Advances in High-Field BOLD fMRI
Abstract
:1. Introduction
2. The BOLD Effect

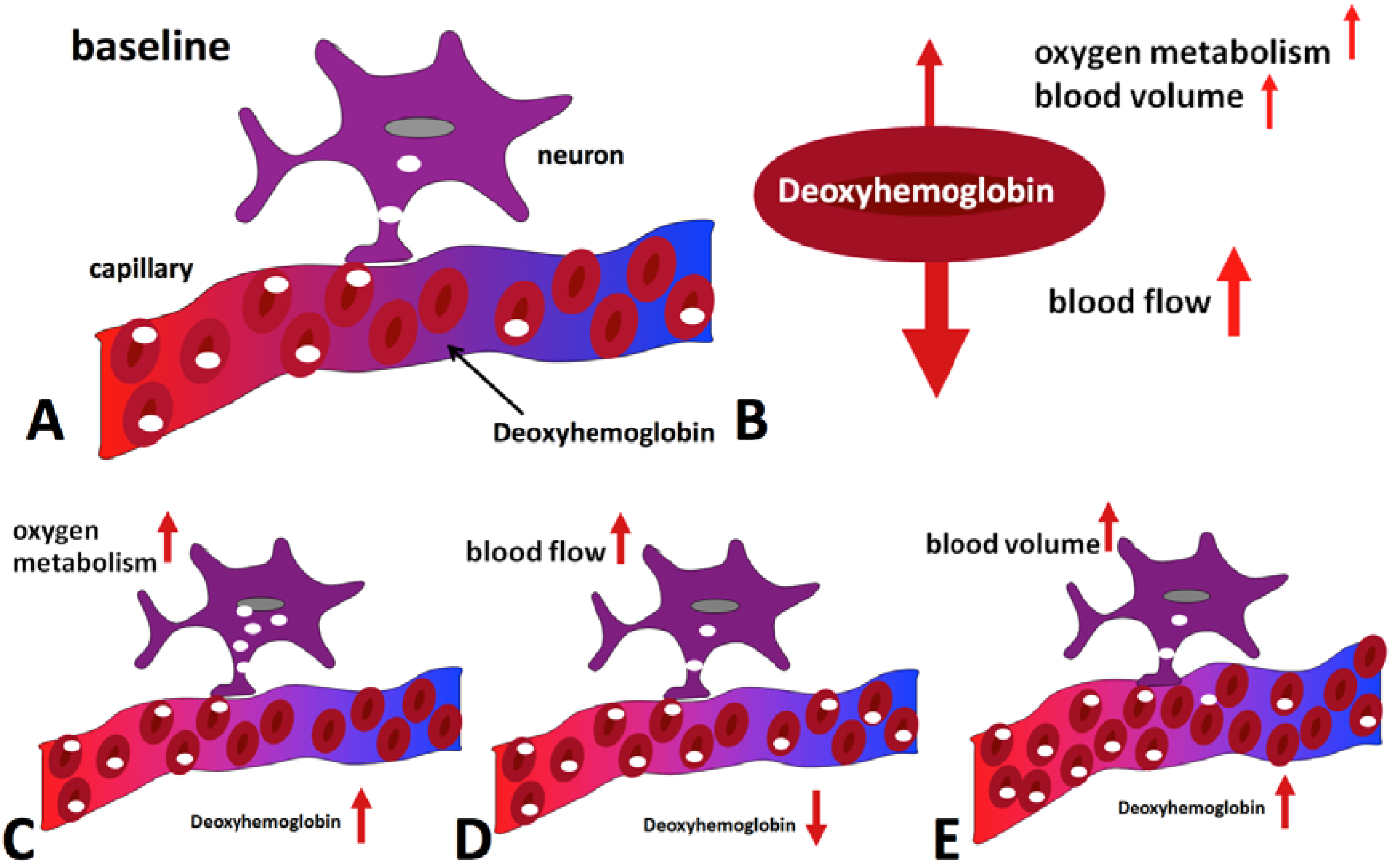
3. Spatial and Temporal Resolution and BOLD Sensitivity
4. Estimation of the Dependence of BOLD Sensitivity on Field Strength
5. Localization and Spatial Specificity
6. Technical Challenges
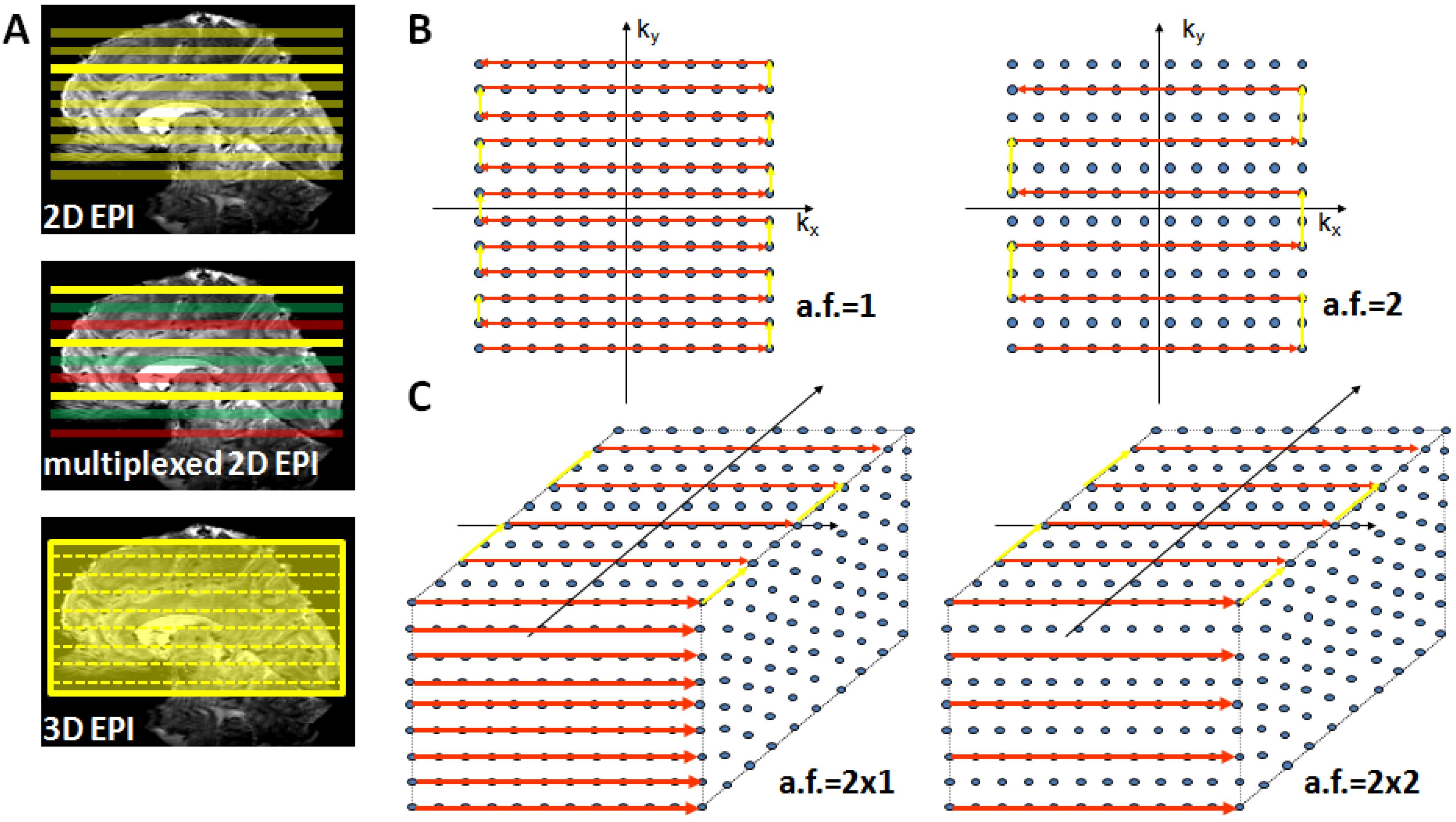
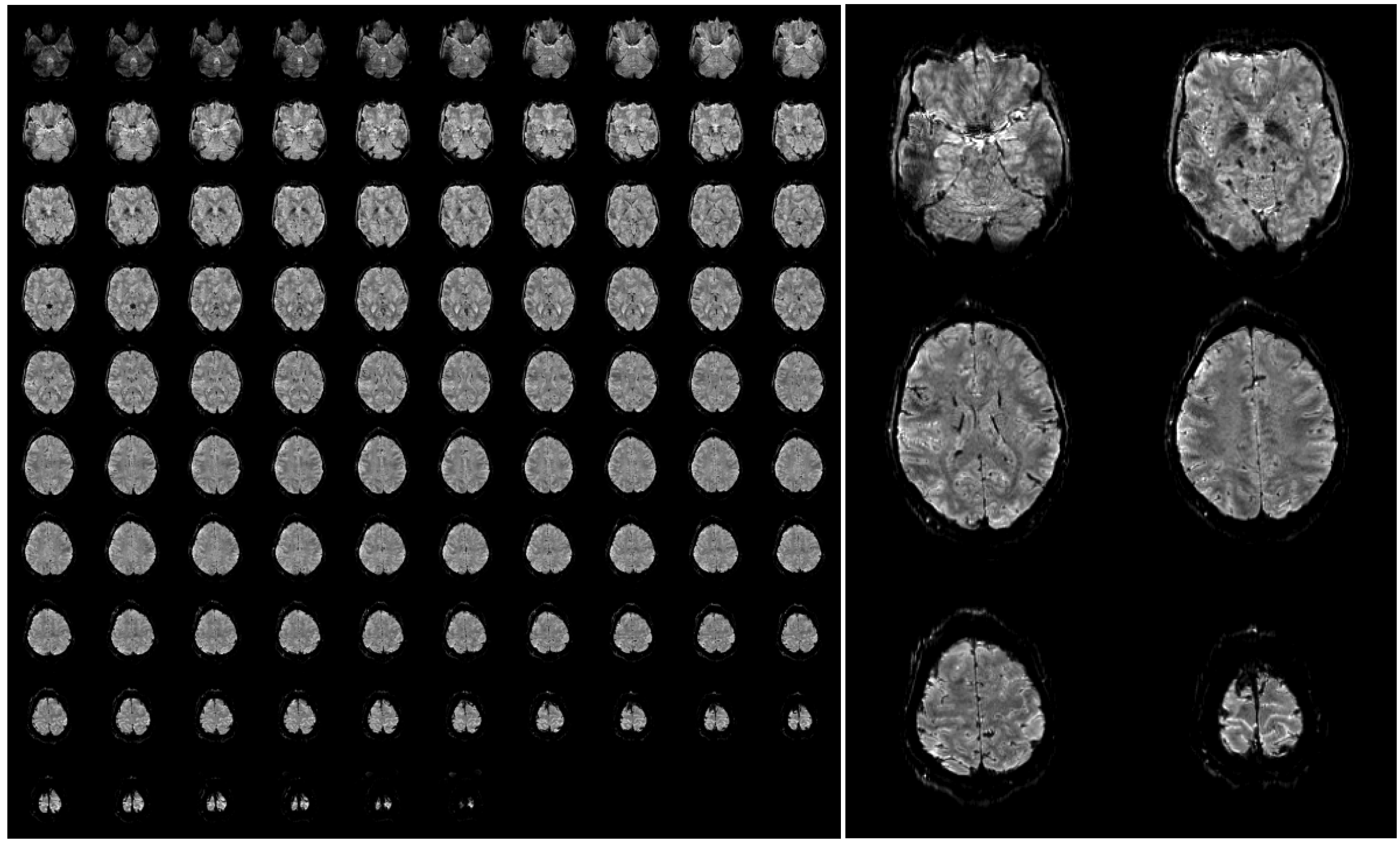
7. Summary and Outlook
References
- Hu, X.; Norris, D.G. Advances in high-field magnetic resonance imaging. Annu. Rev. Biomed. Eng. 2004, 6, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.G. High field human imaging. J. Magn. Reson. Imaging 2003, 18, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Gati, J.S.; Menon, R.S.; Ugurbil, K.; Rutt, B.K. Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn. Reson. Med. 1997, 38, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.M. Magnetic resonance imaging of blood vessels at high fields: In vivo and in vitro measurements and image simulation. Magn. Reson. Med. 1990, 16, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef] [PubMed]
- Bandettini, P.A.; Wong, E.C.; Hinks, R.S.; Tikofsky, R.S.; Hyde, J.S. Time course EPI of human brain function during task activation. Magn. Reson. Med. 1992, 25, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Kwong, K.K.; Belliveau, J.W.; Chesler, D.A.; Goldberg, I.E.; Weisskoff, R.M.; Poncelet, B.P.; Kennedy, D.N.; Hoppel, B.E.; Cohen, M.S.; Turner, R.; et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl. Acad. Sci. USA 1992, 89, 5675–5679. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, P. Real-time echo-planar imaging by NMR. Br. Med. Bull. 1984, 40, 187–190. [Google Scholar] [PubMed]
- Norris, D.G. Principles of magnetic resonance assessment of brain function. J. Magn. Reson. Imaging 2006, 23, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Worsley, K.J. FMRISTAT—A General Statistical Analysis for fMRI Data; McGill University: Montreal, QC, Canada, 2006; Available online: http://www.math.mcgill.ca/keith/fmristat/ (accessed on 2 November 2011).
- Glover, G.H. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage 1999, 9, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Detre, J.A.; Leigh, J.S.; Williams, D.S.; Koretsky, A.P. Perfusion imaging. Magn. Reson. Med. 1992, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.S.; Detre, J.A.; Leigh, J.S.; Koretsky, A.P. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc. Natl. Acad. Sci. USA 1992, 89, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Golay, X.; Pekar, J.J.; van Zijl, P.C. Functional magnetic resonance Imaging based on changes in vascular space occupancy. Magn. Reson. Med. 2003, 50, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, R.; Windischberger, C.; Deecke, L.; Moser, E. The preparation and readiness for voluntary movement: A high-field event-related fMRI study of the Bereitschafts-BOLD response. Neuroimage 2003, 20, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.H.; Witzel, T.; Chang, W.T.; Wen-Kai, T.K.; Wang, Y.H.; Kuo, W.J.; Belliveau, J.W. K-space reconstruction of magnetic resonance inverse imaging (K-InI) of human visuomotor systems. Neuroimage 2010, 49, 3086–3098. [Google Scholar] [CrossRef]
- Glover, G.H.; Li, T.Q.; Ress, D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 2000, 44, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Constable, R.T.; Spencer, D.D. Repetition time in echo planar functional MRI. Magn. Reson. Med. 2001, 46, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Deichmann, R.; Josephs, O.; Hutton, C.; Corfield, D.R.; Turner, R. Compensation of susceptibility-induced BOLD sensitivity losses in echo-planar fMRI imaging. Neuroimage 2002, 15, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, C.; Hoge, R.D.; Krueger, G.; Wiggins, C.J.; Potthast, A.; Wiggins, G.C.; Wald, L.L. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. Neuroimage 2005, 26, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Kruger, G.; Glover, G.H. Physiological noise in oxygenation-sensitive magnetic resonance imaging. Magn. Reson. Med. 2001, 46, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, C.; Hoge, R.D.; Wald, L.L. Effect of spatial smoothing on physiological noise in high-resolution fMRI. Neuroimage 2006, 32, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Menon, R.S.; Tank, D.W.; Kim, S.G.; Merkle, H.; Ellermann, J.M.; Ugurbil, K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys. J. 1993, 64, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Uludag, K.; Muller-Bierl, B.; Ugurbil, K. An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage 2009, 48, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, E.; Shmuel, A.; Pfeuffer, J.; van de Moortele, P.F.; Adriany, G.; Andersen, P.; Vaughan, J.T.; Merkle, H.; Ugurbil, K.; Hu, X. Imaging brain function in humans at 7 Tesla. Magn. Reson. Med. 2001, 45, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, E.; Duong, T.Q.; Van De Moortele, P.F.; Lindquist, M.; Adriany, G.; Kim, S.G.; Ugurbil, K.; Hu, X. Spin-echo fMRI in humans using high spatial resolutions and high magnetic fields. Magn. Reson. Med. 2003, 49, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwaag, W.; Francis, S.; Head, K.; Peters, A.; Gowland, P.; Morris, P.; Bowtell, R. fMRI at 1.5, 3 and 7 T: Characterising BOLD signal changes. Neuroimage 2009, 47, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Beisteiner, R.; Robinson, S.; Wurnig, M.; Hilbert, M.; Merksa, K.; Rath, J.; Hollinger, I.; Klinger, N.; Marosi, C.; Trattnig, S.; Geissler, A. Clinical fMRI: Evidence for a 7T benefit over 3T. Neuroimage 2011, 57, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.; Norris, D.G. Very high-resolution three-dimensional functional MRI of the human visual cortex with elimination of large venous vessels. NMR Biomed. 2007, 20, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.; Reichenbach, J.R.; Venkatesan, R.; Moser, E.; Haacke, E.M. High-resolution, multiple gradient-echo functional MRI at 1.5 T. Magn. Reson. Imaging 1999, 17, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Hopkins, A.L.; Haacke, E.M.; Li, D.; Wasserman, B.A.; Buckley, P.; Friedman, L.; Meltzer, H.; Hedera, P.; Friedland, R. Identification of vascular structures as a major source of signal contrast in high resolution 2D and 3D functional activation imaging of the motor cortex at 1.5T: Preliminary results. Magn. Reson. Med. 1993, 30, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Frahm, J.; Merboldt, K.D.; Hanicke, W.; Kleinschmidt, A.; Boecker, H. Brain or vein—Oxygenation or flow? On signal physiology in functional MRI of human brain activation. NMR Biomed. 1994, 7, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Segebarth, C.; Belle, V.; Delon, C.; Massarelli, R.; Decety, J.; Le Bas, J.F.; Decorps, M.; Benabid, A.L. Functional MRI of the human brain: Predominance of signals from extracerebral veins. Neuroreport 1994, 5, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Hoogenraad, F.G.; Hofman, M.B.; Pouwels, P.J.; Reichenbach, J.R.; Rombouts, S.A.; Haacke, E.M. Sub-millimeter fMRI at 1.5 Tesla: Correlation of high resolution with low resolution measurements. J. Magn. Reson. Imaging 1999, 9, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Hoogenraad, F.G.; Pouwels, P.J.; Hofman, M.B.; Reichenbach, J.R.; Sprenger, M.; Haacke, E.M. Quantitative differentiation between BOLD models in fMRI. Magn. Reson. Med. 2001, 45, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Oja, J.M.; Gillen, J.; Kauppinen, R.A.; Kraut, M.; van Zijl, P.C. Venous blood effects in spin-echo fMRI of human brain. Magn. Reson. Med. 1999, 42, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Duvernoy, H.M.; Delon, S.; Vannson, J.L. Cortical blood vessels of the human brain. Brain Res. Bull. 1981, 7, 519–579. [Google Scholar] [CrossRef] [PubMed]
- Turner, R. How much cortex can a vein drain? Downstream dilution of activation-related cerebral blood oxygenation changes. Neuroimage 2002, 16, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, F.; Cassot, F.; Lauwers-Cances, V.; Puwanarajah, P.; Duvernoy, H. Morphometry of the human cerebral cortex microcirculation: General characteristics and space-related profiles. Neuroimage 2008, 39, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Boxerman, J.L.; Bandettini, P.A.; Kwong, K.K.; Baker, J.R.; Davis, T.L.; Rosen, B.R.; Weisskoff, R.M. The intravascular contribution to fMRI signal change: Monte Carlo modeling and diffusion-weighted studies in vivo. Magn. Reson. Med. 1995, 34, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, P.J.; Barth, M.; Norris, D.G. Layer-specific BOLD activation in human V1. Hum. Brain Mapp. 2010, 31, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, P.J.; Barth, M.; Orzada, S.; Norris, D.G. Multi-echo fMRI of the cortical laminae in humans at 7 T. Neuroimage 2010, 56, 1276–1285. [Google Scholar] [CrossRef]
- Polimeni, J.R.; Fischl, B.; Greve, D.N.; Wald, L.L. Laminar analysis of 7T BOLD using an imposed spatial activation pattern in human V1. Neuroimage 2010, 52, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.G.; Zysset, S.; Mildner, T.; Wiggins, C.J. An investigation of the value of spin-echo-based fMRI using a Stroop color-word matching task and EPI at 3 T. Neuroimage 2002, 15, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Parkes, L.M.; Schwarzbach, J.V.; Bouts, A.A.; Deckers, R.H.; Pullens, P.; Kerskens, C.M.; Norris, D.G. Quantifying the spatial resolution of the gradient echo and spin echo BOLD response at 3 Tesla. Magn. Reson. Med. 2005, 54, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, E.; Shmuel, A.; Logothetis, N.; Ugurbil, K. Robust detection of ocular dominance columns in humans using Hahn Spin Echo BOLD functional MRI at 7 Tesla. Neuroimage 2007, 37, 1161–1177. [Google Scholar] [CrossRef] [PubMed]
- Gizewski, E.R.; de Greiff, A.; Maderwald, S.; Timmann, D.; Forsting, M.; Ladd, M.E. fMRI at 7 T: Whole-brain coverage and signal advantages even infratentorially? Neuroimage 2007, 37, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Gruetter, R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn. Reson. Med. 1993, 29, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Pruessmann, K.P.; Weiger, M.; Scheidegger, M.B.; Boesiger, P. SENSE: Sensitivity encoding for fast MRI. Magn. Reson. Med. 1999, 42, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.A.; Jakob, P.M.; Heidemann, R.M.; Nittka, M.; Jellus, V.; Wang, J.; Kiefer, B.; Haase, A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn. Reson. Med. 2002, 47, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- De Zwart, J.A.; van Gelderen, P.; Kellman, P.; Duyn, J.H. Application of sensitivity-encoded echo-planar imaging for blood oxygen level-dependent functional brain imaging. Magn. Reson. Med. 2002, 48, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Preibisch, C.; Pilatus, U.; Bunke, J.; Hoogenraad, F.; Zanella, F.; Lanfermann, H. Functional MRI using sensitivity-encoded echo planar imaging (SENSE-EPI). Neuroimage 2003, 19, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Speck, O.; Stadler, J.; Zaitsev, M. High resolution single-shot EPI at 7.T. Magma (New York, NY) 2008, 21, 73–86. [Google Scholar]
- Poser, B.A.; Norris, D.G. Investigating the benefits of multi-echo EPI for fMRI at 7 T. Neuroimage 2009, 45, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.T.; Garwood, M.; Collins, C.M.; Liu, W.; DelaBarre, L.; Adriany, G.; Andersen, P.; Merkle, H.; Goebel, R.; Smith, M.B.; Ugurbil, K. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn. Reson. Med. 2001, 46, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Saekho, S.; Yip, C.Y.; Noll, D.C.; Boada, F.E.; Stenger, V.A. Fast-kz three-dimensional tailored radiofrequency pulse for reduced B1 inhomogeneity. Magn. Reson. Med. 2006, 55, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yip, C.Y.; Grissom, W.; Noll, D.C.; Boada, F.E.; Stenger, V.A. Reduction of transmitter B1 inhomogeneity with transmit SENSE slice-select pulses. Magn. Reson. Med. 2007, 57, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Zelinski, A.C.; Wald, L.L.; Setsompop, K.; Alagappan, V.; Gagoski, B.A.; Goyal, V.K.; Adalsteinsson, E. Fast slice-selective radio-frequency excitation pulses for mitigating B + 1 inhomogeneity in the human brain at 7 Tesla. Magn. Reson. Med. 2008, 59, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Orzada, S.; Maderwald, S.; Poser, B.A.; Bitz, A.K.; Quick, H.H.; Ladd, M.E. RF excitation using time interleaved acquisition of modes (TIAMO) to address B1 inhomogeneity in high-field MRI. Magn. Reson. Med. 2010, 64, 327–333. [Google Scholar] [PubMed]
- Feinberg, D.A.; Reese, T.G.; Wedeen, V.J. Simultaneous echo refocusing in EPI. Magn. Reson. Med. 2002, 48, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.; Yacoub, E.; Olman, C.A.; Auerbach, E.; Strupp, J.; Harel, N.; Ugurbil, K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 2010, 63, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Larkman, D.J.; Hajnal, J.V.; Herlihy, A.H.; Coutts, G.A.; Young, I.R.; Ehnholm, G. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J. Magn. Reson. Imaging 2001, 13, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, D.A.; Moeller, S.; Smith, S.M.; Auerbach, E.; Ramanna, S.; Glasser, M.F.; Miller, K.L.; Ugurbil, K.; Yacoub, E. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PloS One 2011, 5, e15710:1–e15710:11. [Google Scholar]
- Setsompop, K.; Gagoski, B.A.; Polimeni, J.R.; Witzel, T.; Wedeen, V.J.; Wald, L.L. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planer imaging with reduced g-factor penalty. Magn. Reson. Med. 2011. [Google Scholar] [CrossRef]
- Norris, D.G.; Koopmans, P. J.; Boyacioğlu, R.; Barth, M. Power independent of number of slices radiofrequency pulses for low-power simultaneous multislice excitation. Magn. Reson. Med. 2011, 66, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Poser, B.A.; Koopmans, P.J.; Witzel, T.; Wald, L.L.; Barth, M. Three dimensional echo-planar imaging at 7 Tesla. Neuroimage 2010, 51, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwaag, W.; Marques, J.P.; Kober, T.; Glover, G.; Gruetter, R.; Krueger, G. Temporal SNR characteristics in segmented 3D-EPI at 7T. Magn. Reson. Med. 2011. [Google Scholar] [CrossRef]
- Barry, R.L.; Strother, S.C.; Gore, J.C. Complex and magnitude-only preprocessing of 2D and 3D BOLD fMRI data at 7 T. Magn. Reson. Med. 2011. [Google Scholar] [CrossRef]
- Kristoffersen, A.; Goa, P.E. Cardiac-induced physiological noise in 3D gradient echo brain imaging: Effect of k-space sampling scheme. J. Magn. Reson. 2011, 212, 74–85. [Google Scholar] [PubMed]
- Hutton, C.; Josephs, O.; Stadler, J.; Featherstone, E.; Reid, A.; Speck, O.; Bernarding, J.; Weiskopf, N. The impact of physiological noise correction on fMRI at 7 T. Neuroimage 2011, 57, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Tijssen, R.H.; Okell, T.W.; Miller, K.L. Real-time cardiac synchronization with fixed volume frame rate for reducing physiological instabilities in 3D FMRI. Neuroimage 2011, 57, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- FMRIB Software Library—A General Statistical Analysis for fMRI Data; FMRIB Centre University of Oxford: Oxford, UK, 2008; Available online: http://www.fmrib.ox.ac.uk/fsl/ (accessed on 2 November 2011).
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barth, M.; Poser, B.A. Advances in High-Field BOLD fMRI. Materials 2011, 4, 1941-1955. https://doi.org/10.3390/ma4111941
Barth M, Poser BA. Advances in High-Field BOLD fMRI. Materials. 2011; 4(11):1941-1955. https://doi.org/10.3390/ma4111941
Chicago/Turabian StyleBarth, Markus, and Benedikt A. Poser. 2011. "Advances in High-Field BOLD fMRI" Materials 4, no. 11: 1941-1955. https://doi.org/10.3390/ma4111941



