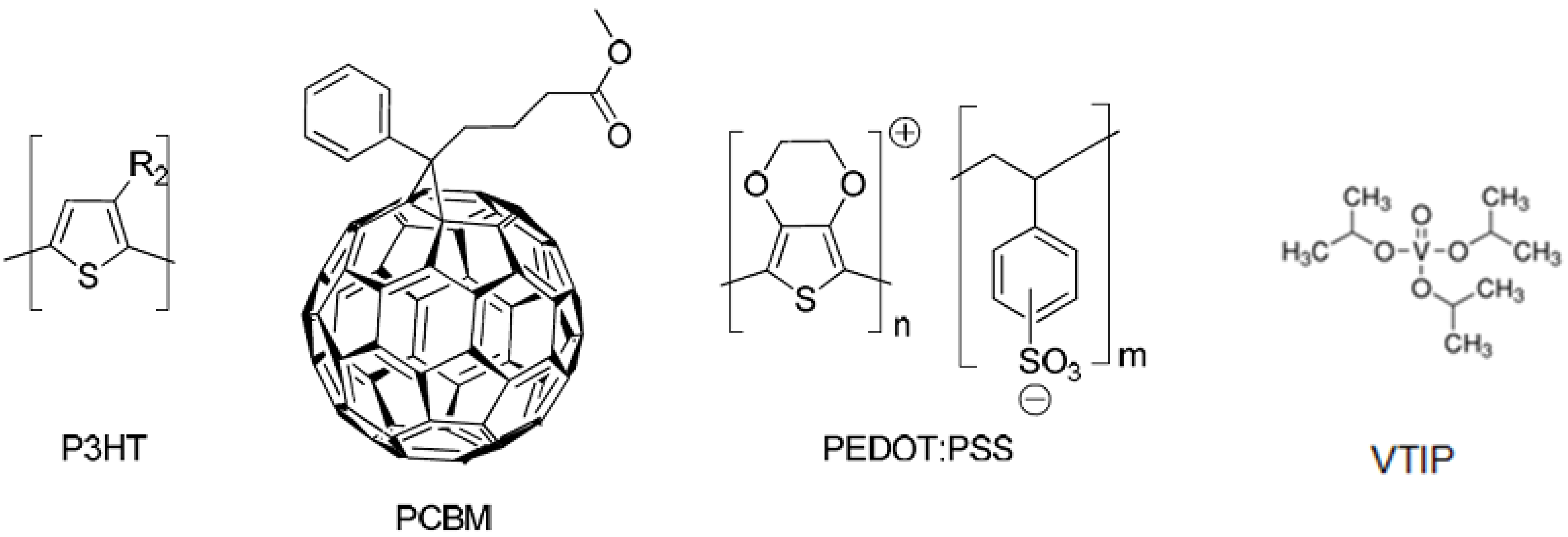
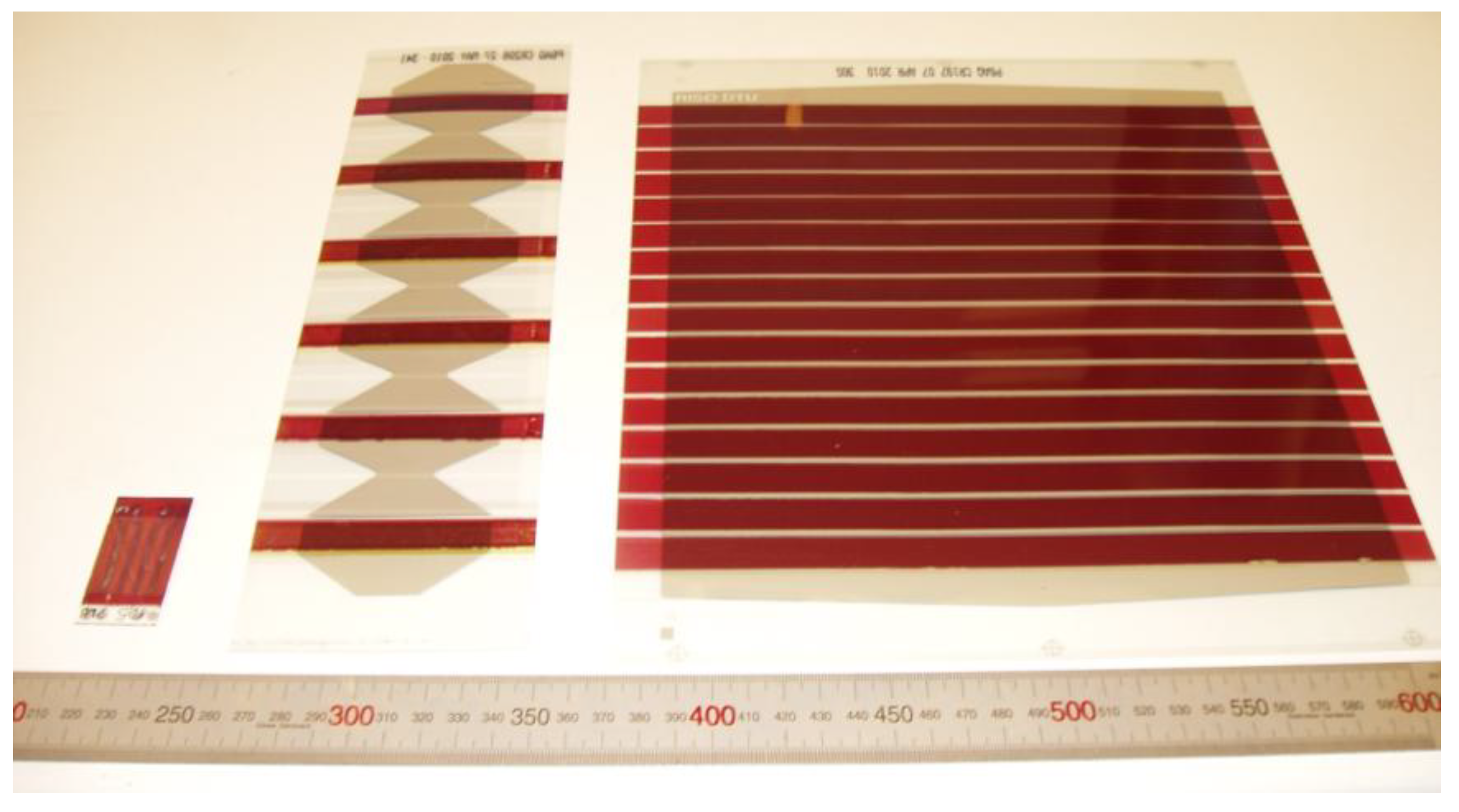
2. Results and Discussion
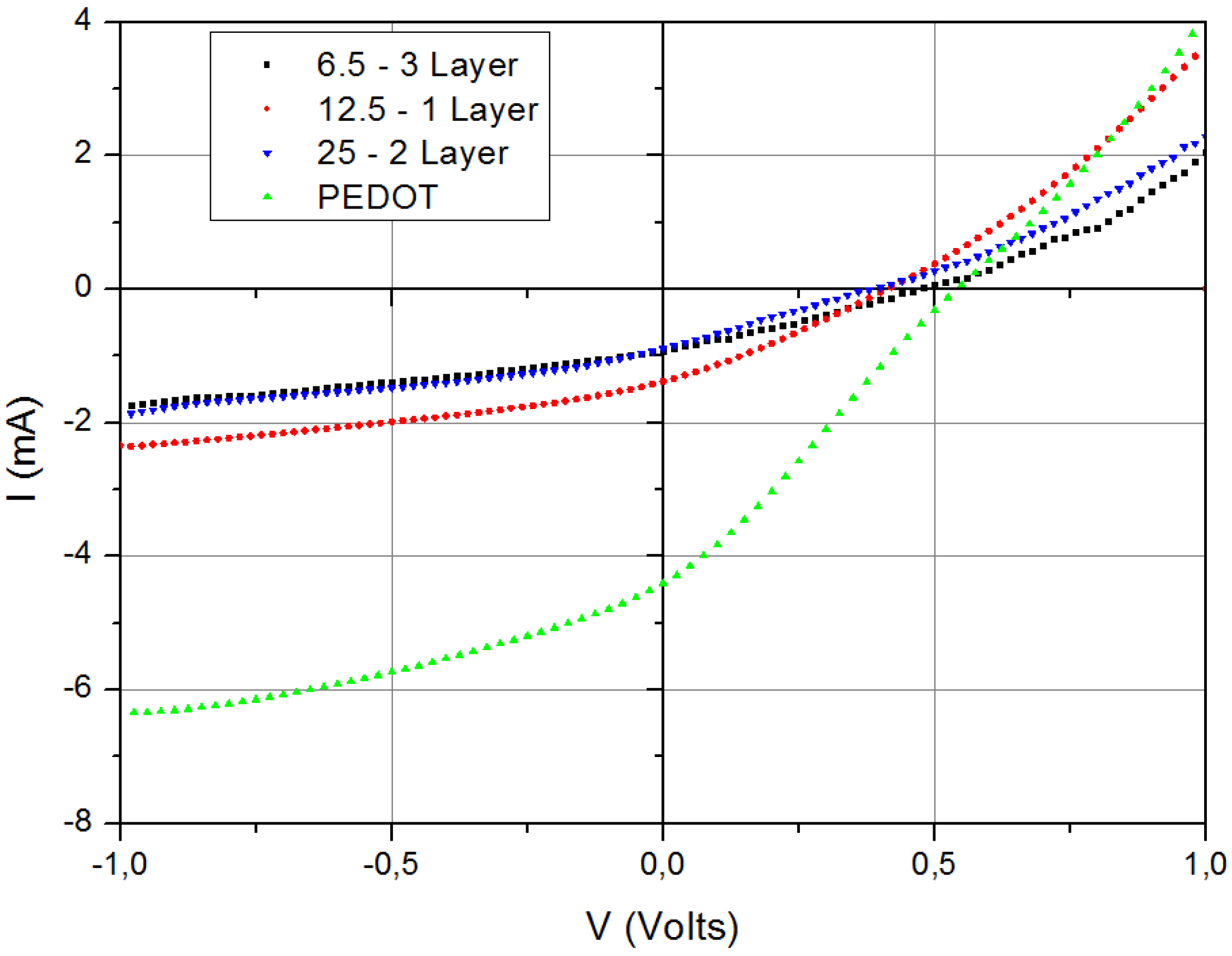
2.1. Spin Coated Cells on Glass Substrates
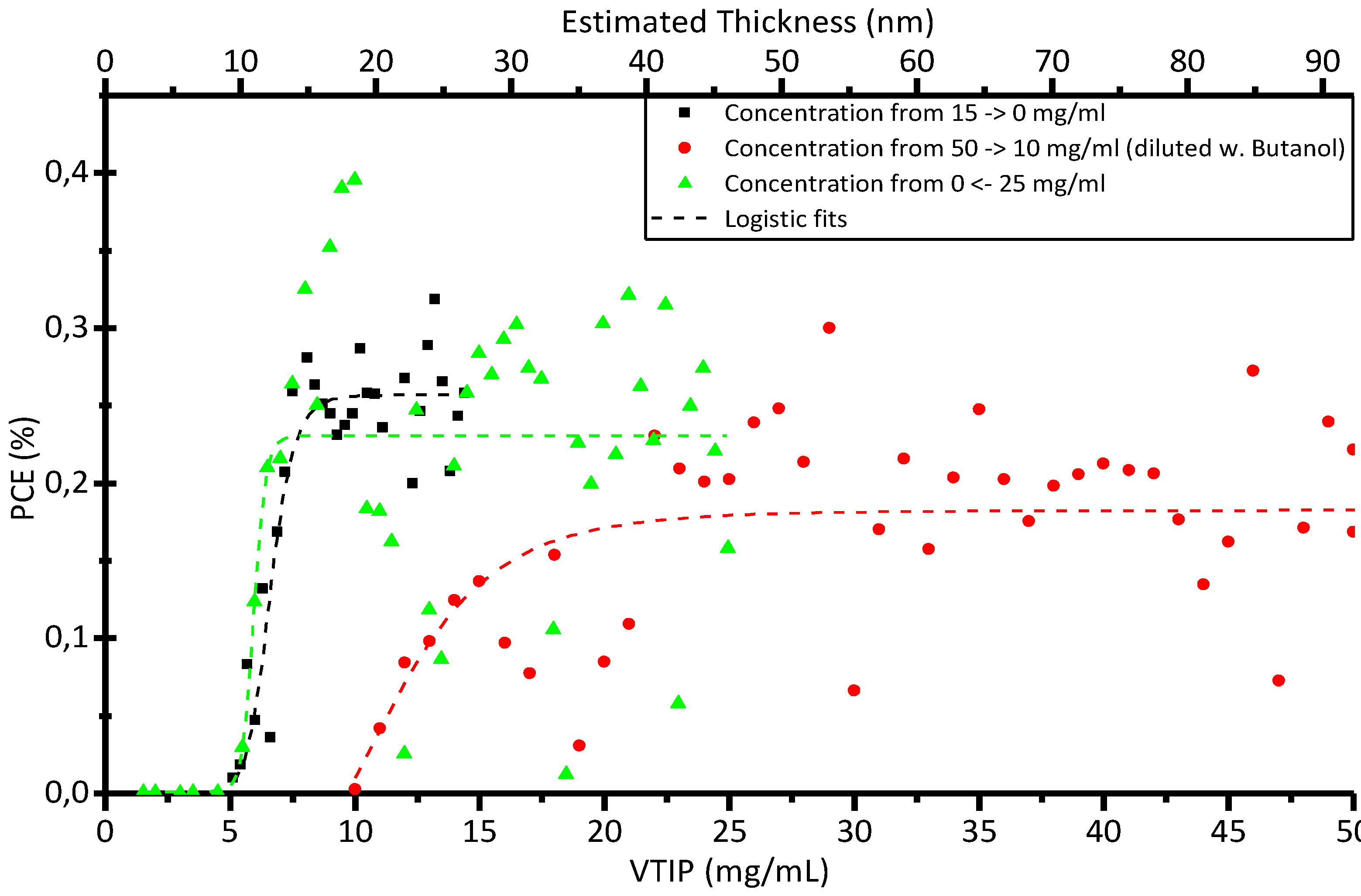
2.2. Gradient Study on R2R PET Cells
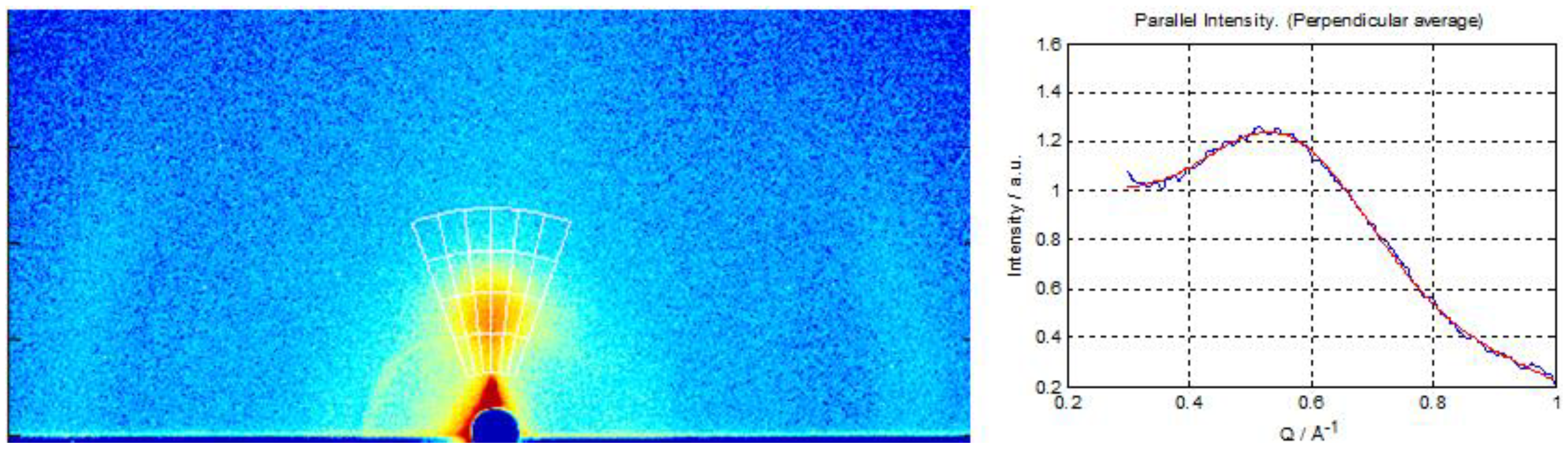
2.3. Characterization of Hydrated Vanadium(V)Oxide

2.4. Full R2R Fabrication of 16 Cell Modules
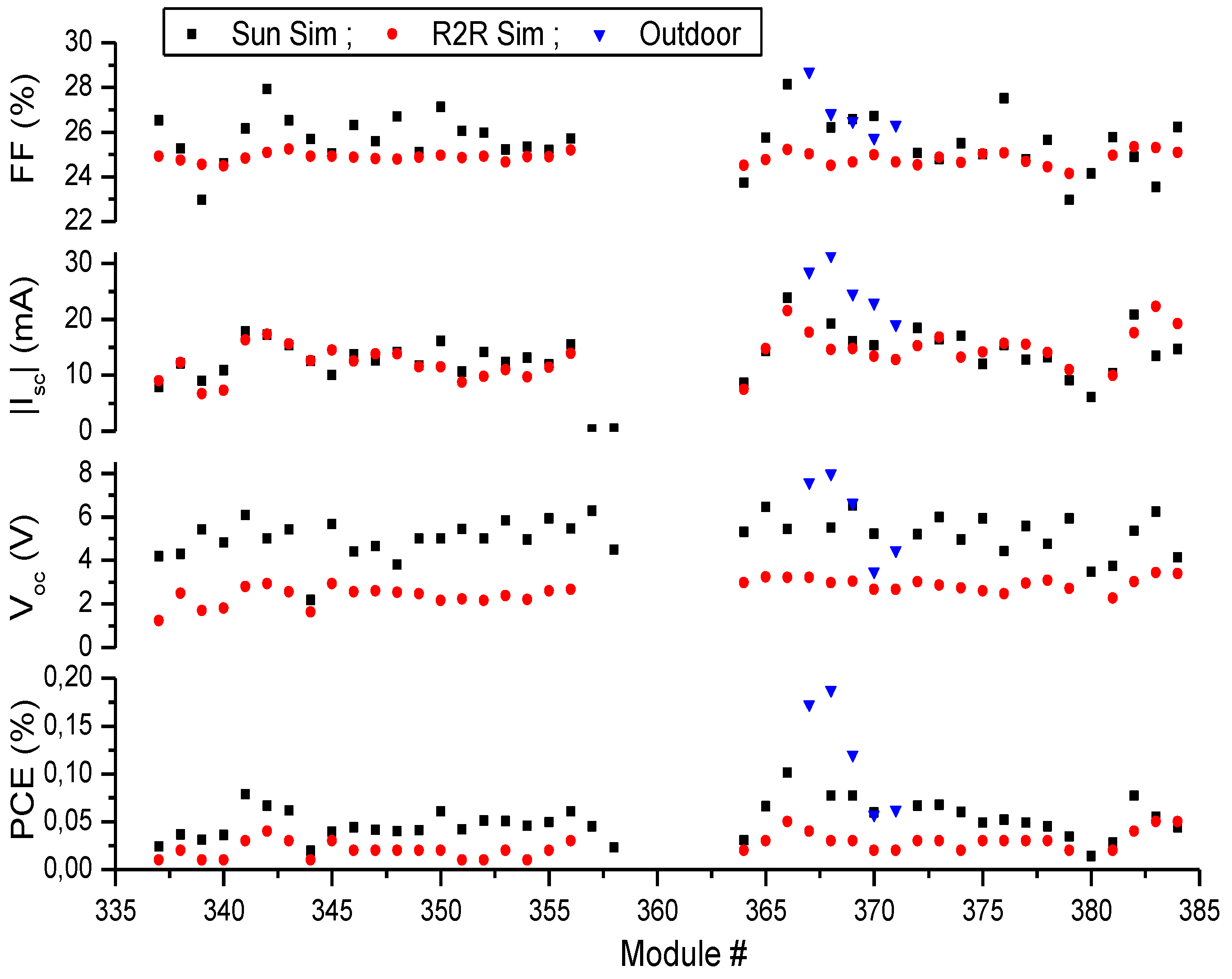
| Measurement | # of Modules | PCE (%) | Voc (V) | Isc (mA) | FF (%) |
|---|---|---|---|---|---|
| R2R Simulator | 40 | 0.025 ± 0.011 | 2.6 ± 0.5 | −13.5±3.6 | 24.8 ± 0.3 |
| Sun Simulator | 40 | 0.050 ± 0.018 | 5.1 ± 0.9 | −13.8 ± 3.6 | 25.6 ± 1.2 |
| Outdoor | 5 | 0.12 ± 0.06 | 6.0 ± 2.0 | −25.4 ± 4.8 | 26.8 ± 1.1 |
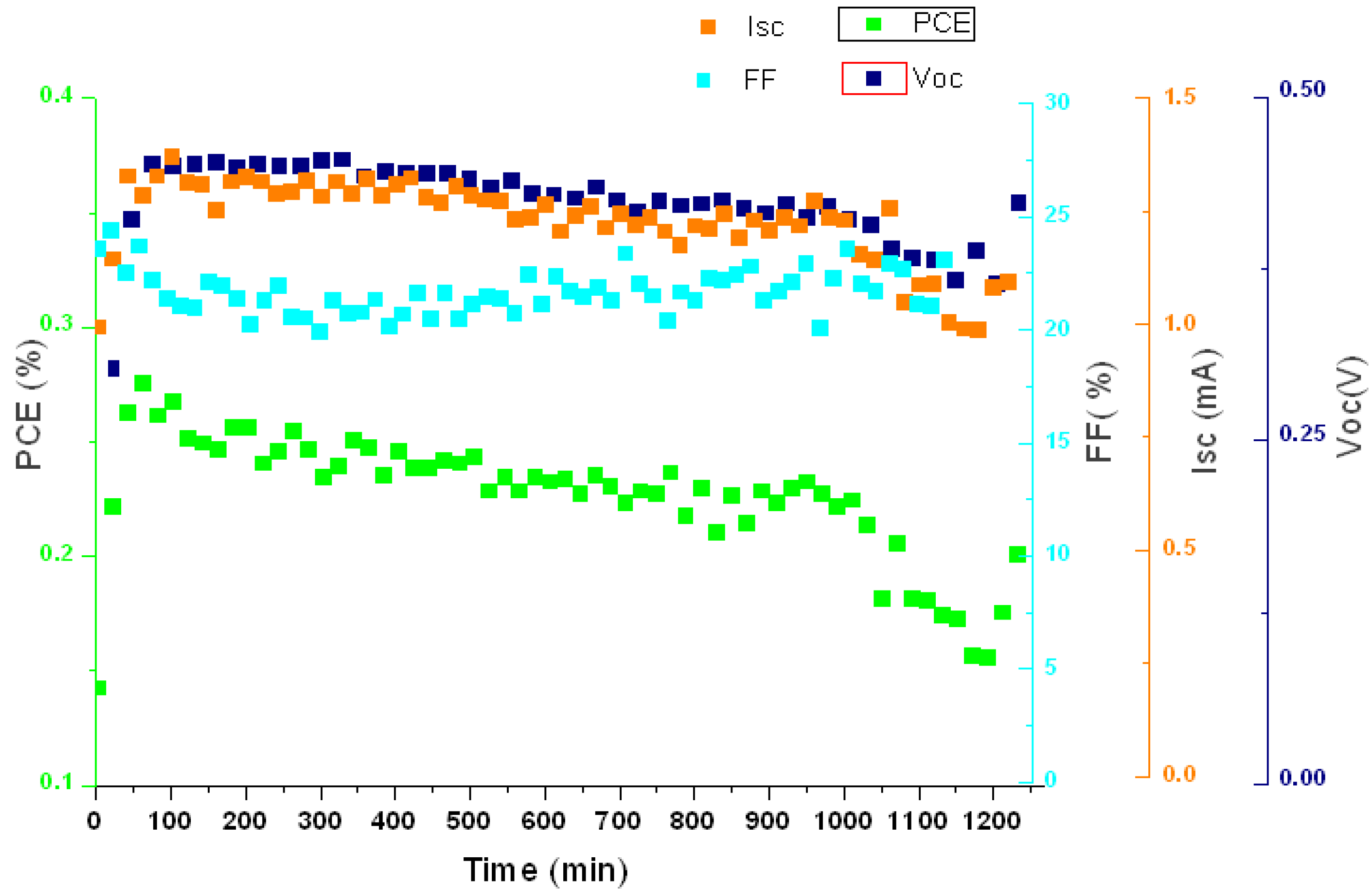
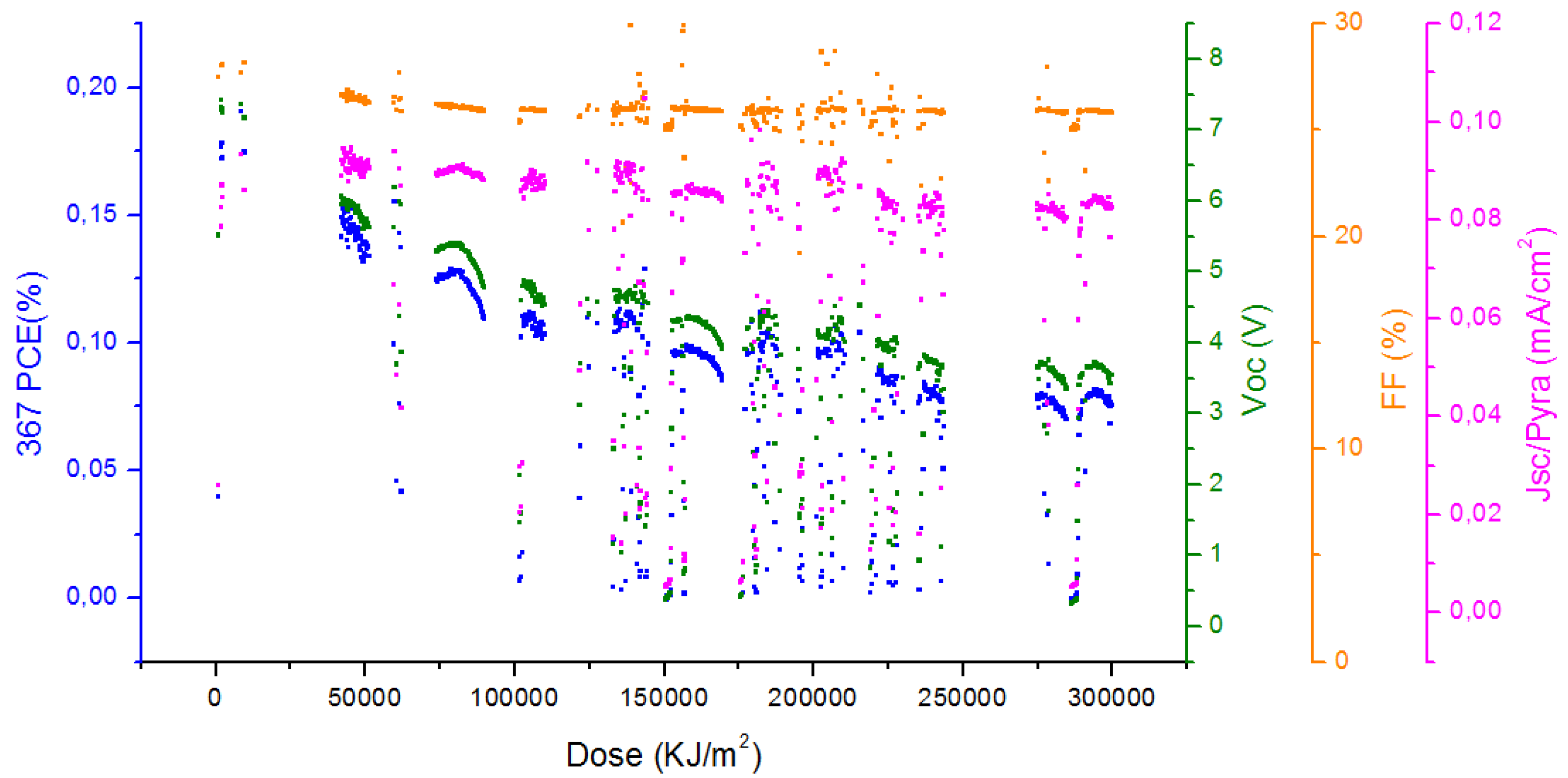
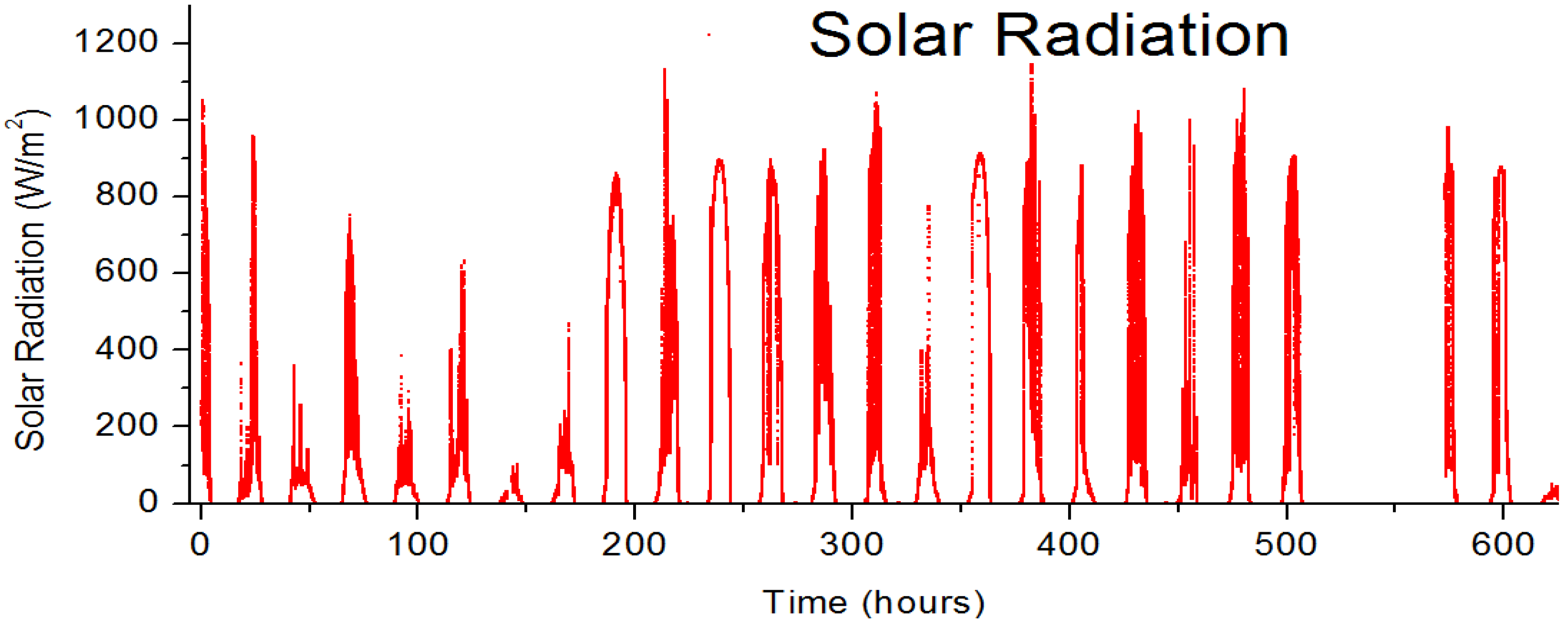
2.5. Stability Measurements

3. Experimental Section
3.1. Materials
3.2. Grazing Incidence Wide Angle X-ray Scattering
3.3. Processing Methods
| Substrate | Device | [VTIP] (mg/mL) | Active Area (cm2) |
|---|---|---|---|
| Glass | Spin Cast Single Cells | 0.3–50 | 0.5 |
| PET | R2R Cells Stripe 1 | 0–100 | 4.2 |
| PET | R2R Cells Stripe 2 | 0–15 | 4.2 |
| PET | R2R Cells Stripe 3 | 0–50 | 4.2 |
| PET | R2R Cells Stripe 4 | 0–50 (butanol) | 4.2 |
| PET | R2R Cells Stripe 5 | 0–25 | 4.2 |
| PET | R2R Cells Stripe 6 | 0–100 | 4.2 |
| PET | R2R 16 Cell Modules | 15 | 360 |
3.4. Test Conditions
4. Future Perspectives and Conclusion
Acknowledgements
References
- Helgesen, M.; Søndergaard, R.; Krebs, F.C. Advanced materials and processes for polymer solar cell devices. J. Mater. Chem. 2010, 20, 36–60. [Google Scholar] [CrossRef]
- Nielsen, T.D.; Cruickshank, C.; Foged, S.; Thorsen, J.; Krebs, F.C. Business, market and intellectual property analysis of polymer solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1553–1571. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells. A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Krebs, F.C.; Gevorgyan, S.A.; Alstrup, J. A roll-to-roll process to flexible polymer solar cells: Model studies, manufacture and operational stability studies. J. Mater. Chem. 2009, 19, 5442–5451. [Google Scholar] [CrossRef]
- Krebs, F.C. All solution roll-to-roll processed polymer solar cells free from indium-tin-oxide and vacuum coating steps. Org. Electronics 2009, 10, 761–768. [Google Scholar]
- Krebs, F.C.; Tromholt, T.; Jørgensen, M. Upscaling of polymer solar cell fabrication using full roll-to-roll processing. Nanoscale 2010, 2, 878–886. [Google Scholar] [CrossRef]
- Krebs, F.C. Polymer solar cell modules prepared using roll-to-roll methods: Knife-over-edge coating, slot-die coating and screen printing. Sol. Energy Mater. Sol. Cells 2009, 93, 465–475. [Google Scholar] [CrossRef]
- Krebs, F.C.; Norrman, K. Using light induced thermocleavage in a roll-to-roll process for polymer solar cells. ACS Appl. Mater. Interfaces 2010, 2, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Krebs, F.C.; Jørgensen, M.; Norrman, K.; Hagemann, O.; Alstrup, J.; Nielsen, T.D.; Fyenbo, J.; Larsen, K.; Kristensen, J. A complete process for production of flexible large area polymer solar cells entirely using screen printing—First public demonstration. Sol. Energy Mater. Sol. Cells 2009, 93, 422–441. [Google Scholar] [CrossRef]
- Krebs, F.C.; Gevorgyan, S.A.; Gholamkhass, B.; Holdcroft, S.; Schlenker, C.; Thompson, M.E.; Thompson, B.C.; Olson, D.; Ginley, D.S.; Shaheen, S.E.; Alshareef, H.N.; Murphy, J.W.; Youngblood, W.J.; Heston, N.C.; Reynolds, J.R.; Jia, S.; Laird, D.; Tuladhar, S.M.; Dane, J.G.A.; Atienzar, P.; Nelson, J.; Kroon, J.M.; Wienk, M.M.; Janssen, R.A.J.; Tvingstedt, K.; Zhang, F.; Andersson, M.; Inganäs, O.; Lira-Cantu, M.; De Bettignies, R.; Guillerez, S.; Aernouts, T.; Cheyns, D.; Lutsen, L.; Zimmermann, B.; Würfel, U.; Niggemann, M.; Schleiermacher, H.-F.; Liska, P.; Grätzel, M.; Lianos, P.; Katz, E.A.; Lohwasser, W.; Jannon, B. A round robin study of flexible large-area roll-to-roll processed polymer solar cell modules. Sol. Energy Mater. Sol. Cells 2009, 93, 1968–1977. [Google Scholar] [CrossRef]
- Krebs, F.C.; Nielsen, T.D.; Fyenbo, J.; Wadstrøm, M.; Pedersen, M.S. Manufacture, integration and demonstration of polymer solar cells in a lamp for the “Lighting Africa” initiative. Energy. Environ. Sci. 2010, 3, 512–525. [Google Scholar] [CrossRef]
- Medford, A.J.; Lilliedal, M.R.; Jørgensen, M.; Aarø, D.H.; Pakalski, D.; Fyenbo, J.; Krebs, F.C. Grid-connected polymer solar panels: Initial considerations of cost, lifetime, and practicality. Optics Express 2010, 18, A272–A285. [Google Scholar]
- Krebs, F.C.; Fyenbo, J.; Jørgensen, M. Product integration of compact roll-to-roll processed polymer solar cell modules: Methods and manufacture using flexographic printing, slot-die coating and rotary screen printing. J. Mater. Chem. 2010, 20, 8994–9001. [Google Scholar] [CrossRef]
- Solarmer, Inc. Available online: http://www.solarmer.com (accessed on the 10 June 2010).
- Hauch, J.A.; Schilinsky, P.; Choulis, S.A.; Rajoelson, S.; Brabec, C.J. The impact of water vapor transmission rate on the lifetime of flexible polymer solar cells. App. Phys. Lett. 2008, 93, 103306–103308. [Google Scholar] [CrossRef]
- Ameri, T.; Dennler, G.; Lungenschmied, C.; Brabec, C.J. Organic tandem solar cells: A review. Energy Environ. Sci. 2009, 2, 347–363. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, G. Synthesis and enhanced intercalation properties of nanostructured vanadium oxides. Chem. Mater. 2006, 18, 2787–2804. [Google Scholar] [CrossRef]
- Shrotriya, V.; Li, G.; Yao, Y.; Chu, C.; Yang, Y. Transition metal oxides as the buffer layer for polymer photovoltaic cells. Appl. Phys. Lett. 2006, 88, 073508–073510. [Google Scholar] [CrossRef]
- Wu, J.; Hou, J.; Cheng, Y.; Xie, Z.; Wang, L. Efficient top-emitting organic light-emitting diodes with a V2O5 modified silver anode. Semicond. Sci. Technol. 2007, 22, 824–826. [Google Scholar] [CrossRef]
- Huang, J.S.; Wu, C.H.; Chou, C.Y.; Liu, M.Y.; Lin, W.H.; Lin, Y.H.; Lin, C.F. Solution-processed vanadium oxide interlayer for improving the performance of polymer/ZnO nanorod hybrid solar cells. In Proceedings of Photovoltaic Specialists Conference (PVSC) 2009 34th IEEE, Philadelphia, PA, USA, 7–12 June 2010; pp. 000554–000556.
- Petkov, V.; Trikalitis, P.N.; Bozin, E.S.; Billinge, S.J.L.; Vogt, T.; Kanatzidis, M.G. Structure of V2O5·nH2O Xerogel Solved by the Atomic Pair Distribution Function Technique. J. Am. Chem. Soc. 2002, 124, 10157–10162. [Google Scholar] [CrossRef]
- Barbosa, G.N.; Graeff, C.F.O.; Oliveira, H.P. Thermal annealing effects on vanadium pentoxide xerogel films. Ecl. Quím. 2005, 30, 7–15. [Google Scholar]
- Apitz, D.; Bertram, R.P.; Benter, N.; Hieringer, W.; Andreasen, J.W.; Nielsen, M.M.; Johansen, P.M.; Buse, K. Investigation of chromophore-chromophore interaction by electro-optic measurements, linear dichroism, X-ray scattering, and density-functional calculations. Phys. Rev. E 2005, 72, 036610–036619. [Google Scholar]
- Breiby, D.W.; Bunk, O.; Andreasen, J.W.; Lemke, H.T.; Nielsen, M.M. Simulating X-ray diffraction of textured films. J. Appl. Cryst. 2008, 41, 262–271. [Google Scholar] [CrossRef]
- Alstrup, J; Jørgensen, M.; Medford, A.J.; Krebs, F.C. Ultra fast and parsimonious materials screening for polymer solar cells using differentially pumped slot-die coating. ACS Appl. Mater. Interfaces 2010, 2, 2819–2827. [Google Scholar] [CrossRef] [PubMed]
- International Summit on OPV Stability (ISOS-3), Roskilde, Denmark, 21–29 October 2010.
© 2011 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Espinosa, N.; Dam, H.F.; Tanenbaum, D.M.; Andreasen, J.W.; Jørgensen, M.; Krebs, F.C. Roll-to-Roll Processing of Inverted Polymer Solar Cells using Hydrated Vanadium(V)Oxide as a PEDOT:PSS Replacement. Materials 2011, 4, 169-182. https://doi.org/10.3390/ma4010169
Espinosa N, Dam HF, Tanenbaum DM, Andreasen JW, Jørgensen M, Krebs FC. Roll-to-Roll Processing of Inverted Polymer Solar Cells using Hydrated Vanadium(V)Oxide as a PEDOT:PSS Replacement. Materials. 2011; 4(1):169-182. https://doi.org/10.3390/ma4010169
Chicago/Turabian StyleEspinosa, Nieves, Henrik Friis Dam, David M. Tanenbaum, Jens W. Andreasen, Mikkel Jørgensen, and Frederik C. Krebs. 2011. "Roll-to-Roll Processing of Inverted Polymer Solar Cells using Hydrated Vanadium(V)Oxide as a PEDOT:PSS Replacement" Materials 4, no. 1: 169-182. https://doi.org/10.3390/ma4010169