Surfactant Effects on Microemulsion-Based Nanoparticle Synthesis
Abstract
:1. Introduction
2. Simulation Procedure
2.1. Microemulsion Description
2.2. Initial Reactant Distribution
2.3. Motion and Collision
2.4. Time Unit Base
2.5. Intermicellar Exchange Criteria of Reactants
2.6. Chemical Reduction Rates
2.7. Critical Nucleation Number and Intermicellar Exchange of Products
2.8. Intermicellar Exchange Criteria of Free Atoms/Molecules of Products
2.9. Autocatalysis
2.10. Intermicellar Exchange Criteria of Growing Particles
2.10.1. Surfactant Film Flexibility
2.10.2. Ripening
2.11. Droplet Size
3. Model
4. Results and Discussion
4.1. Simulation Results: Simple Particles
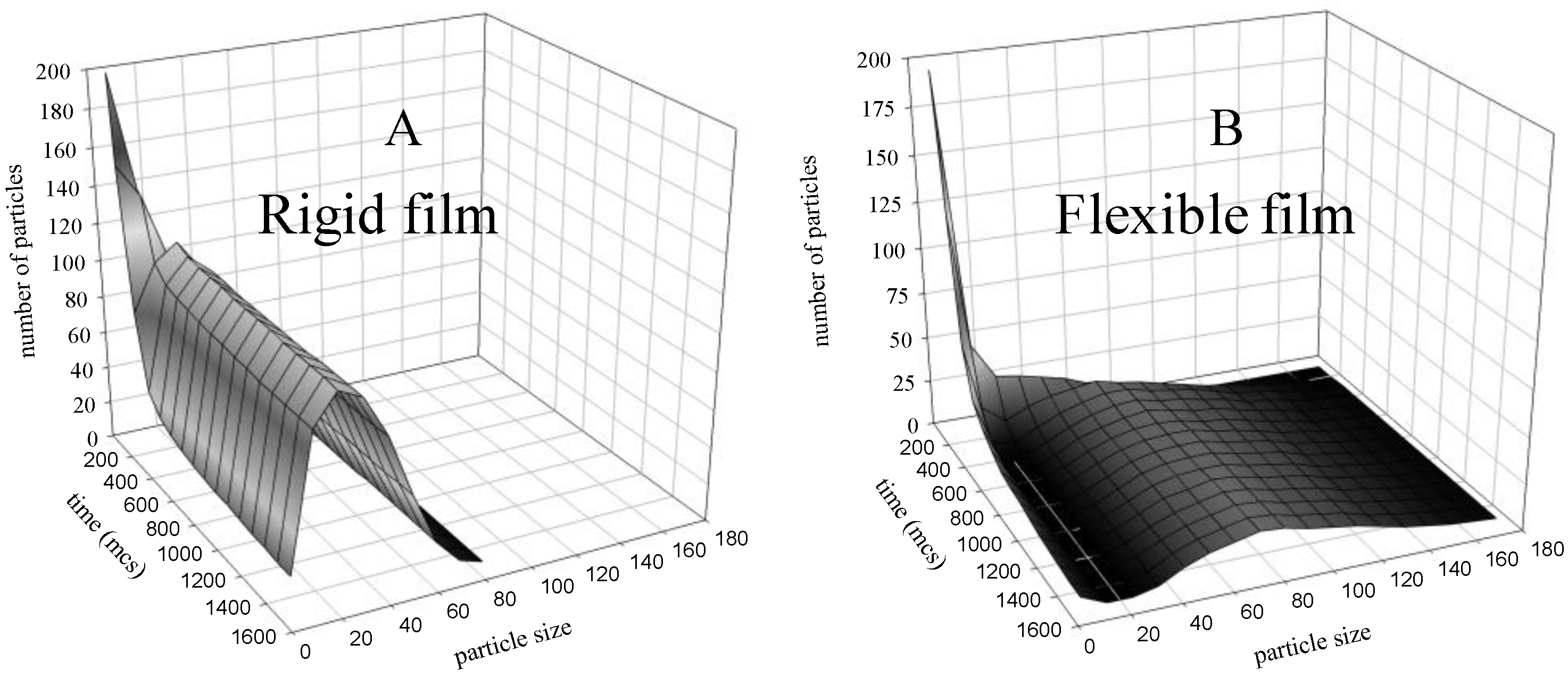
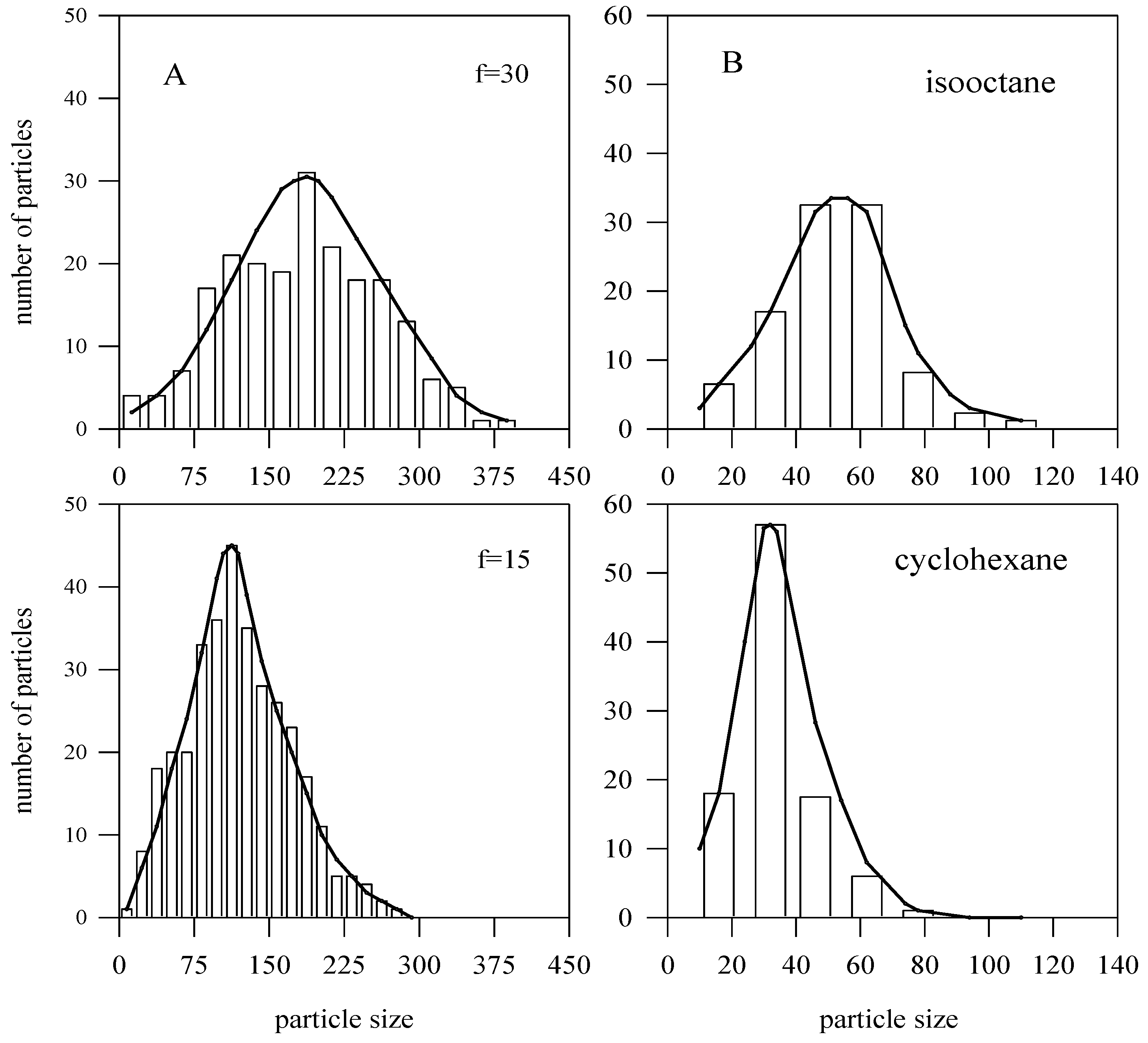
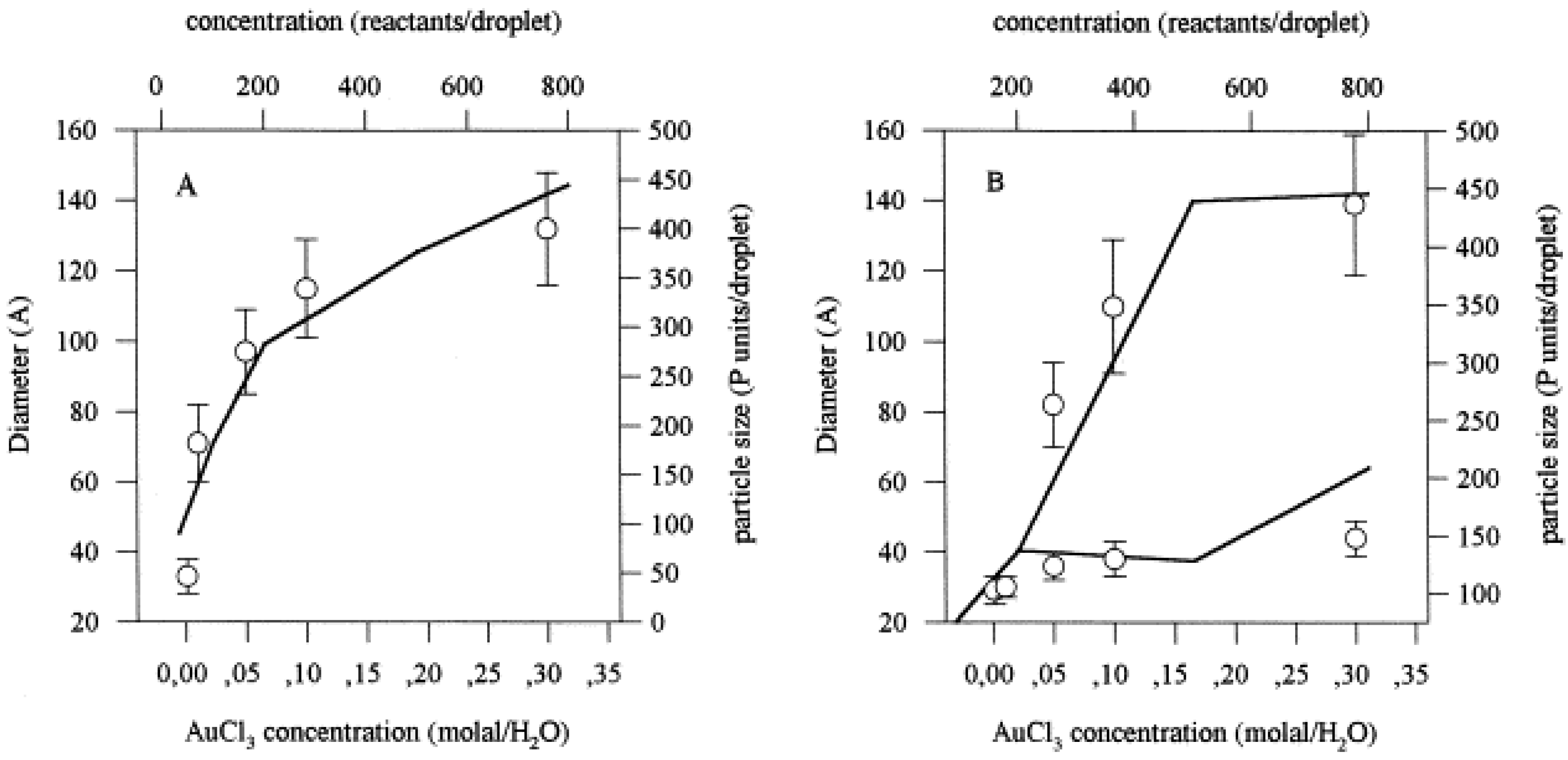
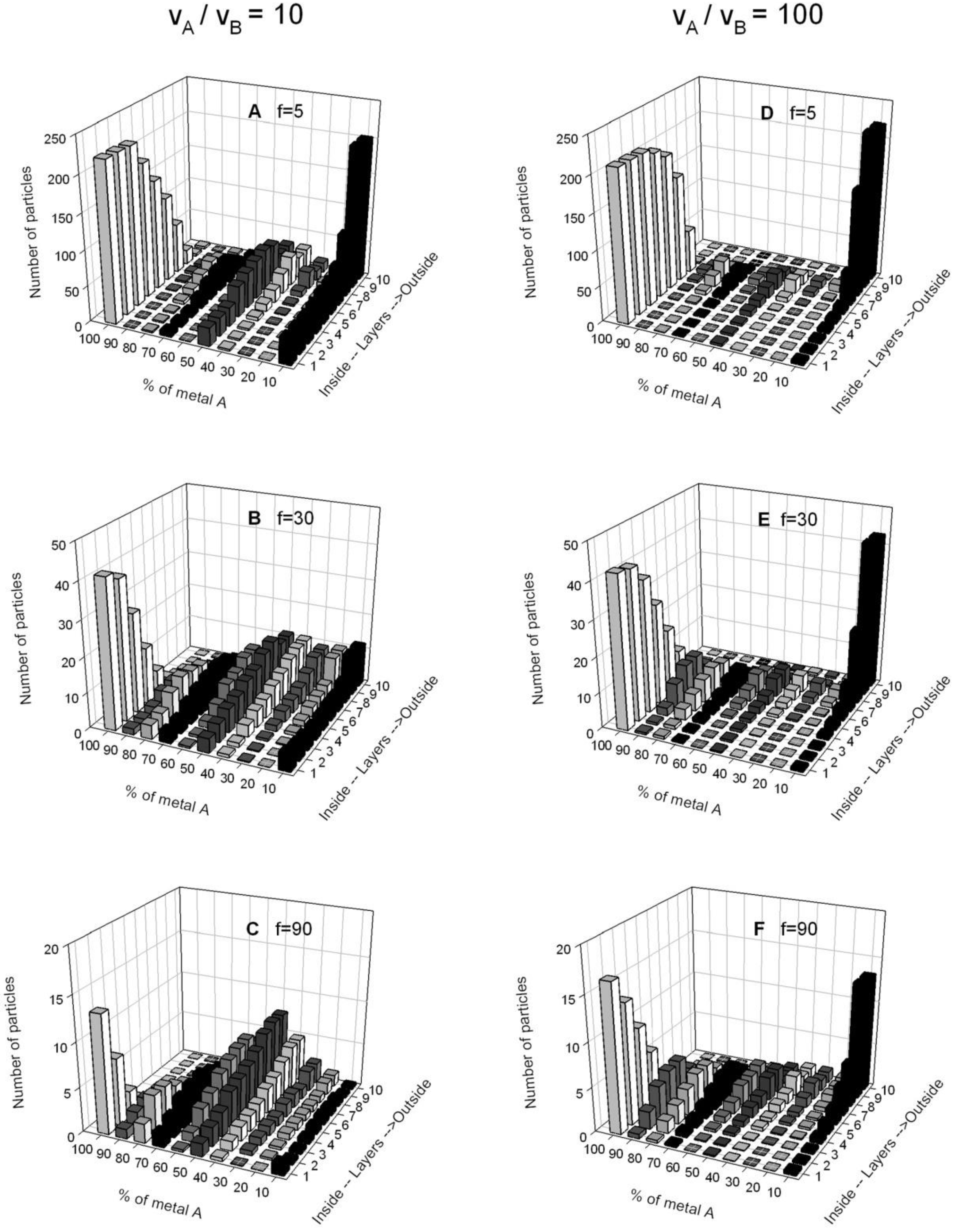
4.2. Simulation Results: Bimetallic Particles
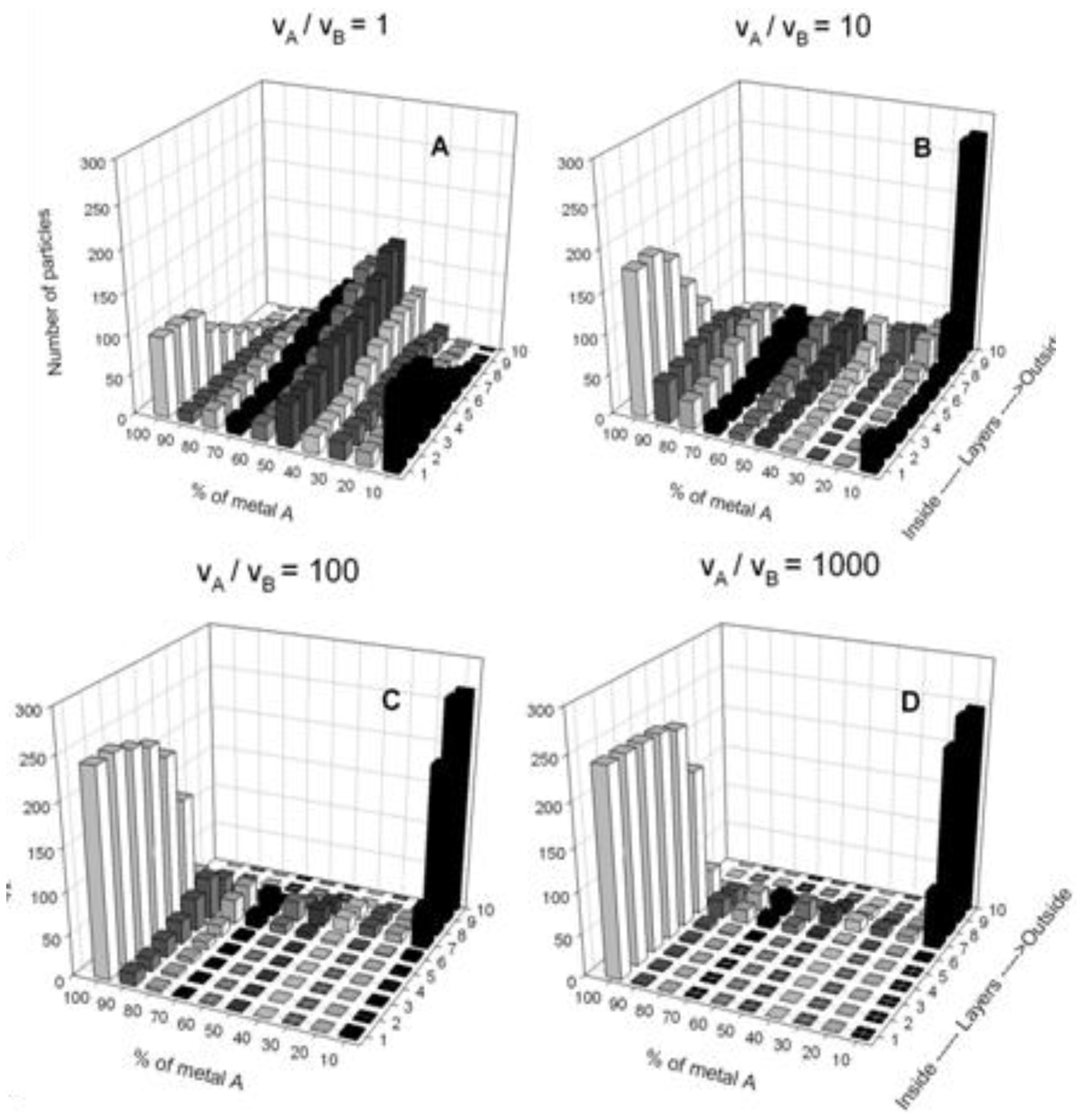
| Metals | Structure | Microemulsion reductor agent; metal precursor | f | Ref |
|---|---|---|---|---|
| Au-Ag | Au core-enriched in Ag shell | water/AOT/isooctane N2H5OH; Ag+, AuCl4- | rigid | [62] |
| nanoalloy | water/TritonX-100/cyclohexane NaBH4, Ag+, AuCl4- | flexible | [61] | |
| Au-Pt | core-shell | water/AOT/isooctane N2H5OH, AuCl4-, PtCl62- | rigid | [63] |
| nanoalloy | water/Tergitol 15-S-5/isooctane N2H5OH, AuCl4-, PtCl62- | flexible | [64] |
5. Conclusions
Acknowledgements
References and Notes
- López-Quintela, M.A. Synthesis of nanomaterials in microemulsions: Formation mechanisms and growth control. Curr. Opin. Colloid Interface Sci. 2003, 8, 137–144. [Google Scholar] [CrossRef]
- Nagabhushana, K.S.; Bönnemann, H. Wet chemical synthesis of nanoparticles. In Nanotechnology in Catalysis; Zhou, B., Hermans, S., Somorjai, G.A., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2004; Volume 1, pp. 51–82. [Google Scholar]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Toshima, N.; Yonezawa, T.; Kushihashi, K. Polymer-protected palladium-platinum bimetallic clusters: Preparation, catalytic properties and structural considerations. J. Chem. Soc. Faraday Trans. 1993, 89, 2537–2543. [Google Scholar] [CrossRef]
- Chung, Y.M.; Rhee, H.K. Dendrimer-templated Ag-Pd bimetallic nanoparticles. J. Colloid Interface Sci. 2004, 271, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Kim, Y.; Kim, K. Dodecanethiol-derivatized Au/Ag bimetallic nanoparticles: TEM, UV/VIS, XPS, and FTIR analysis. J. Colloid Interface Sci. 1998, 208, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Capek, I. Preparation of metal nanoparticles in water-in-oil (w/o) microemulsions. Adv. Colloid Interface Sci. 2004, 10, 49–74. [Google Scholar] [CrossRef]
- Yadav, O.P.; Palmqvist, A.; Cruise, N.; Holmberg, K. Synthesis of platinum nanoparticles in microemulsions and their catalytic activity for the oxidation of carbon monoxide. Colloids Surf. A 2003, 221, 131–134. [Google Scholar] [CrossRef]
- Sánchez-Dominguez, M.; Boutonnet, M.; Solans, C. A novel approach to metal and metal oxide nanoparticle synthesis: The oil-in-water microemulsion reaction method. J. Nanopart. Res. 2009, 11, 1823–1829. [Google Scholar] [CrossRef]
- Stubenrauch, C.; Wielpütz, T.; Sottmann, T.; Roychowdhury, C.; DiSalvo, F.J. Microemulsions as templates for the synthesis of metallic nanoparticles. Colloids Surf. A 2008, 317, 328–338. [Google Scholar] [CrossRef]
- Arriagada, F.J.; Osseo-Asare, K. Synthesis of Nanosize Silica in a Nonionic Water-in-Oil Microemulsion: Effects of the Water/Surfactant Molar Ratio and Ammonia Concentration. J. Colloid Interface Sci. 1999, 211, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Bumajdad, A.; Eastoe, J.; Zaki, M.I.; Heenan, R.K.; Pasupulety, L. Generation of metal oxide nanoparticles in optimised microemulsions. J. Colloid Interface Sci. 2007, 312, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, A.; Gulik, T.; Pileni, M.P. Synthesis of Nanosize Latexes by Reverse Micelle Polymerization. Langmuir 1995, 11, 3656–3659. [Google Scholar] [CrossRef]
- Summers, M.; Eastoe, J.; Davis, S. Formation of BaSO4 Nanoparticles in Microemulsions with Polymerized Surfactant Shells. Langmuir 2002, 18, 5023–5026. [Google Scholar] [CrossRef]
- Ethayaraja, M.; Bandyopadhyaya, R. Population Balance Models and Monte Carlo Simulation for Nanoparticle Formation in Water-in-Oil Microemulsions: Implications for CdS Synthesis. J. Am. Chem. Soc. 2006, 128, 17102–17113. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.; Kumar, P.; Hou, M.J.; Ayyub, P.; Shah, D.O. Preparation of nanoparticles of silver halides, superconductors and magnetic materials using water-in-oil microemulsions as nano-reactors. Adv. Colloid Interface Sci. 1995, 55, 241–269. [Google Scholar] [CrossRef]
- Viger, M.; Live, L.; Therrien, O.; Boudreau, D. Reduction of self-quenching in fluorescent silica-coated silver nanoparticles. Plasmonics 2008, 3, 33–40. [Google Scholar] [CrossRef]
- Jain, R.; Shukla, D.; Mehra, A. A Monte Carlo Model for the Formation of Core-Shell Nanocrystals in Reverse Micellar Systems. Ind. Eng. Chem. Res. 2006, 45, 2249–2254. [Google Scholar] [CrossRef]
- Shukla, D.; Mehra, A. Modeling Shell Formation in Core-Shell Nanocrystals in Reverse Micelle Systems. Langmuir 2006, 22, 9500–9506. [Google Scholar] [CrossRef] [PubMed]
- Wanjala, B.N.; Luo, J.; Loukrakpam, R.; Fang, B.; Mott, D.; Njoki, P.N.; Engelhard, M.; Naslund, H.R.; Wu, J.K.; Wang, L.; Malis, O.; Zhong, C.J. Nanoscale alloying, phase-segregation, and core-shell evolution of Gold-Platinum nanoparticles and their electrocatalytic effect on oxygen reduction reation. Chem. Mater. 2010, 22, 4282–4294. [Google Scholar] [CrossRef]
- Holmberg, K. Surfactant-templated nanomaterials synthesis. J. Colloid Interface Sci. 2004, 274, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Lisiecki, I.; Pileni, M.P. Synthesis of copper metallic clusters using reverse micelles as microreactors. J. Am. Chem. Soc. 1993, 115, 3887–3896. [Google Scholar] [CrossRef]
- Shah, P.S.; Holmes, J.D.; Johnston, K.P.; Korgel, B.A. Size-Selective Dispersion of Dodecanethiol-Coated Nanocrystals in Liquid and Supercritical Ethane by Density Tuning. J. Phys. Chem. B 2002, 106, 2545–2551. [Google Scholar] [CrossRef]
- Kitchens, C.L.; McLeod, M.C.; Roberts, C.B. Solvent Effects on the Growth and Steric Stabilization of Copper Metallic Nanoparticles in AOT Reverse Micelle Systems. J. Phys. Chem. B 2003, 107, 11331–11338. [Google Scholar] [CrossRef]
- Quintillán, S.; Tojo, C.; Blanco, M.C.; López-Quintela, M.A. Effects of the Intermicellar Exchange on the Size Control of Nanoparticles Synthesized in Microemulsions. Langmuir 2001, 17, 7251–7254. [Google Scholar] [CrossRef]
- Tojo, C.; Barroso, F.; de Dios, M. Critical nucleus size effects on nanoparticle formation in microemulsions: A comparison study between experimental and simulation results. J. Colloid Interface Sci. 2006, 296, 591–598. [Google Scholar] [CrossRef] [PubMed]
- de Dios, M. Síntesis de nanopartículas en microemulsiones. Estudio por simulación. Ph.D. Thesis, University of Vigo, Vigo, Spain, 2009. [Google Scholar]
- Curl, R.L. Dipersed phase mixing. Theory and effects in simple reactors. AIChE J. 1963, 9, 175–181. [Google Scholar] [CrossRef]
- Niemann, N.; Rauscher, F.; Adityawarman, D.; Voigt, A.; Sundmacher, K. Microemulsion-assisted precipitation of particles: Experimental and model-based process analysis. Chem. Eng. Process 2006, 45, 917–935. [Google Scholar] [CrossRef]
- Niemann, B.; Veit, P.; Sundmacher, K. Nanoparticle precipitation in reverse microemulsions: Particle formation dynamics and tailoring of particle size distributions. Langmuir 2008, 24, 4320–4328. [Google Scholar] [CrossRef] [PubMed]
- Sundmacher, K.; Niemann, B. Nanoparticle precipitation in microemulsions: Population balance model and identification of bivariate exchange kernel. J. Colloid Interface Sci. 2010, 342, 361–371. [Google Scholar] [CrossRef] [PubMed]
- La Mer, V.K.; Dinegar, R.H. Theory, production, and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Tojo, C.; Blanco, M.C.; López-Quintela, M.A. Preparation of Nanoparticles in Microemulsions: A Monte Carlo Study of the Influence of the Synthesis Variables. Langmuir 1997, 13, 4527–4534. [Google Scholar] [CrossRef]
- Hellweg, T. Phase structures of microemulsions. Curr. Opin.Colloid Interface Sci. 2002, 7, 50–56. [Google Scholar] [CrossRef]
- Bagwe, R.P.; Khilar, K.C. Effects of the Intermicellar Exchange Rate and Cations on the Size of Silver Chloride Nanoparticles formed in Reverse Micelles of AOT. Langmuir 1997, 13, 6432–6438. [Google Scholar] [CrossRef]
- Bagwe, R.P.; Khilar, K.C. Effects of Intermicellar Exchange Rate on the Formation of Silver Nanoparticles in Reverse Microemulsions of AOT. Langmuir 2000, 16, 905–910. [Google Scholar] [CrossRef]
- Cason, J.; Miller, M.E.; Thompson, J.B.; Roberts, C.B. Solvent effects on copper nanoparticle growth behavior in AOT reverse micelle systems. J. Phys. Chem. B 2001, 105, 2297–2302. [Google Scholar] [CrossRef]
- De Smet, Y.; Deriemaecker, L.; Finsy, R. A Simple Computer Simulation of Aging Processes. Langmuir 1997, 13, 6884–6888. [Google Scholar] [CrossRef]
- Taisne, L.; Cabane, B. Emulsification and Ripening following a Temperature Quench. Langmuir 1998, 14, 4744–4752. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, X.; Chen, J. Synthesis of nanosilver colloidal particles in water/oil microemulsion. Colloids Surf. A 2007, 299, 22–28. [Google Scholar] [CrossRef]
- Ethayaraja, M.; Ravikumar, C.; Muthukumaran, D.; Dutta, K.; Bandyopadhyaya, R. CdS-ZnS Core-Shell Nanoparticle Formation: Experiment, Mechanism, and Simulation. J. Phys. Chem. C 2007, 111, 3246–3252. [Google Scholar] [CrossRef]
- Henle, J.; Simon, P.; Frenzel, A.; Scholz, S.; Kaskel, S. Nanosized BiOX (X = Cl, Br, I) Particles Synthesized in Reverse Microemulsions. Chem. Mater. 2007, 19, 366–373. [Google Scholar] [CrossRef]
- Kimijima, K.; Sugimoto, T. Effects of the water content on the growth rate of AgCl nanoparticles in a reversed micelle system. J. Colloid Interface Sci. 2005, 286, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Monnoyer, Ph.; Fonseca, A.; Nagy, J.B. Preparation of colloidal AgBr particles from microemulsions. Colloids Surf. A 1995, 100, 233–243. [Google Scholar] [CrossRef]
- Koutzarova, T.; Kolev, S.; Ghelev, Ch.; Paneva, D.; Nedkov, I. Microstructural study and size control of iron oxide nanoparticles produced by microemulsion technique. Phys. Status Solidi C 2006, 3, 1302–1307. [Google Scholar] [CrossRef]
- Destrée, C.; Ghijsen, J.; Nagy, J.B. Preparation of Organic Nanoparticles Using Microemulsions: Their Potential Use in Transdermal Delivery. Langmuir 2007, 23, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Tojo, C.; Blanco, M.C.; López-Quintela, M.A. Microemulsions as microreactors: A Monte Carlo simulation on the synthesis of particles. J. Non-Cryst. Solids 1998, 235-237, 688–691. [Google Scholar] [CrossRef]
- López-Quintela, M.A.; Tojo, C.; Blanco, M.C.; García Río, L.; Leis, J.R. Microemulsion dynamics and reactions in microemulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 264–279. [Google Scholar] [CrossRef]
- Curri, M.L.; Agostiano, A.; Manna, L.; Della Monica, M.; Catalano, M.; Chiavarone, L.; Spagnolo, V.; Lugarà, M. Synthesis and Characterization of CdS Nanoclusters in a Quaternary Microemulsion: The Role of the Cosurfactant. J. Phys. Chem. B 2000, 104, 8391–8397. [Google Scholar] [CrossRef]
- Andelman, D.; Cates, M.E.; Roux, D.; Safran, S.A. Structure and phase equilibrium of microemulsions. J. Chem. Phys. 1987, 87, 7229–7241. [Google Scholar] [CrossRef]
- Cates, M.E.; Andelman, D.; Safran, S.A.; Roux, D. Theory of microemulsions: Comparison with experimental behaviour. Langmuir 1988, 4, 802–806. [Google Scholar] [CrossRef]
- Szleifer, I.; Kramer, D.; Ben-Shaul, A.; Roux, D.; Gelbart, W.M. Curvature elasticity of pure and mixed surfactant films. Phys. Rev. Lett. 1988, 60, 1966–1969. [Google Scholar] [CrossRef]
- Jain, T.K.; Cassin, G.; Badiali, J.P.; Pileni, M.P. Relation between Exchange Process and Structure of AOT Reverse Micellar System. Langmuir 1996, 12, 2408–2411. [Google Scholar] [CrossRef]
- López-Quintela, M.A.; Rivas, J.; Blanco, M.C.; Tojo, C. Synthesis of nanoparticles in microemulsions. In Nanoscale Materials; Liz Marzán, L., Kamat, P.V., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2003; pp. 135–155. [Google Scholar]
- Petit, C.; Lixon, P.; Pileni, M.P. In situ synthesis of silver nanocluster in AOT reverse micelles. J. Phys. Chem. B 1993, 97, 12974–12983. [Google Scholar] [CrossRef]
- Towey, T.F.; Khan-Lodhi, A.; Robinson, B.H. Kinetics and mechanism of formation of quantum-sized cadmium sulfide particles in water-Aerosol OT-oil microemulsions. J. Chem. Soc. Faraday Trans. 1990, 86, 3757–3762. [Google Scholar] [CrossRef]
- Fletcher, D.I.; Howe, A.M.; Robinson, B.H. The kinetics of solubilisate exchange between water droplets of a water-in-oil microemulsion. J. Chem. Soc. Faraday Trans. 1987, 83, 985–1006. [Google Scholar] [CrossRef]
- Tojo, C.; Rivadulla, F.; Blanco, M.C.; López-Quintela, M.A. Kinetics of the Formation of Particles in Microemulsions. Langmuir 1997, 13, 1970–1977. [Google Scholar] [CrossRef]
- Nagy, J.B.; Barette, D.; Fonseca, A.; Jenieau, L.; Monnoyer, P.H.; Piedigrosso, P.; Ravet-Bodart, I.; Verfaillie, J.-P.; Wathelet, A. Nanoparticles in Microemulsions: A General Approach. In Nanoparticles in Solids and Solutions; Fendler, J.H., Dékány, I., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 71–129. [Google Scholar]
- de Dios, M.; Barroso, F.; Tojo, C.; Lopez-Quintela, M.A. Simulation of the kinetics of nanoparticle formation in microemulsions. J. Colloid Interface Sci. 2009, 333, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Shah, S.; Devi, S. Preparation of silver, gold and silver-gold bimetallic nanoparticles in w/o microemulsion containing TritonX-100. Colloids Surf. A 2007, 302, 483–487. [Google Scholar] [CrossRef]
- Chen, D.; Chen, C. Formation and characterization of Au-Ag bimetallic nanoparticles in water-in-oil microemulsions. J. Mater. Chem. 2002, 12, 1557–1562. [Google Scholar] [CrossRef]
- Hernández-Fernández, P.; Rojas, S.; Ocón, P.; Gómez de la Fuente, J.L.; San Fabián, J.; Sanza, J.; Peña, M.A.; García-García, F.J.; Terreros, P.; Fierro, J.L.G. Influence of the Preparation Route of Bimetallic Pt-Au Nanoparticle Electrocatalysts for the Oxygen Reduction Reaction. J. Phys. Chem. C 2007, 111, 2913–2923. [Google Scholar] [CrossRef]
- Wu, M.; Chen, D.; Huang, T. Preparation of Au/Pt bimetallic nanoparticles in water-in-oil microemulsions. Chem. Mater. 2001, 13, 599–606. [Google Scholar] [CrossRef]
- Tojo, C.; de Dios, M.; López-Quintela, M.A. On the structure of bimetallic nanoparticles synthesized in microemulsions. J. Phys. Chem. C 2009, 113, 19145–19154. [Google Scholar] [CrossRef]
© 2010 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tojo, C.; Dios, M.d.; Barroso, F. Surfactant Effects on Microemulsion-Based Nanoparticle Synthesis. Materials 2011, 4, 55-72. https://doi.org/10.3390/ma4010055
Tojo C, Dios Md, Barroso F. Surfactant Effects on Microemulsion-Based Nanoparticle Synthesis. Materials. 2011; 4(1):55-72. https://doi.org/10.3390/ma4010055
Chicago/Turabian StyleTojo, Concha, Miguel de Dios, and Fernando Barroso. 2011. "Surfactant Effects on Microemulsion-Based Nanoparticle Synthesis" Materials 4, no. 1: 55-72. https://doi.org/10.3390/ma4010055






