Dense CO2 as a Solute, Co-Solute or Co-Solvent in Particle Formation Processes: A Review
Abstract
:1. Dense CO2 in Particle Formation Processes
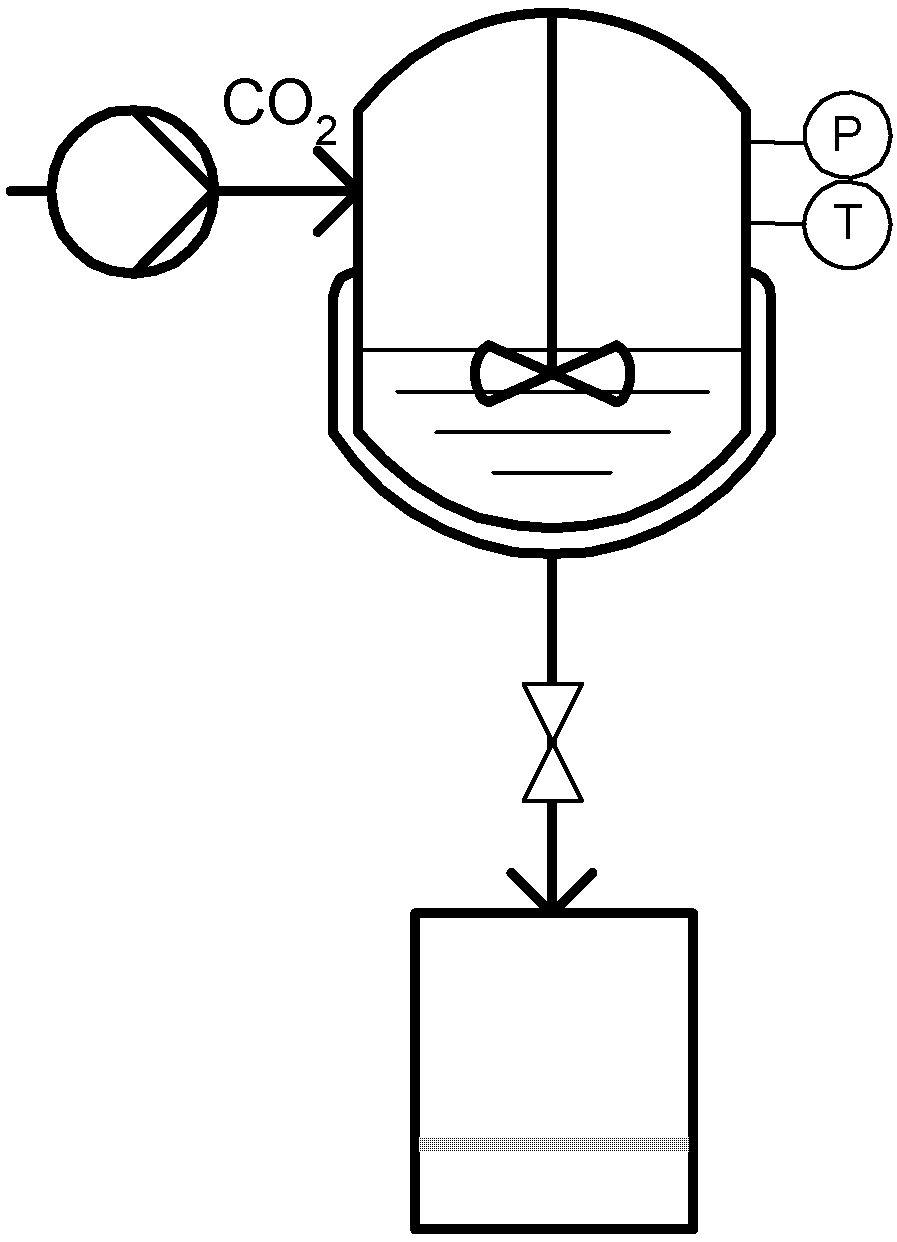
2. The Particles from Gas Saturated Solution (PGSS) Technique
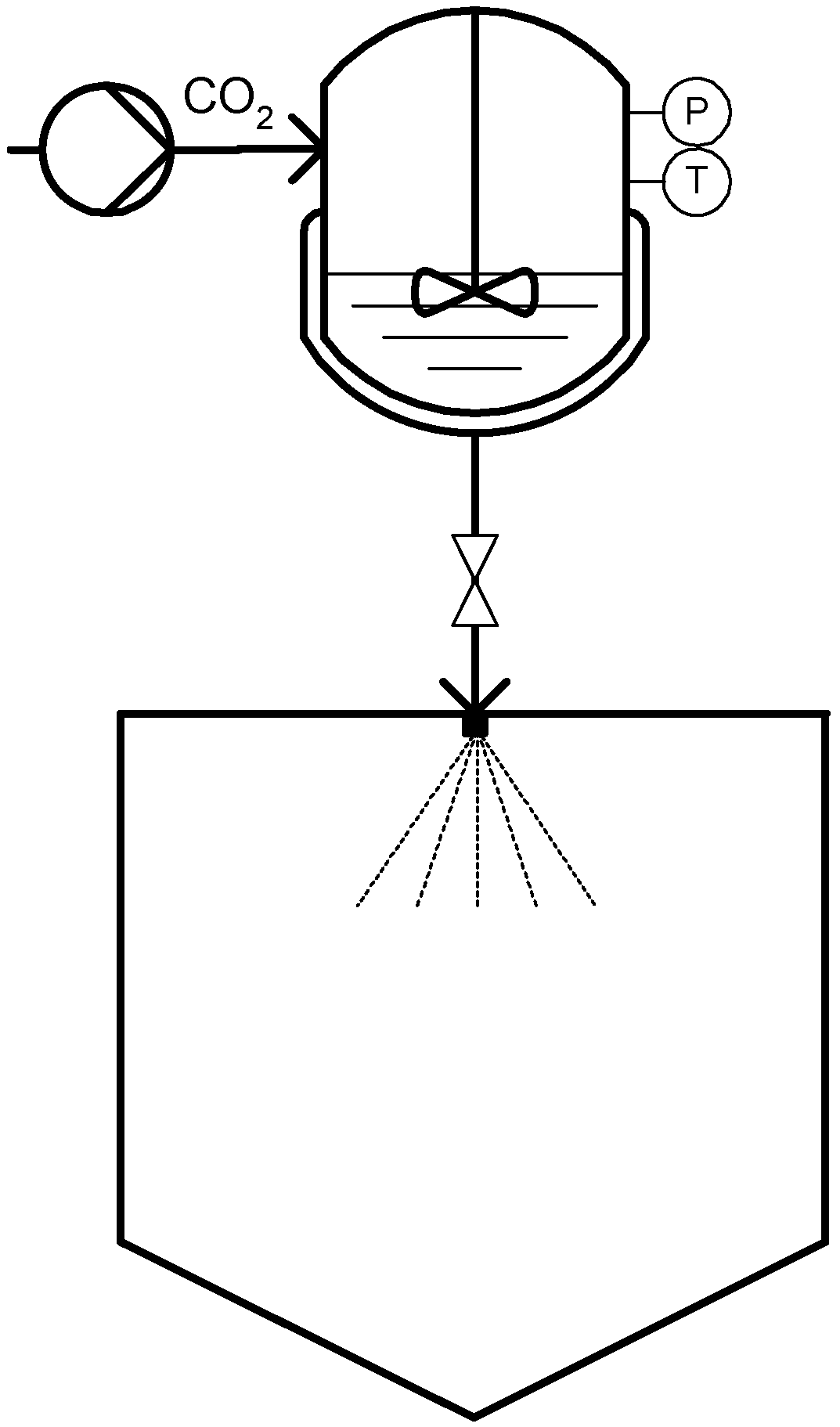

3. Role of Dense CO2 in PGSS Related Techniques
3.1. Solute: CPF and CPCSP

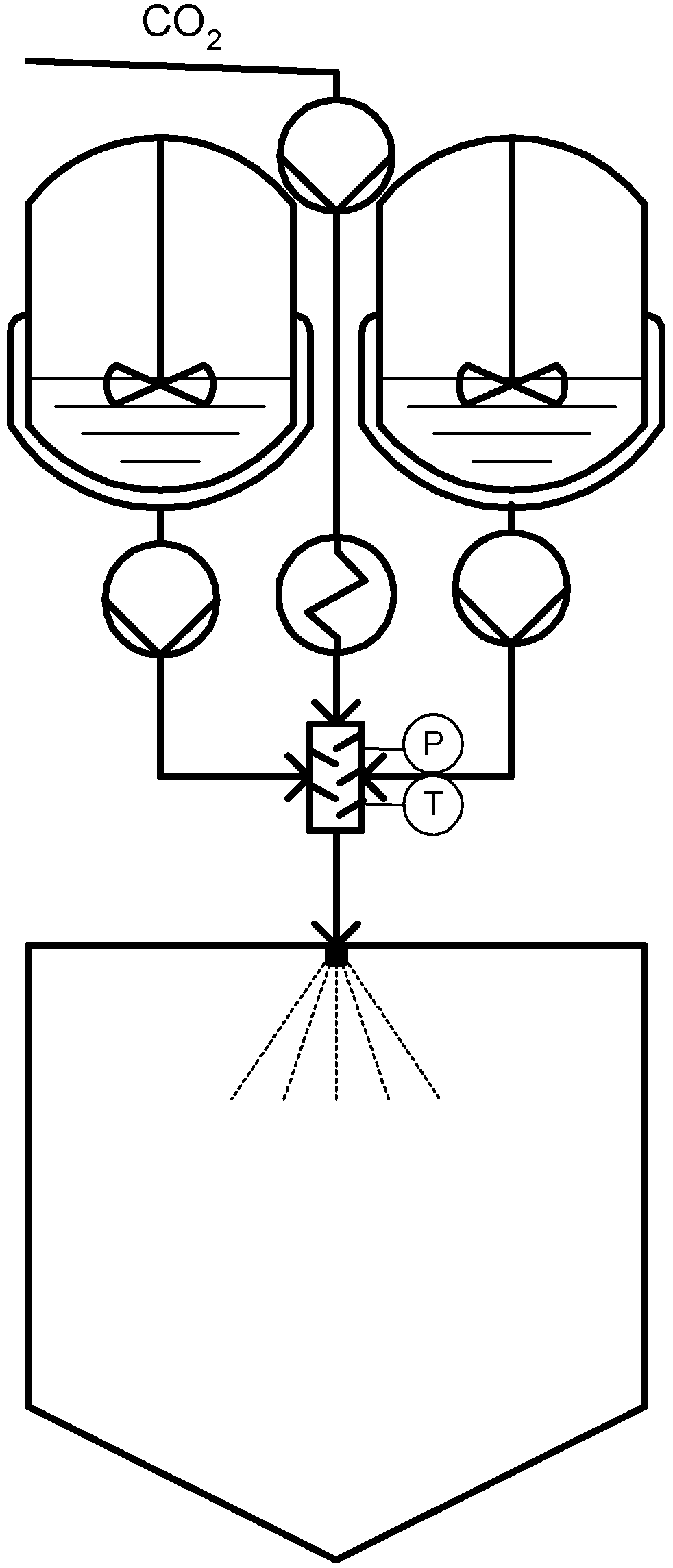
3.2. Co-Solute (and Propellant): CAN-BD®, SAA, SEA and PGSS-Drying
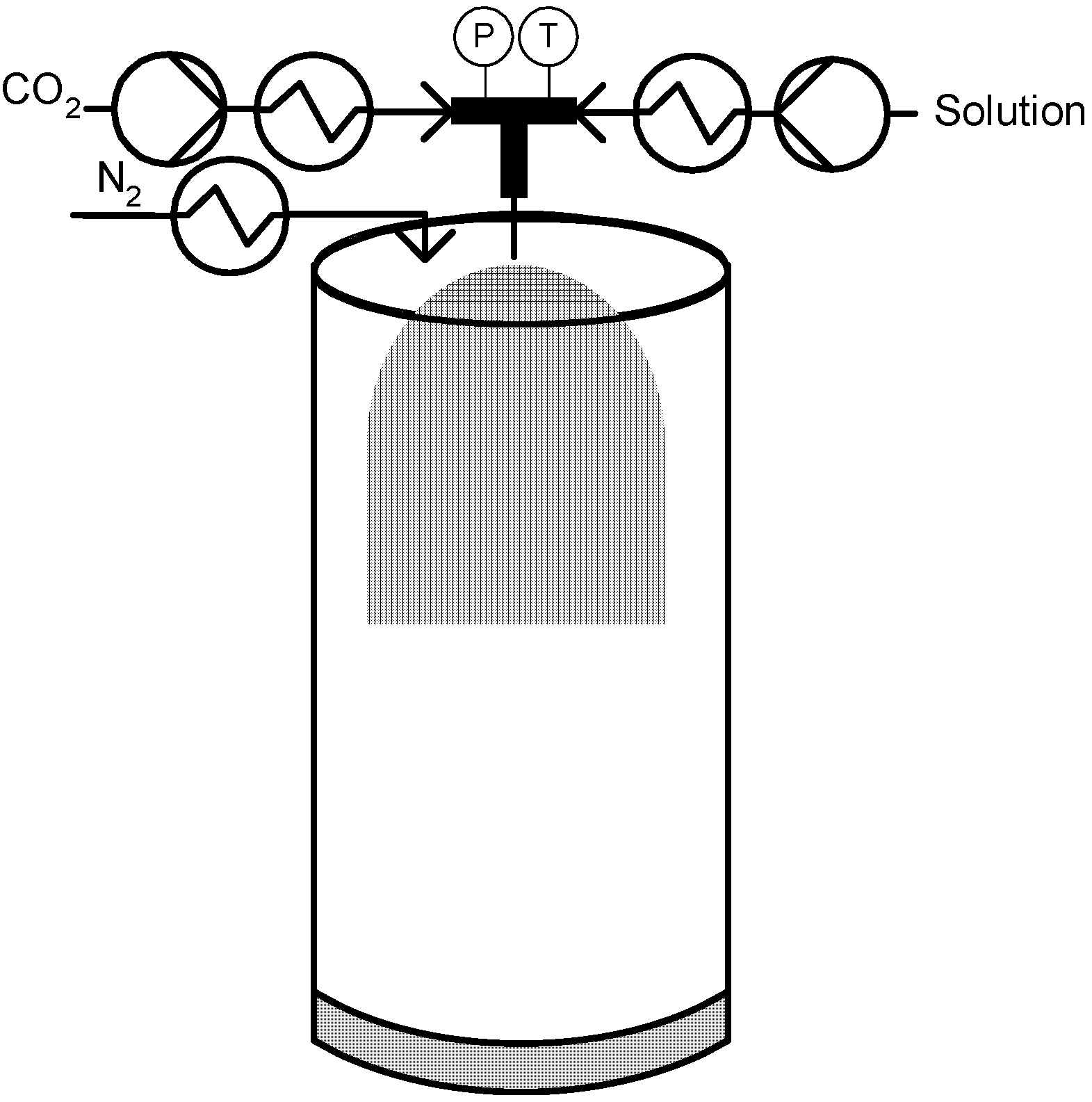
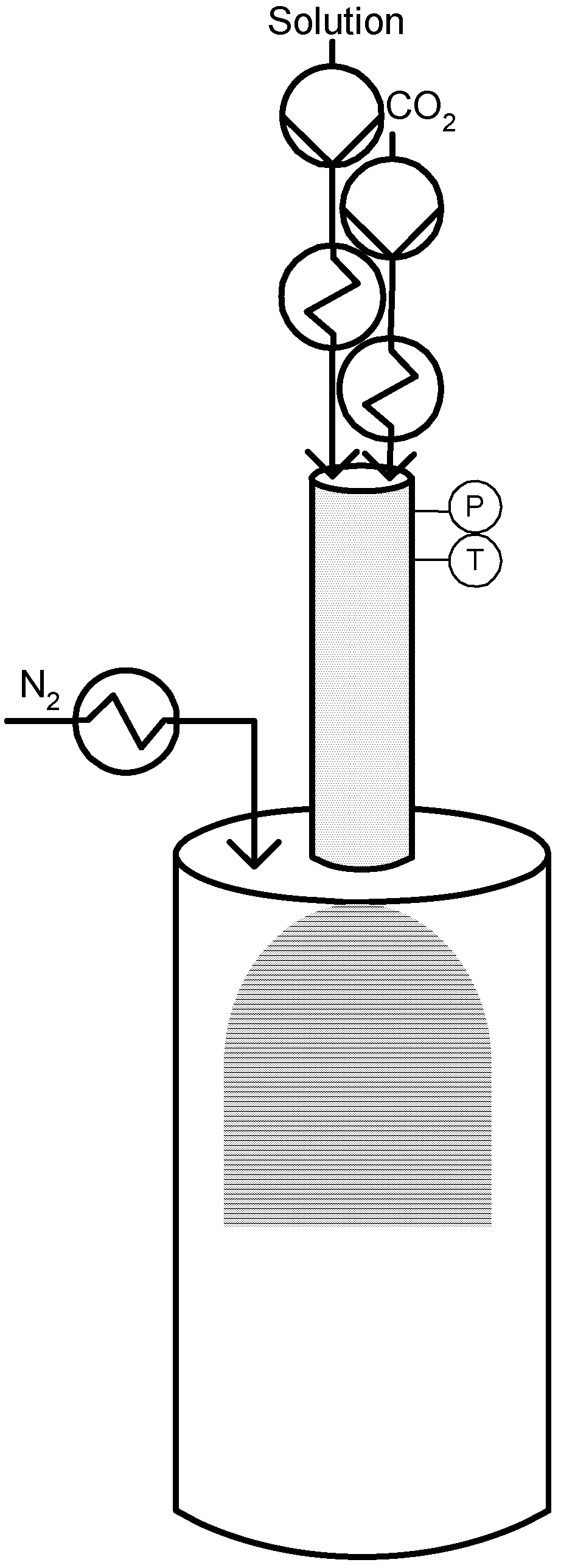
3.3. Co-Solvent: DELOS
| Technique | CO2 role | Liquid solvents | Pre-requisites | Equilibrium measurements | Saturation | Precipitation | Drying |
|---|---|---|---|---|---|---|---|
| PGSS | Solute | NA | Melted or substances that experiment a mp depression effect under CO2 conditions | S-L-G equlibrium | High pressure reactor with mixing or in a static mixer | Spray tower | NA |
| CPF | The solute to be powderized is a liquid | VLE of the binary system (solute + CO2) | High pressure reactor with mixing | NA (Infiltration occur in a spray tower) | |||
| CPCSP | Melted or substances that experiment a mp depression effect under CO2 conditions | S-L-G equlibrium | Static mixer | Spray tower | |||
| CAN-BD | Co-solute (aerosolization aid) | Water and alcohol | Limited solubility between scCO2 and the liquid solvent | VLE of the binary system (solvent + CO2) | CO2 solubilisation occur in a low volume tee | Spar tower | With N2 |
| SEA | CO2 solubilisation occur in a pre-expansion mixing chamber | High pressure vessel equipped with a filter | NA | ||||
| SAA | Packed tower | Spay tower | With N2 | ||||
| PGSS-drying | Static mixer | Spray tower | With CO2 (T must be higher than the dew point of the binary system gas and solvent) | ||||
| DELOS | Co-solvent | Organic solvents | CO2 acts as a co-solvent | VLE of the ternary system (solute + CO2 +organic solvent) | Autoclave | High pressure vessel equipped with a filter | With CO2 |
5. Applications of PGSS and PGSS-based Techniques
| Substance | Technique | References |
|---|---|---|
| Anthocyanin extracts/silica | CPF | Vatai et al. (2008) [76] |
| Caffeine/glyceryl monostearate | PGSS | de Sousa et al. (2007) [77] |
| Caffeine/glyceryl monostearate/cutine/TiO2 | PGSS | Garcia-Gonzalez et al. (2009) [78] |
| trans-Chalcone | PGSS | de Sousa et al. (2009) [79] |
| Citrus flavour | CPF | Gruner et al. (2003) [80] |
| Citric acid/PEG | PGSS | Weidner et al. (1996) [26] |
| Cyclosporine | PGSS | Tandya et al. (2006) [81] |
| Coatings systems (acrylic coatings, polyester-epoxy systems, low-melting polyester coatings) | CPCSP | Weidner et al. (2001) [43] |
| Cocoa butter | PGSS | Letourneau et al. (2005) [82] |
| Cocoa powder | PGSS | Perva-Uzunalic et al. (2008) [83] |
| Cilantro(Coriandrum sativum)/PEG | Choi et al. (2009) [84] | |
| Cydia pomonella granulovirus | PGSS | Pemsel et al. (2010) [85] |
| Felodipine, Felofipine/lactose, Felodipine/PEG4000 | PGSS | Kerc et al. (1999) [86] |
| Fenofibrate, Fenofibrate/PEG4000 | PGSS | Kerc et al. (1999) [86] |
| Glutathione/glyceryl monostearate/cutine/TiO2 | PGSS | Garcia-Gonzalez et al. (2009) [78] |
| Glyceryl monostearate | PGSS | de Sousa et al. (2007) [77] |
| Green tea extracts (Aqueous) | PGSS-drying | Meterc et al.(2008) [64] |
| hgH/PLGA/PLA | PGSS | Jordan et al. (2010) [87] |
| rh-gH/ Phosphatidylcholine/PEG/Tristearin | PGSS | Salmasso et al. (2009) [88] |
| Hydrogenated palm oil | PGSS | Li et al. (2005) [89] |
| Insulin/tristearin, Tween-80, phosphatidylcholine, PEG, Insulin/tristearin, dioctyl sulfosuccinate and phosphatidylcholine | PGSS | Salmaso et al. (2009) [38] |
| Ketoprofen/glyceryl monostearate/cutine/TiO2 | PGSS | Garcia-Gonzalez et al. (2009) [78] |
| Lavandin essential oil/(OSA)-starch | PGSS-drying | Varona et al. (2010) [69] |
| Lavandin essential oil/PEG | PGSS | Varona et al. (2010) [69] |
| Lysozyme/P(DLLA) | PGSS | Whitaker et al. (2005) [90] |
| Monostearate | PGSS | Mandzuka et al. (2008) [91] |
| Nifedipine, Nifedipine/PEG 4000 | PGSS | Kerc et al. (1999) [86], Sencar-Bozic et al. (1997) [28] |
| Polybutylenterephthalate, Polybutylenterephthalate /zinc oxide, Polybutylenterephthalate/bentonite | PGSS | Pollak et al. (2010) [92] |
| Poly (DL-lactic acid) | PGSS | Hao et al. (2004) [37] |
| Poly (ethylene glycol) | PGSS | Hao et al. (2005) [93], Nalawade et al. (2007) [94] |
| Poly (ethylene glycol) aqueous solution | PGSS-drying | Martin et al. (2010) [68] |
| Precirol | PGSS | Calderone et al. (2007) [95] |
| Rapeseed 70 | PGSS | Manuklu et al. (2007) [96] |
| PEGylated Ribonuclease/ Triestearin/Phosphatidylcholine/PEG | PGSS | Vezzu et a. (2010) [97] |
| Ribonuclease A/P(DLLA) | PGSS | Whitaker et al. (2005) [90] |
| Theophylline/hydrogenated palm oil | PGSS | Rodrigues et al. (2004) [30] |
| TiO2-PLA, TiO2-PS-b-PMMA-co-PGMA | PGSS | Matsuyama et al. (2007) [98] |
| Triacetyl-β-cyclodextrin | PGSS | Nunes et al. (2010) [99] |
| Tristearate | PGSS | Mandzuka et al. (2008) [91], Mandzuka et al. (2010) [100] |
| Vegetable oil emulsion/cellulose | CPF | Wehowski et al. (2008) [101] |
| YNS3107/PEG400/PEG4000/Polaxamer 407 | PGSS | Brion et al. (2009) [102] |
| Substance | Liquid solvent | References |
|---|---|---|
| Albuterol sulfate | Water | Sievers et al. (1998, 2000, 2001) [47,103,104] |
| Alpha-1-antitrypsin | Water | Cape et al. (2008) [48] |
| Amphotericin B | Ethanol | Sievers et al. (2003) [50] |
| Anti-CD4 | Water | Cape et al. (2008) [48] |
| Betamethasone-17,21-dipropionate | Ethanol | Villa et al. (2005) [51] |
| Budesonide | Ethanol | Sievers et al. (2003) [50] |
| Cromolyn sodium | Water | Sievers et al. (2000) [47] |
| Doxycycline | Water | Sievers et al. (2003) [105] |
| Glutathione | Water | Sievers et al. (1999) [46] |
| Myo-inositol | Water | Huang et al. (2003) [49] |
| HBsAg (Hepatitis B surface antigen protein)/Albumin hydroxide | Water | Sievers et al. (2007) [52] |
| Iron oxides mixture (Fe3O4 and FeO) | Water | Sievers et al. (1999) [46] |
| Lactate dehydrogenase (LDH) | Water | Sellers et al. (2001) [106], Sievers et al. (2001) [104] |
| Lactose | Water | Sievers et al. (2000) [47], Villa et al. (2005) [51] |
| Lactose/Betamethasone | Water/Ethanol | Villa et al. (2005) [51] |
| Lactose/( Betamethasone/Stearic acid) | Water/Ethanol | Villa et al. (2005) [51] |
| Lactose/Palmitic acid | Water/Ethanol | Villa et al. (2005) [51] |
| Lysozyme | Water | Sellers et al. (2001) [106], Sievers et al. (2001) [104] |
| Mannitol | Water | Huang et al. (2003) [49] |
| Measles Vaccine virus, live-attenuated | Water | Sievers et al. (2007) [52], Burger et al. (2008) [53] |
| Naproxen | Water | Sievers et al. (2003) [50] |
| Ovalbumin/trehalose | Water | Sievers et al. (2001) [104], Sievers et al. (2003) [50] |
| Palmitic acid | Ethanol | Villa et al. (2005) [51] |
| rhDNase | water | Sievers et al. (1999) [46] |
| Rifampin | Ethyl acetate | Sievers et al. (2007) [52] |
| Sacharin(SAC)-Aspirin, SAC-Caffeine, SAC-Carbamazeoine, SAC-Indomethacin, SAC-Sulfamethazine, SAC-Theophylline (Cocrystals) | Ethanol | Padrela et al. (2009) [61], Padrela et al. (2010) [62] |
| Sodium chloride | Water | Sievers et al. (2001) [104], Sievers et al. (2003) [50], Villa et al. (2005) [51] |
| Sodium chloride/Palmitic acid | Water/acetone | Villa et al. (2005) [51] |
| Sodium chloride/PLGA | Water/acetone | Villa et al. (2005) [51] |
| Tobramycin sulfate | Water | Sievers et al. (1998) [103] |
| Trypsinogen | Water | Cape et al. (2008) [48] |
| Yttrium oxide phosphors (Y2O3:Eu, Y2O3:Tb) | Water | Xu et al. (1997) [45] |
| Zanamivir (Relenza®) | Water | Sievers et al. (2007) [52] |
| Substance | Liquid solvent | References |
|---|---|---|
| Albumin/Gentamicin sulfate | Water | Della Porta et al. (2010) [107] |
| Aluminum sulfate | Water | Reverchon et al. (2002) [54] |
| Amonium chloride | Water | Reverchon et al. (2004) [108] |
| Ampicillin | Water, methanol, ethanol | Reverchon et al. (2002, 2003) [54,109] |
| Ampicillin trihydrate /Chitosan | Water | Reverchon et al. (2007) [110] |
| HPMC/ampicillin trihydrate | Buffer solution | Reverchon et al. (2008) [111] |
| Beclomethasone | Methanol, acetone, methanol/water, acetone/water | Reverchon et al. (2010) [112] |
| Carbamazepine | Methanol | Reverchon et al. (2002) [54] |
| Cefadroxil | Water | Li et al. (2009) [113] |
| Chitosan | 1%acid acetic aqueous solution | Reverchon et al. (2006) [114] |
| Cromolyn Sodium | Water | Reverchon et al. (2007) [115] |
| α-Cyclodextrin | Water | Reverchon et al. (2006) [116] |
| Dexamethasone, Dexamethasone acetate | Acetone, methanol | Reverchon et al. (2002, 2006) [54,117] |
| Erythromycin | Methanol, ethanol, acetone | Reverchon et al. (2003, 2004) [118,119], Li et al. (2007) [120] |
| Ginkgo biloba leaves extract | x | Miao et al. (2010) [121] |
| Griseofulvin | Acetone, acetone/ethanol | Reverchon et al. (2004) [122], Li et al. (2008) [123] |
| HP-beta-CD | Water | Reverchon et al. (2006) [114] |
| HMR1031 (new chemical entity by Aventis Pharma) | Methanol | Reverchon et al. (2005) [124] |
| Levofloxacin hydrochloride | Methanol | Cai et al. (2008) [60] |
| Lysozyme | Water, water/ethanol mixtures | Reverchon et al. 2009 [125] |
| Pigment red 60 | Acetone | Reverchon et al. (2005) [126] |
| PLLA | DCM | Reverchon et al. (2007) [127] |
| PMMA | Acetone | Reverchon et al. (2007) [127] |
| Potassium iodide | Water, methanol | Reverchon et al. (2004) [108] |
| Rifampicine | Methanol | Reverchon et al. (2003) [128] |
| Sodium chloride | Water | Reverchon et al. (2002, 2004) [54,108] |
| Sodium cellulose sulfate | Water | Wang et al. [129] |
| Terbutaline | Water | Reverchon et al. (2003) [130] |
| Tetracycline | Water, water/ethanol | Reverchon et al. (2003) [128,119], Li et al. 2008 [131] |
| Triclabenzadol | Methanol | Reverchon et al. (2002) [54] |
| Yttrium acetate | Water, methanol | Reverchon et al. (2002, 2003) [54,119] |
| Zinc acetate | Methanol | Reverchon et al. (2002) [54] |
| Zirconyl nitrate hydrate | Water | Reverchon et al. (2002) [54] |
| Substance | Liquid solvent | References |
|---|---|---|
| 1,4-bis-(n-butylamino)-9,10-anthraquinone (solventblue35) | Acetone | Ventosa et al. (2001, 2003) [71,72] |
| 1,4-bis-(n-butylamino)-9,10-anthraquinone (solventblue35) | 1,1,1,2-Tetrafluoroethane | Gimeno et al. (2006) [132] |
| 1,3,5,7-Tetraazatricyclo[3.3.1.13,7]decane (hexamethylenetetramine) | 1,1,1,2-Tetrafluoroethane | Gimeno et al. (2006) [132] |
| Acetylsalicylic acid (aspirin) | 1,1,1,2-Tetrafluoroethane | Gimeno et al. (2006) [132] |
| Cholesterol | Cano-Sarabia et al. (2008) [75] | |
| Ibuprofen | Ethanol and Acetone | Munto et al. (2008) [133] |
| Naproxen | ethanol | Munto et al. (2008) [133] |
| Poloxamer F-127 | ethanol | Munto et al. (2008) [134] |
| Stearic acid | Ethyl acetate | Sala et al. (2010) [135] |
6. Conclusions and Future Perspectives
References
- York, P. Strategies for particle design using supercritical fluid technologies. Pharm. Sci. Technol. Today 1999, 2, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Bertucco, A.; Vetter, G. High Pressure Process Technology: Fundamentals and Applications, Industrial Chemistry Library; Elsevier: Amsterdam, The Netherlands, 2001; Volume 9. [Google Scholar]
- Hakuta, Y.; Hayashi, H.; Arai, K. Fine particle formation using supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003, 7, 341–351. [Google Scholar] [CrossRef]
- Fages, J.; Lochard, H.; Letourneau, J.J.; Sauceau, M.; Rodier, E. Particle generation for pharmaceutical applications using supercritical fluid technology. Powder Technol. 2004, 141, 219–226. [Google Scholar] [CrossRef]
- Martin, A.; Cocero, M.J. Precipitation processes with supercritical fluids: Patents review. Recent Pat. Eng. 2008, 1, 9–20. [Google Scholar]
- Pasquali, I.; Bettini, R. Are pharmaceutics really going supercritical? Int. J. Pharm. 2008, 364, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K. Biodegradable particle formation for drug and gene delivery using supercritical fluid and dense gas. Adv. Drug Deliv. Rev. 2008, 60, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Cocero, M.J. Micronization processes with supercritical fluids: Fundamentals and mechanisms. Adv. Drug Deliv. Rev. 2008, 60, 339–350. [Google Scholar] [CrossRef]
- Foster, N.; Mammucari, R.; Dehghani, F.; Barrett, A.; Bezanehtak, K.; Coen, E.; Combes, G.; Meure, L.; Ng, A.; Regtop, H.L.; Tandya, A. Processing pharmaceutical compounds using dense gas technology. Ind. Eng. Chem. Res. 2003, 42, 6476–6493. [Google Scholar] [CrossRef]
- Beckman, E.J. Supercritical and near-critical CO2 in green chemical synthesis and processing. J. Supercrit. Fluids 2004, 28, 121–191. [Google Scholar] [CrossRef]
- Clifford, T. Fundamentals of Supercritical Fluids; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Leitner, W.; Poliakoff, M. Supercritical fluids in green chemistry. Green Chem. 2008, 10, 730–730. [Google Scholar] [CrossRef]
- Green Chemistry Using Liquid and Supercritical Carbon Dioxide; DeSimone, J.M.; Tumas, W. (Eds.) Oxford University Press: Oxford, UK, 2003.
- Jung, J.; Perrut, M. Particle design using supercritical fluids: Literature and patent survey. J. Supercrit. Fluids 2001, 20, 179–219. [Google Scholar] [CrossRef]
- Shariati, A.; Peters, C. J. Recent developments in particle design using supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003, 7, 371–383. [Google Scholar] [CrossRef]
- Reverchon, E.; Volpe, M.C.; Caputo, G. Supercritical fluid processing of polymers: Composite particles and porous materials elaboration. Curr. Opin. Solid State Mater. Sci. 2003, 7, 391–397. [Google Scholar] [CrossRef]
- Yeo, S.D.; Kiran, E. Formation of polymer particles with supercritical fluids: A review. J. Supercrit. Fluids 2005, 34, 287–308. [Google Scholar] [CrossRef]
- Tandya, A.; Mammucari, R.; Dehghani, F.; Foster, N.R. Dense gas processing of polymeric controlled release formulations. Int. J. Pharm. 2007, 328, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cocero, M.J.; Martin, A.; Mattea, F.; Varona, S. Encapsulation and co-precipitation processes with supercritical fluids: Fundamentals and applications. J. Supercrit. Fluids 2009, 47, 546–555. [Google Scholar] [CrossRef]
- Weidner, E. High pressure micronization for food applications. J. Supercrit. Fluids 2009, 47, 556–565. [Google Scholar] [CrossRef]
- Weidner, E.; Knez, Z.; Novak, Z. A Process and Equipment for Production and Fractionation of Fine Particles from Gas Saturated Solutions. World Patent WO 95/21688, 1994. [Google Scholar]
- Weidner, E.; Knez, Z.; Novak, Z. PGSS (Particles from Gas Saturated Solutions)—A new process for powder generation. In Proceedings of the 3rd International Symposium on Supercritical Fluids, Strasbourg, France, 17–19 October 1994; Volume 3, pp. 229–235.
- Lack, E.; Weidner, E.; Knez, Z.; Gruner, S.; Weinreich, B.; Seidlitz, H. Particle generation with supercritical CO2. In Proceedings of the 1st Vienna International Conference: Micro- and Nano-Technology, Vienna, Austria, 9–11 March 2005.
- Knez, Z.; Weidner, E. Particles formation and particle design using supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003, 7, 353–361. [Google Scholar] [CrossRef]
- Knez, Z.; Skerget, M.; Mandzuka, Z. Determination of S-L phase transitions under gas pressure. J. Supercrit. Fluids 2010, 55, 648–652. [Google Scholar] [CrossRef]
- Weidner, E.; Steiner, R.; Knez, Z. Powder generation from polyethyleneglycols with compressible fluids. High Press. Chem. Eng. 1996, 12, 223–228. [Google Scholar]
- Weidner, E.; Petermann, M.; Knez, Z. Multifunctional composites by high-pressure spray processes. Curr. Opin. Solid State Mater. Sci. 2003, 7, 385–390. [Google Scholar] [CrossRef]
- SencarBozic, P.; Srcic, S.; Knez, Z.; Kerc, J. Improvement of nifedipine dissolution characteristics using supercritical CO2. Int. J. Pharm. 1997, 148, 123–130. [Google Scholar] [CrossRef]
- Howdle, S.M.; Watson, M.S.; Whitaker, M.J.; Popov, V.K.; Davies, M.C.; Mandel, F.S.; Wang, J.D.; Shakesheff, K.M. Supercritical fluid mixing: Preparation of thermally sensitive polymer composites containing bioactive materials. Chem. Commun. 2001, 109–110. [Google Scholar]
- Rodrigues, M.; Peirico, N.; Matos, H.; de Azevedo, E.G.; Lobato, M.R.; Almeida, A.J. Microcomposites theophylline/hydrogenated palm oil from a PGSS process for controlled drug delivery systems. J. Supercrit. Fluids 2004, 29, 175–184. [Google Scholar] [CrossRef]
- Shine, A.D.; Gelb, J. Forming microparticles of material, partic. protein|by swelling and liquefaction of polymer, then releasing pressure, used for e.g., drugs, vaccines, agrochemicals and deodorants. WO9815348, 16 April 1998. [Google Scholar]
- Foster, N.R.; Regtop, H.L.; Dehghani, F.; Tandya, A. Preparation of biologically active micro particles by pulverization for pharmaceutical purposes. WO2003088951, 30 October 2003. [Google Scholar]
- Perrut, M. Prepn. of liq. loaded powder useful in topical compsns. of e.g., sunscreen. WO200205944, 24 January 2002. [Google Scholar]
- Calderone, M.; Rodier, E. Coating of powdery solid active substances, for use in e.g., a pharmaceutical formulation, comprises contacting a mixture (having coating) and individualized particles of active substance. WO2006030112, 23 March 2006. [Google Scholar]
- Calderone, M.; Rodier, E.; Fages, J. Microencapsulation by a solvent-free supercritical fluid process: Use of density, calorimetric, and size analysis to quantify and qualify the coating. Part. Sci. Technol. 2007, 25, 213–225. [Google Scholar] [CrossRef]
- Calderone, M.; Rodier, E.; Lochard, H.; Marciacq, F.; Fages, J. A new supercritical co-injection process to coat microparticles. Chem. Eng. Process. 2008, 47, 2228–2237. [Google Scholar] [CrossRef]
- Hao, J.Y.; Whitaker, M.J.; Wong, B.; Serhatkulu, G.; Shakesheff, K.M.; Howdle, S.M. Plasticization and spraying of poly (dl-lactic acid) using supercritical carbon dioxide: Control of particle size. J. Pharm. Sci. 2004, 93, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, S.; Elvassore, N.; Bertucco, A.; Caliceti, P. Production of solid lipid submicron particles for protein delivery using a novel supercritical gas-assisted melting atomization process. J. Pharm. Sci. 2009, 98, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Bertucco, A.; Caliceti, P.; Elvassore, N. Process for the production of nano-particles. WO2007028421, 2007. [Google Scholar]
- Weidner, E.; Steiner, R.; Dirscherl, H.; Weinreich, B. Verfahren zur Herstellung eines pulverförmigen Produktes aus einem flüssigen Stoff oder Stoffgemisch. European Patent EP 9705484, 6 October 1997. [Google Scholar]
- Petermann, M.; Weidner, E.; Grüner, S.; Weinreich, B. CPF—Concentrated powder form—A high pressure spray agglomeration technique. In Proceedings of the Spray Drying ’01 and Related Processes, Dortmund, Germany, 8–10 October 2001.
- Petermann, M.; Weidner, E.; Blatter, K.; Simmrock, H.U. Manufacture of powder coatings by spraying of gas saturated melts. In Proceedings of the 6th International Symposium on Supercritical Fluids, Versailles, France, 28–30 April 2003.
- Weidner, E.; Petermann, M.; Blatter, K.; Rekowski, V. Manufacture of powder coatings by spraying of gas-enriched melts. Chem. Eng. Technol. 2001, 24, 529–533. [Google Scholar] [CrossRef]
- Sievers, R.E.; Karst, U. Methods for fine particle formation. US Patent 5,639,441, 17 June 1997. [Google Scholar]
- Xu, C.Y.; Watkins, B.A.; Sievers, R.E.; Jing, X.P.; Trowga, P.; Gibbons, C.S.; Vecht, A. Submicron-sited spherical yttrium oxide based phosphors prepared by supercritical CO2-assisted aerosolization and pyrolysis. Appl. Phys. Lett. 1997, 71, 1643–1645. [Google Scholar] [CrossRef]
- Sievers, R.E.; Karst, U.; Milewski, P.D.; Sellers, S.P.; Miles, B.A.; Schaefer, J.D.; Stoldt, C.R.; Xu, C.Y. Formation of aqueous small droplet aerosols assisted by supercritical carbon dioxide. Aerosol Sci. Technol. 1999, 30, 3–15. [Google Scholar] [CrossRef]
- Sievers, R.E.; Milewski, P.D.; Sellers, S.P.; Miles, B.A.; Korte, B.J.; Kusek, K.D.; Clark, G.S.; Mioskowski, B.; Villa, J.A. Supercritical and near-critical carbon dioxide assisted low-temperature bubble drying. Ind. Eng. Chem. Res. 2000, 39, 4831–4836. [Google Scholar] [CrossRef]
- Cape, S.P.; Villa, J.A.; Huang, E.T.S.; Yang, T.H.; Carpenter, J.F.; Sievers, R.E. Preparation of active proteins, vaccines and pharmaceuticals as fine powders using supercritical or near-critical fluids. Pharm. Res. 2008, 25, 1967–1990. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.T.S.; Chang, H.; Liang, C.D.; Sievers, R.E. Fine particle pharmaceutical manufacturing using dense carbon dioxide mixed with aqueous or alcoholic solutions. In Supercritical Carbon Dioxide: Separations and Processes; Gopalan, A.S., Wai, C.M., Jacobs, H.K., Eds.; 2003; Volume 860, pp. 324–338. [Google Scholar]
- Sievers, R.E.; Huang, E.T.S.; Villa, J.A.; Engling, G.; Brauer, P.R. Micronization of water-soluble or alcohol-soluble pharmaceuticals and model compounds with a low-temperature Bubble Dryer (R). J. Supercrit. Fluids 2003, 26, 9–16. [Google Scholar] [CrossRef]
- Villa, J.A.; Huang, E.T.S.; Cape, S.P.; Sievers, R.E. Synthesis of composite microparticles with a mixing cross. Aerosol Sci. Technol. 2005, 39, 473–484. [Google Scholar] [CrossRef]
- Sievers, R.E.; Quinn, B.P.; Cape, S.P.; Searles, J.A.; Braun, C.S.; Bhagwat, P.; Rebits, L.G.; McAdams, D.H.; Burger, J.L.; Best, J.A.; Lindsay, L.; Hernandez, M.T.; Kisich, K.O.; Iacovangelo, T.; Kristensen, D.; Chen, D. Near-critical fluid micronization of stabilized vaccines, antibiotics and anti-virals. J. Supercrit. Fluids 2007, 42, 385–391. [Google Scholar] [CrossRef]
- Burger, J.L.; Cape, S.P.; Braun, C.S.; McAdams, D.H.; Best, J.A.; Bhagwat, P.; Pathak, P.; Rebits, L.G.; Sievers, R.E. Stabilizing formulations for inhalable powders of live-attenuated measles virus vaccine. J. Aerosol. Med. Pulm. Drug Deliv. 2008, 21, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E. Supercritical-assisted atomization to produce micro- and/or nanoparticles of controlled size and distribution. Ind. Eng. Chem. Res. 2002, 41, 2405–2411. [Google Scholar] [CrossRef]
- Charbit, G.; Badens, E.; Boutin, O. Methods of particle production. In Supercritical Fluid Technology for Drug Product Development; York, P., Kompella, U.B., Shekunov, B.Y., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004. [Google Scholar]
- Reverchon, E. Continuous separation of fatty acid mono:glyceride from mixed glyceride(s). WO2003004142-A, 16 January 2003. [Google Scholar]
- Reverchon, E.; Adami, R.; Cardea, S.; Della Porta, G. Supercritical fluids processing of polymers for pharmaceutical and medical applications. J. Supercrit. Fluids 2009, 47, 484–492. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Caputo, G. Supercritical assisted atomization: Performance comparison between laboratory and pilot scale. J. Supercrit. Fluids 2006, 37, 298–306. [Google Scholar] [CrossRef]
- Cai, M.Q.; Guan, Y.X.; Yao, S.J.; Zhu, Z.Q. Supercritical fluid assisted atomization introduced by hydrodynamic cavitation mixer (SAA-HCM) for micronization of levofloxacin hydrochloride. J. Supercrit. Fluids 2008, 43, 524–534. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Li, J.; Padrela, L.; Almeida, A.; Matos, H.A.; de Azevedo, E.G. Anti-solvent effect in the production of lysozyme nanoparticles by supercritical fluid-assisted atomization processes. J. Supercrit. Fluids 2009, 48, 253–260. [Google Scholar] [CrossRef]
- Padrela, L.; Rodrigues, M.A.; Velaga, S.R.; Matos, H.A.; de Azevedo, E.G. Formation of indomethacin-saccharin cocrystals using supercritical fluid technology. Eur. J. Pharm. Sci. 2009, 38, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Padrela, L.; Rodrigues, M.A.; Velaga, S.P.; Fernandes, A.C.; Matos, H.A.; de Azevedo, E.G. Screening for pharmaceutical cocrystals using the supercritical fluid enhanced atomization process. J. Supercrit. Fluids 2010, 53, 156–164. [Google Scholar] [CrossRef]
- Weidner, E.; Kilzer, A.; Petermann, M.; Prob, A.; Lucas, K.; Stepanski, R. Verfahren zur Erzeugung von Polyurethanpartikeln. Deutsches Patent DE 10040551.7, 15 August 2000. [Google Scholar]
- Meterc, D.; Petermann, M.; Weidner, E. Drying of aqueous green tea extracts using a supercritical fluid spray process. J. Supercrit. Fluids 2008, 45, 253–259. [Google Scholar] [CrossRef]
- Meterc, D.; Petermann, M.; Weidner, E. Extraction of green tea and drying with a high pressure spray process. Hem. Ind. 2007, 61, 222–228. [Google Scholar] [CrossRef]
- Martin, A.; Pham, H.M.; Kilzer, A.; Kareth, S.; Weidner, E. Phase equilibria of carbon dioxide plus poly ethylene glycol plus water mixtures at high pressure: Measurements and modelling. Fluid Phase Equilibria 2009, 286, 162–169. [Google Scholar] [CrossRef]
- Martin, A.; Weidner, E. PGSS-drying: Mechanisms and modeling. J. Supercrit. Fluids 2010, 55, 271–281. [Google Scholar] [CrossRef]
- Martin, A.; Huu, M.P.; Kilzer, A.; Kareth, S.; Weidner, E. Micronization of polyethylene glycol by PGSS (Particles from Gas Saturated Solutions)-drying of aqueous solutions. Chem. Eng. Process. 2010, 49, 1259–1266. [Google Scholar] [CrossRef]
- Varona, S.; Kareth, S.; Martin, A.; Cocero, M.J. Formulation of lavandin essential oil with biopolymers by PGSS for application as biocide in ecological agriculture. J. Supercrit. Fluids 2010, 54, 369–377. [Google Scholar] [CrossRef]
- Ventosa, N.; Veciana, J.; Rovira, C.; Sala, S.; Carburos Metàlicos, S.E. Method for Precipitating Finely Divided Solid Particles. US Patent 7,291,295, 23 August 2001. PCT/ES01/00327. [Google Scholar]
- Ventosa, N.; Sala, S.; Veciana, J.; Torres, J.; Llibre, J. Depressurization of an expanded liquid organic solution (DELOS): A new procedure for obtaining submicron- or micron-sized crystalline particles. Cryst. Growth Des. 2001, 1, 299–303. [Google Scholar] [CrossRef]
- Ventosa, N.; Sala, S.; Veciana, J. DELOS process: A crystallization technique using compressed fluids—1. Comparison to the GAS crystallization method. J. Supercrit. Fluids 2003, 26, 33–45. [Google Scholar] [CrossRef]
- Dalvi, S.V.; Mukhopadhyay, M. Parameters controlling supersaturation by DELOS using carbon dioxide. J. Chem. Technol. Biotechnol. 2006, 81, 1267–1270. [Google Scholar] [CrossRef]
- Ventosa, N.; Veciana, J.; Sala, S.; Cano, M. Method for obtaining micro- and nano-disperse systems. Patents ES2265262; WO2006/0799889, 1 February 2007. [Google Scholar]
- Cano-Sarabia, M.; Ventosa, N.; Sala, S.; Patino, C.; Arranz, R.; Veciana, J. Preparation of uniform rich cholesterol unilamellar nanovesicles using CO2-expanded solvents. Langmuir 2008, 24, 2433–2437. [Google Scholar] [CrossRef] [PubMed]
- Vatai, T.; Skerget, M.; Knez, Z.; Kareth, S.; Wehowski, M.; Weidner, E. Extraction and formulation of anthocyanin-concentrates from grape residues. J. Supercrit. Fluids 2008, 45, 32–36. [Google Scholar] [CrossRef]
- De Sousa, A.R.S.; Simplicio, A.L.; de Sousa, H.C.; Duarte, C.M.M. Preparation of glyceryl mono stearate-based particles by PGSS(R)—Application to caffeine. J. Supercrit. Fluids 2007, 43, 120–125. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, C.A.; da Sousa, A.R.S.; Argemi, A.; Periago, A.L.; Saurina, J.; Duarte, C.M.M.; Domingo, C. Production of hybrid lipid-based particles loaded with inorganic nanoparticles and active compounds for prolonged topical release. Int. J. Pharm. 2009, 382, 296–304. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.R.S.; Silva, R.; Tay, F.H.; Simplicio, A.L.; Kazarian, S.G.; Duarte, C.M.M. Solubility enhancement of trans-chalcone using lipid carriers and supercritical CO2 processing. J. Supercrit. Fluids 2009, 48, 120–125. [Google Scholar] [CrossRef]
- Grüner, S.; Otto, F.; Weinreich, B. CPF-technology—A new cryogenic spraying process for pulverization of liquid. In Proceedings of the 6th International Symposium on Supercritical Fluids, Versailles, France, 28–30 April 2003.
- Tandya, A.; Dehghani, F.; Foster, N.R. Micronization of cyclosporine using dense gas techniques. J. Supercrit. Fluids 2006, 37, 272–278. [Google Scholar] [CrossRef]
- Letourneau, J.J.; Vigneau, S.; Gonus, P.; Fages, J. Micronized cocoa butter particles produced by a supercritical process. Chem. Eng. Process. 2005, 44, 201–207. [Google Scholar] [CrossRef]
- Perva-Uzunalic, A.; Skerget, M.; Knez, Z. Supercritical fluids for producing cocoa powder. In Proceedings of the 2008 Joint Central European Congress, Cavtat, Croatia, 9–11 April 2008; Volume 1, pp. 211–217.
- Jin-Ah, C.; In-Il, J.; Gio-Bin, L.; Jong-Hoon, R. Preparation of drug-loaded polymeric particles using a PGSS process and their characterization. J. Biosci. Bioeng. 2009, 108, S26. [Google Scholar]
- Pemsel, M.; Schwab, S.; Scheurer, A.; Freitag, D.; Schatz, R.; Schlucker, E. Advanced PGSS process for the encapsulation of the biopesticide Cydia pomonella granulovirus. J. Supercrit. Fluids 2010, 53, 174–178. [Google Scholar] [CrossRef]
- Kerc, J.; Srcic, S.; Knez, Z.; Sencar-Bozic, P. Micronization of drugs using supercritical carbon dioxide. Int. J. Pharm. 1999, 182, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Jordan, F.; Naylor, A.; Kelly, C.A.; Howdle, S.M.; Lewis, A.; Illum, L. Sustained release hGH microsphere formulation produced by a novel supercritical fluid technology: In vivo studies. J. Control. Release 2010, 141, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, S.; Bersani, S.; Elvassore, N.; Bertucco, A.; Caliceti, P. Biopharmaceutical characterisation of insulin and recombinant human growth hormone loaded lipid submicron particles produced by supercritical gas micro-atomisation. Int. J. Pharm. 2009, 379, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rodrigues, M.; Paiva, A.; Matos, H.A.; de Azevedo, E.G. Modeling of the PGSS process by crystallization and atomization. AIChE J. 2005, 51, 2343–2357. [Google Scholar] [CrossRef]
- Whitaker, M.J.; Hao, J.Y.; Davies, O.R.; Serhatkulu, G.; Stolnik-Trenkic, S.; Howdle, S.M.; Shakesheff, K.M. The production of protein-loaded microparticles by supercritical fluid enhanced mixing and spraying. J. Control. Release 2005, 101, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Mandzuka, Z.; Knez, Z. Influence of temperature and pressure during PGSS (TM) micronization and storage time on degree of crystallinity and crystal forms of monostearate and tristearate. J. Supercrit. Fluids 2008, 45, 102–111. [Google Scholar] [CrossRef]
- Pollak, S.; Petermann, M.; Kareth, S.; Kilzer, A. Manufacturing of pulverised nanocomposites-dosing and dispersion of additives by the use of supercritical carbon dioxide. J. Supercrit. Fluids 2010, 53, 137–141. [Google Scholar] [CrossRef]
- Hao, J.Y.; Whitaker, M.J.; Serhatkulu, G.; Shakesheff, K.M.; Howdle, S.M. Supercritical fluid assisted melting of poly(ethylene glycol): A new solvent-free route to microparticles. J. Mater. Chem. 2005, 15, 1148–1153. [Google Scholar] [CrossRef]
- Nalawade, S.P.; Picchioni, F.; Janssen, L. Batch production of micron size particles from poly(ethylene glycol) using supercritical CO2 as a processing solvent. Chem. Eng. Sci. 2007, 62, 1712–1720. [Google Scholar] [CrossRef]
- Calderone, M.; Rodier, E.; Letourneau, J.J.; Fages, J. Solidification of Precirol (R) by the expansion of a supercritical fluid saturated melt: From the thermodynamic balance towards the crystallization aspect. J. Supercrit. Fluids 2007, 42, 189–199. [Google Scholar] [CrossRef]
- Munuklu, P.; Jansens, P.J. Particle formation of an edible fat (rapeseed 70) using the supercritical melt micronization (ScMM) process. J. Supercrit. Fluids 2007, 40, 433–442. [Google Scholar] [CrossRef]
- Vezzu, K.; Borin, D.; Bertucco, A.; Bersani, S.; Salmaso, S.; Caliceti, P. Production of lipid microparticles containing bioactive molecules functionalized with PEG. J. Supercrit. Fluids 2010, 54, 328–334. [Google Scholar] [CrossRef]
- Matsuyama, K.; Mishima, K. Formation of TiO2-polymer composite microparticles by rapid expansion of CO2 saturated polymer suspensions with high shear mixing. J. Supercrit. Fluids 2007, 40, 117–124. [Google Scholar] [CrossRef]
- Nunes, A.V.M.; Almeida, A.P.C.; Marques, S.R.; de Sousa, A.R.S.; Casimiro, T.; Duarte, C.M.M. Processing triacetyl-beta-cyclodextrin in the liquid phase using supercritical CO2. J. Supercrit. Fluids 2010, 54, 357–361. [Google Scholar] [CrossRef]
- Mandzuka, Z.; Skerget, M.; Knez, Z. High Pressure Micronization of Tristearate. J. Am. Oil Chem. Soc. 2010, 87, 119–125. [Google Scholar] [CrossRef]
- Wehowski, M.; Weidner, E.; Kilzer, A. Production of powderous emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 331, 143–149. [Google Scholar] [CrossRef]
- Brion, M.; Jaspart, S.; Perrone, L.; Piel, G.; Evrard, B. The supercritical micronization of solid dispersions by Particles from Gas Saturated Solutions using experimental design. J. Supercrit. Fluids 2009, 51, 50–56. [Google Scholar] [CrossRef]
- Sievers, R.E.; Milewski, P.D.; Sellers, S.P.; Kusek, K.D.; Kleutz, P.G.; Miles, B.A. Supercritical CO2-assisted methods for the production and pulmonary administration of pharmaceutical aerosols. J. Aerosol. Sci. 1998, 29, S1271–S1272. [Google Scholar] [CrossRef]
- Sievers, R.E.; Huang, E.T.S.; Villa, J.A.; Kawamoto, J.K.; Evans, M.M.; Brauer, P.R. Low-temperature manufacturing of fine pharmaceutical powders with supercritical fluid aerosolization in a Bubble Dryer (R). Pure Appl. Chem. 2001, 73, 1299–1303. [Google Scholar] [CrossRef]
- Sievers, R.E.; Clark, G.J.; Villa, A.; Alargov, D.; Rinner, L.; Cape, S.P.; Huang, E.T.S. Micronization of inhalable drugs with liquid carbon dioxide at near ambient conditions. J. Aerosol. Med. 2003, 16, 213. [Google Scholar]
- Sellers, S.P.; Clark, G.S.; Sievers, R.E.; Carpenter, J.F. Dry powders of stable protein formulations from aqueous solutions prepared using supercritical CO2-assisted aerosolization. J. Pharm. Sci. 2001, 90, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Della Porta, G.; Adami, R.; del Gaudio, P.; Prota, L.; Aquino, R.; Reverchon, E. Albumin/Gentamicin Microspheres produced by supercritical assisted atomization: Optimization of size, drug loading and release. J. Pharm. Sci. 2010, 99, 4720–4729. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Spada, A. Crystalline microparticles of controlled size produced by supercritical-assisted atomizatione. Ind. Eng. Chem. Res. 2004, 43, 1460–1465. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G.; Spada, A. Ampicillin micronization by supercritical assisted atomization. J. Pharm. Pharmacol. 2003, 55, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Antonacci, A. Drug-polymer microparticles produced by supercritical assisted atomization. Biotechnol. Bioeng. 2007, 97, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Lamberti, G.; Antonacci, A. Supercritical fluid assisted production of HPMC composite microparticles. J. Supercrit. Fluids 2008, 46, 185–196. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Scognamiglio, M.; Fortunato, G.; Della Porta, G. Beclomethasone Microparticles for Wet Inhalation, Produced by Supercritical Assisted Atomization. Ind. Eng. Chem. Res. 2010, 49, 12747–12755. [Google Scholar] [CrossRef]
- Li, Z.Y.; Jiang, J.Z.; Liu, X.W.; Tang, H.H.; Wei, W. Experimental investigation on the micronization of aqueous cefadroxil by supercritical fluid technology. J. Supercrit. Fluids 2009, 48, 247–252. [Google Scholar] [CrossRef]
- Reverchon, E.; Antonacci, A. Cyclodextrins micrometric powders obtained by supercritical fluid processing. Biotechnol. Bioeng. 2006, 94, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Antonacci, A. Chitosan microparticles production by supercritical fluid processing. Ind. Eng. Chem. Res. 2006, 45, 5722–5728. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Caputo, G. Production of cromolyn sodium microparticles for aerosol delivery by supercritical assisted atomization. AAPS Pharmscitech 2007, 8, 272–280. [Google Scholar] [CrossRef]
- Della Porta, G.; Ercolino, S.F.; Parente, L.; Reverchon, E. Corticosteroid microparticles produced by supercritical-assisted atomization: Process optimization, product characterization, and “in vitro” performance. J. Pharm. Sci. 2006, 95, 2062–2076. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Della Porta, G. Particle design using supercritical fluids. Chem. Eng. Technol. 2003, 26, 840–845. [Google Scholar] [CrossRef]
- Reverchon, E.; Spada, A. Erythromycin micro-particles produced by supercritical fluid atomization. Powder Technol. 2004, 141, 100–108. [Google Scholar] [CrossRef]
- Li, Z.Y.; Jiang, J.Z.; Liu, X.W.; Zhao, S.X.; Xia, Y.J.; Tang, H.H. Preparation of erythromycin microparticles by supercritical fluid expansion depressurization. J. Supercrit. Fluids 2007, 41, 285–292. [Google Scholar] [CrossRef]
- Miao, S.F.; Yu, J.P.; Du, Z.; Guan, Y.X.; Yao, S.J.; Zhu, Z.Q. Supercritical fluid extraction and micronization of ginkgo flavonoids from ginkgo biloba leaves. Ind. Eng. Chem. Res. 2010, 49, 5461–5466. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G.; Spada, A.; Antonacci, A. Griseofulvin micronization and dissolution rate improvement by supercritical assisted atomization. J. Pharm. Pharmacol. 2004, 56, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Jiang, J.Z.; Liu, X.W.; Zhao, S.X.; Xia, Y.J.; Wang, J. Preparation of griseofulvin microparticles by supercritical fluid expansion depressurization process. Powder Technol. 2008, 182, 459–465. [Google Scholar] [CrossRef]
- Della Porta, G.; de Vittori, C.; Reverchon, E. Supercritical assisted atomization: A novel technology for microparticles preparation of an asthma-controlling drug. AAPS Pharmscitech 2005, 6, E421–E428. [Google Scholar] [CrossRef] [PubMed]
- Adami, R.; Osseo, L.S.; Reverchon, E. Micronization of Lysozyme by Supercritical Assisted Atomization. Biotechnol. Bioeng. 2009, 104, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Adami, R.; de Marco, I.; Laudani, C.G.; Spada, A. Pigment Red 60 micronization using supercritical fluids based techniques. J. Supercrit. Fluids 2005, 35, 76–82. [Google Scholar] [CrossRef]
- Reverchon, E.; Antonacci, A. Polymer microparticles production by supercritical assisted atomization. J. Supercrit. Fluids 2007, 39, 444–452. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G. Micronization of antibiotics by supercritical assisted atomization. J. Supercrit. Fluids 2003, 26, 243–252. [Google Scholar] [CrossRef]
- Wang, Q.; Guan, Y.X.; Yao, S.J.; Zhu, Z.Q. Microparticle formation of sodium cellulose sulfate using supercritical fluid assisted atomization introduced by hydrodynamic cavitation mixer. Chem. Eng. J. 2010, 159, 220–229. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G. Terbutaline microparticles suitable for aerosol delivery produced by supercritical assisted atomization. Int. J. Pharm. 2003, 258, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Jiang, J.Z.; Liu, X.W.; Xia, Y.J.; Zhao, S.X.; Jian, W. Preparation of tetracycline microparticles suitable for inhalation administration by supercritical fluid expansion depressurization. Chem. Eng. Process. 2008, 47, 1317–1322. [Google Scholar] [CrossRef]
- Gimeno, M.; Ventosa, N.; Sala, S.; Veciana, J. Use of 1,1,1,2-tetrafluoroethane (R-134a)-expanded liquids as solvent media for ecoefficient particle design with the DELOS crystallization process. Cryst. Growth Des. 2006, 6, 23–25. [Google Scholar] [CrossRef]
- Munto, M.; Ventosa, N.; Sala, S.; Veciana, J. Solubility behaviors of ibuprofen and naproxen drugs in liquid “CO2-organic solvent” mixtures. J. Supercrit. Fluids 2008, 47, 147–153. [Google Scholar] [CrossRef]
- Munto, M.; Ventosa, N.; Veciana, J. Synergistic solubility behaviour of a polyoxyalkylene block co-polymer and its precipitation from liquid CO2-expanded ethanol as solid microparticles. J. Supercrit. Fluids 2008, 47, 290–295. [Google Scholar] [CrossRef]
- Sala, S.; Elizondo, E.; Moreno, E.; Calvet, T.; Cuevas-Diarte, M.A.; Ventosa, N.; Veciana, J. Kinetically driven crystallization of a pure polymorphic phase of stearic acid from CO2-expanded solutions. Cryst. Growth Des. 2010, 10, 1226–1232. [Google Scholar] [CrossRef]
- Spray Drying: A Review; Pharmainfo.net: Regina, Canada, 2009. Available online: http://www.pharmainfo.net/reviews/spray-drying-review (accessed on 28 September 2009).
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nunes, A.V.M.; Duarte, C.M.M. Dense CO2 as a Solute, Co-Solute or Co-Solvent in Particle Formation Processes: A Review. Materials 2011, 4, 2017-2041. https://doi.org/10.3390/ma4112017
Nunes AVM, Duarte CMM. Dense CO2 as a Solute, Co-Solute or Co-Solvent in Particle Formation Processes: A Review. Materials. 2011; 4(11):2017-2041. https://doi.org/10.3390/ma4112017
Chicago/Turabian StyleNunes, Ana V. M., and Catarina M. M. Duarte. 2011. "Dense CO2 as a Solute, Co-Solute or Co-Solvent in Particle Formation Processes: A Review" Materials 4, no. 11: 2017-2041. https://doi.org/10.3390/ma4112017




