Characterization of the Materials Synthesized by High Pressure-High Temperature Treatment of a Polymer Derived t-BC2N Ceramic
Abstract
:1. Introduction
2. Experimental Section
| Sample | C (w/w %) | N (w/w %) | C/N ratio | O (w/w %) |
|---|---|---|---|---|
| Pyro-BN-C | 40.0 ± 0.4 | 22.9 ± 1.1 | 1.75 | 4.2 ± 0.3 |
| Heat-treat-BN-C | 46.4 ± 0.6 | 23.5 ± 2.0 | 1.97 | 0.4 ± 0.2 |
3. Results
3.1. Precursor Material

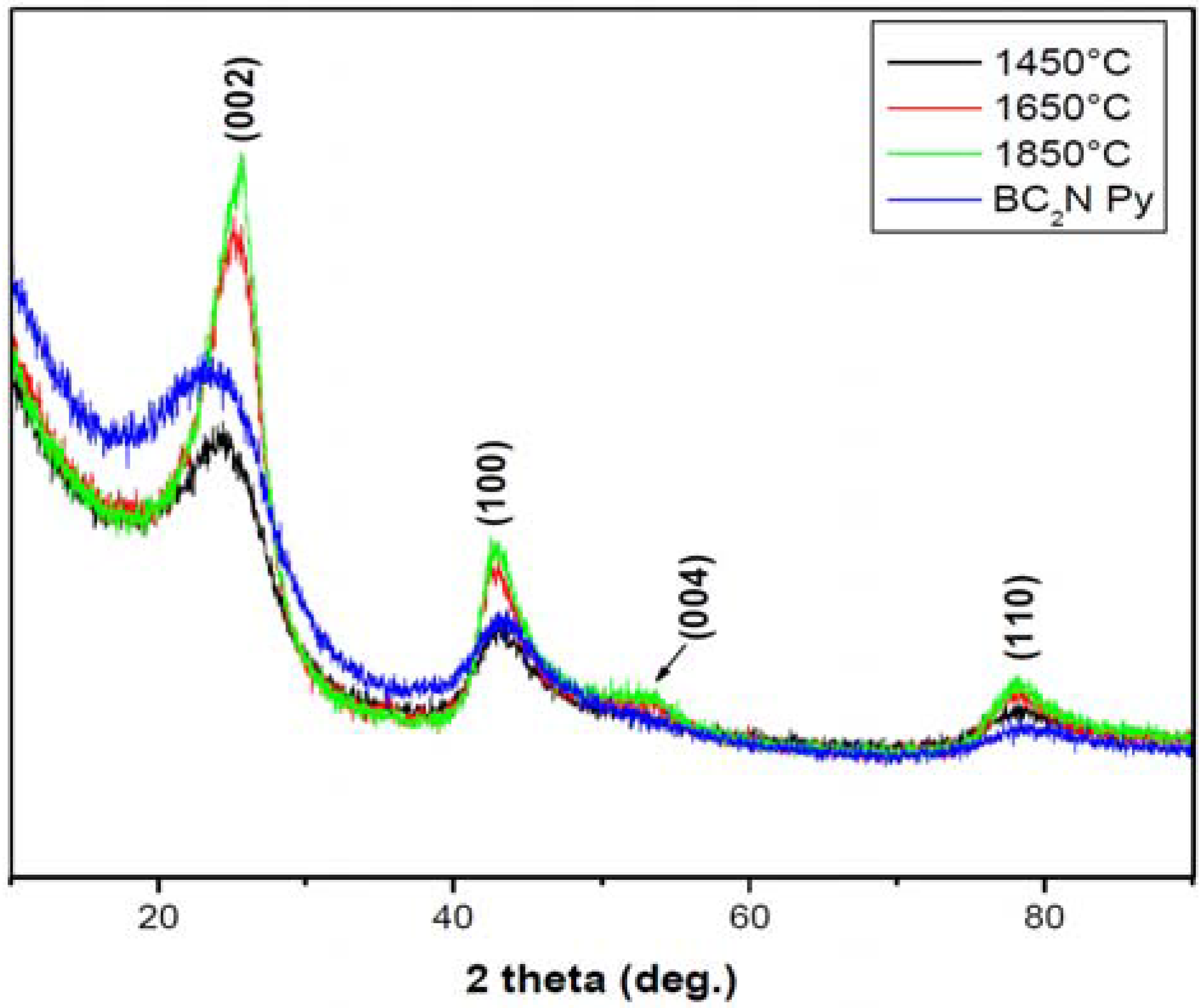
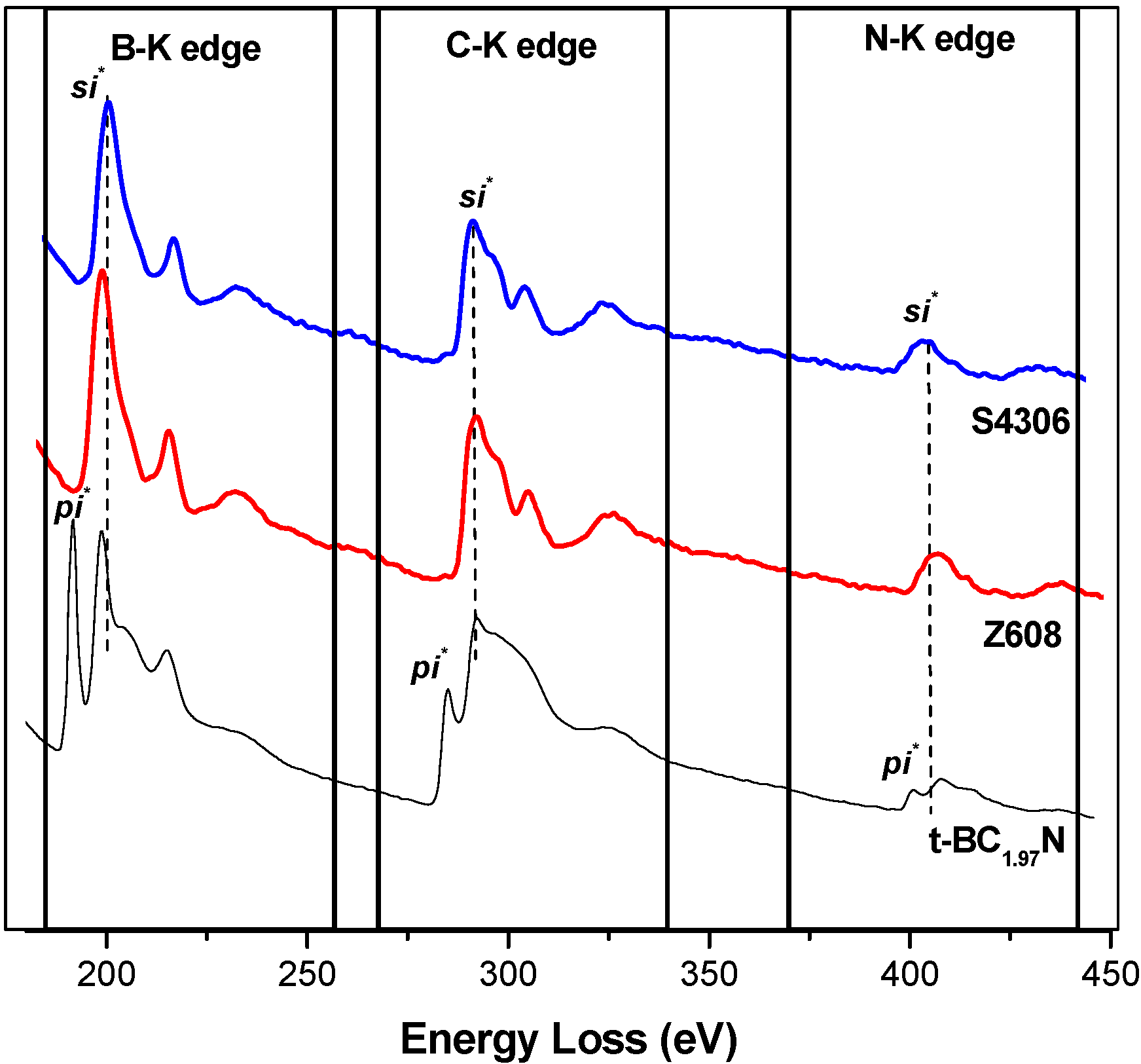
| Sample | B-K(eV) | C-K (eV) | N-K (eV) | Reference | |||
|---|---|---|---|---|---|---|---|
| π* | σ* | π* | σ* | Π* | σ* | ||
| g-BC2N | 191.6 | 197.7 | 285 | 291.7 | 400.7 | 406.3 | [30] |
| t-BN-C | 191.3 | 198.9 | 284.9 | 292.5 | 401.2 | 407.4 | Present work |
| h-BN | 191.9 | 198.1 | - | - | 401.6 | 408.1 | [30] |
| graphite | - | - | 285 | 291.7 | - | - | [30] |
| Z608 | - | 196.9 | - | 291.7 | - | 407.5 | Present work |
| S4306 | - | 196.1 | - | 291.7 | - | 406.3 | Present work |
| diamond | - | - | - | 291.7 | - | - | [30] |
| c-BN | - | 197.7 | - | - | - | 406.3 | [30] |
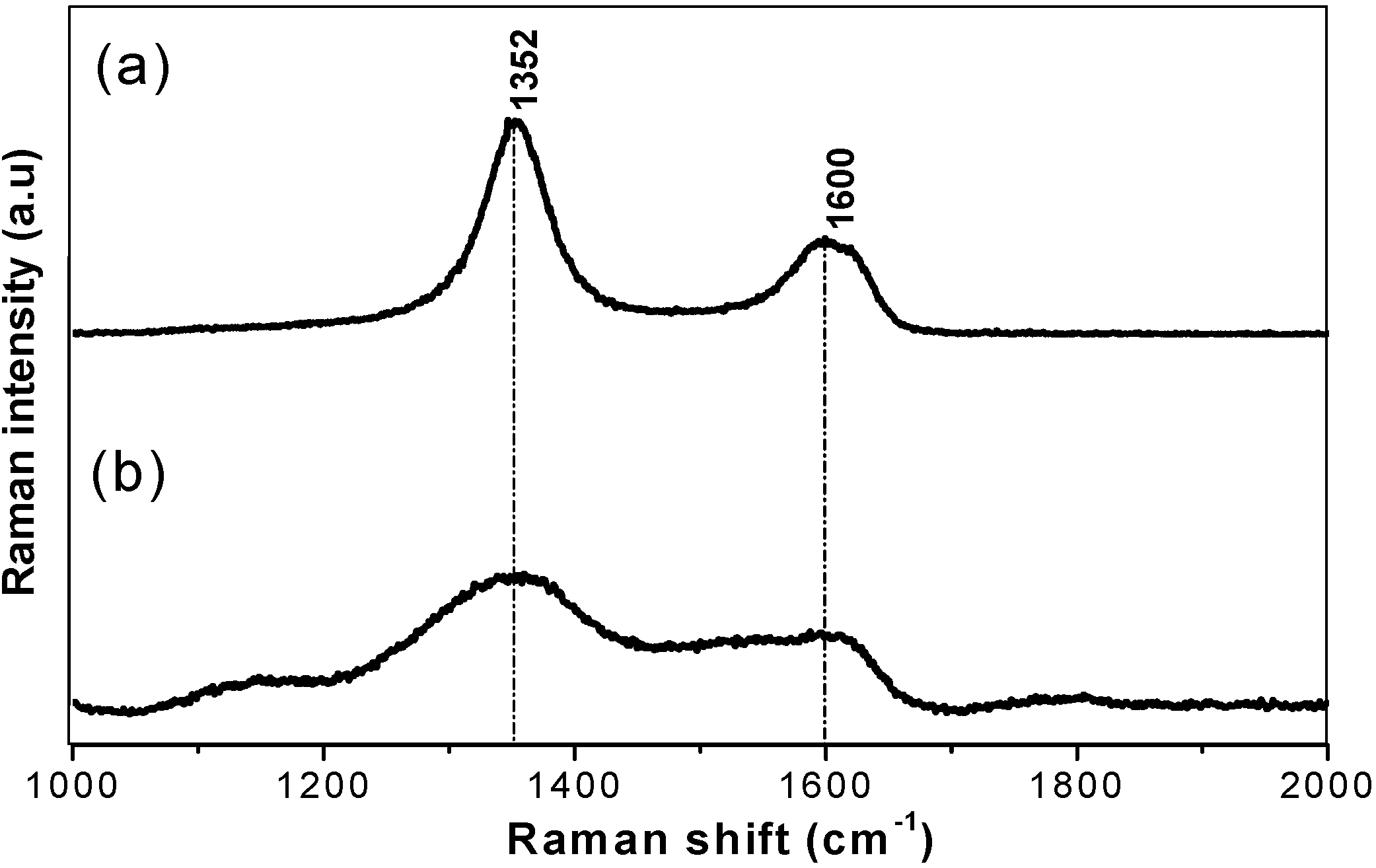
3.2. High Pressure Synthesised Materials
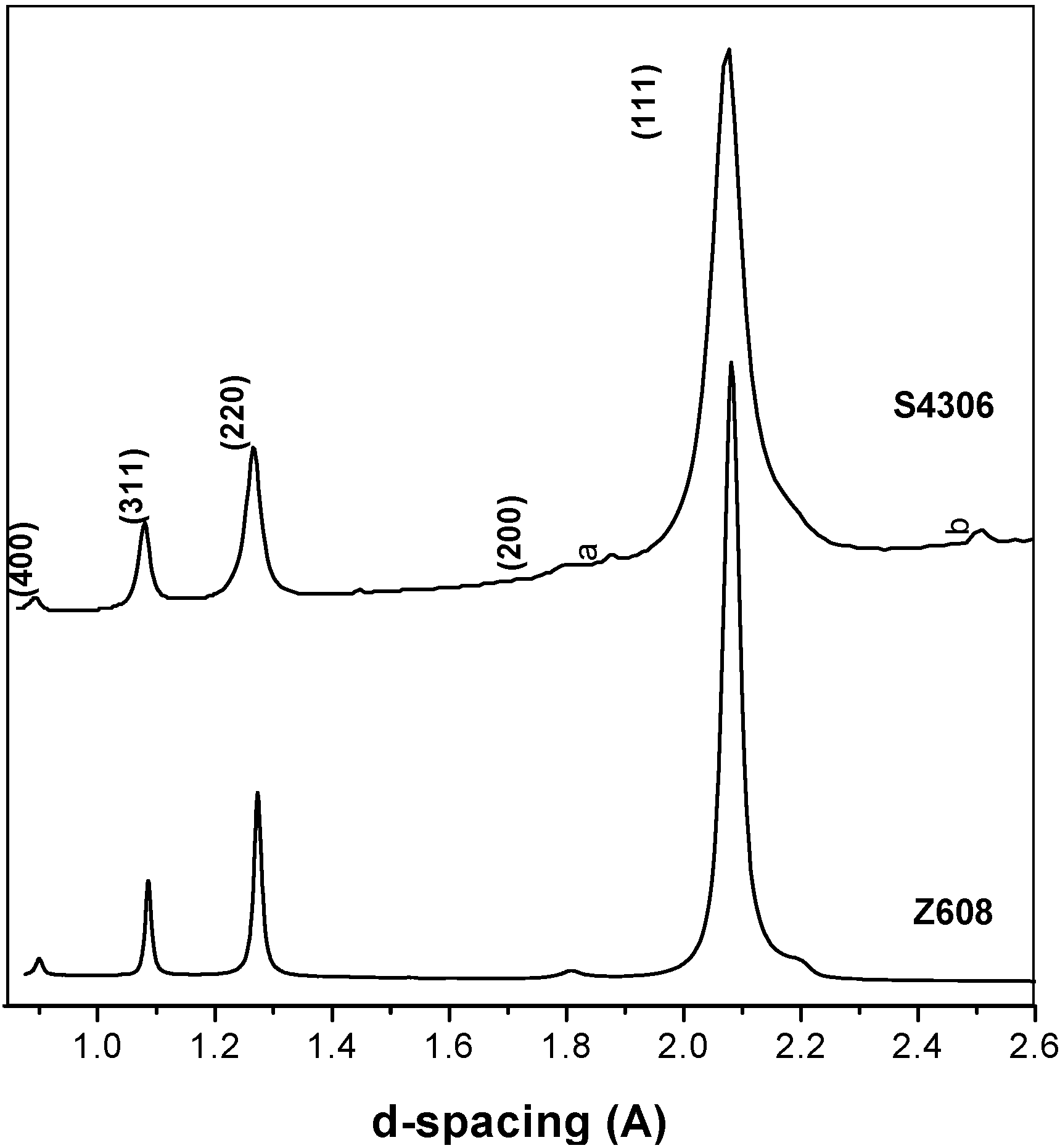
| Sample/Starting material | P [GPa] | T [°C] | Holding time [s] | Products | Lattice parameter [Å] | Method | Reference |
|---|---|---|---|---|---|---|---|
| Z608 | 20 | 2,000 | 60 | Diamond-like phases | 3.6006(3) | MA | Present work |
| S4306 | 20 | 2,000 | 30 | Diamond-like phases | 3.596(6) | MA | Present work |
| S4295 | 20 | 1,920 | 300 | t-BN-C + Diamond-like phases | - | MA | Present work |
| S4294 | 20 | 1,500 | 120 | t-BCN | - | MA | Present work |
| S4311 | 20 | 25 | 7,200 | t-BCN | - | MA | Present work |
| Milled BN/C2 | 20 | 1,927 | 300 | c-BC2N | 3.595(7) | MA | [5] |
| g-BC2N | 25 | 1,820 | 1,800 | c-B0.4C1.1N0.5 | 3.642(2) | MA | [7] |
| g-BC2N | 50 | 3,227 | 10−6 | c-BC2.5N | 3.605 | SC | [27] |
| Milled BN/C2 | 30 | 1,727 | 300–600 | c-(BN)0.67C0.33 | 3.613(3) | LHDAC | [4] |
| g-BC2N | 7.7 | 2,300 | 900 | c-BN, Diamond c-B/C/N mixture | - | BP | [3] |
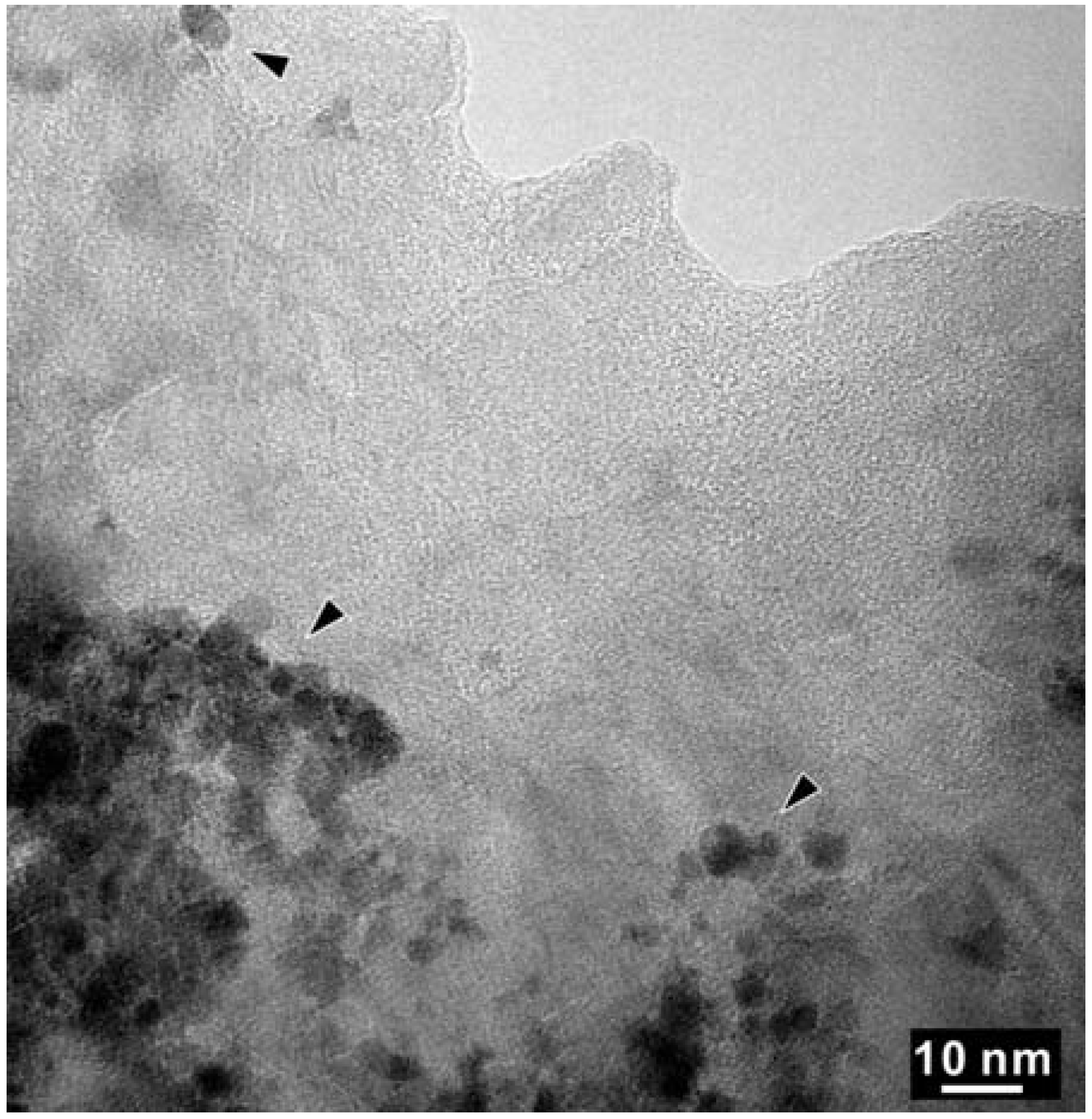
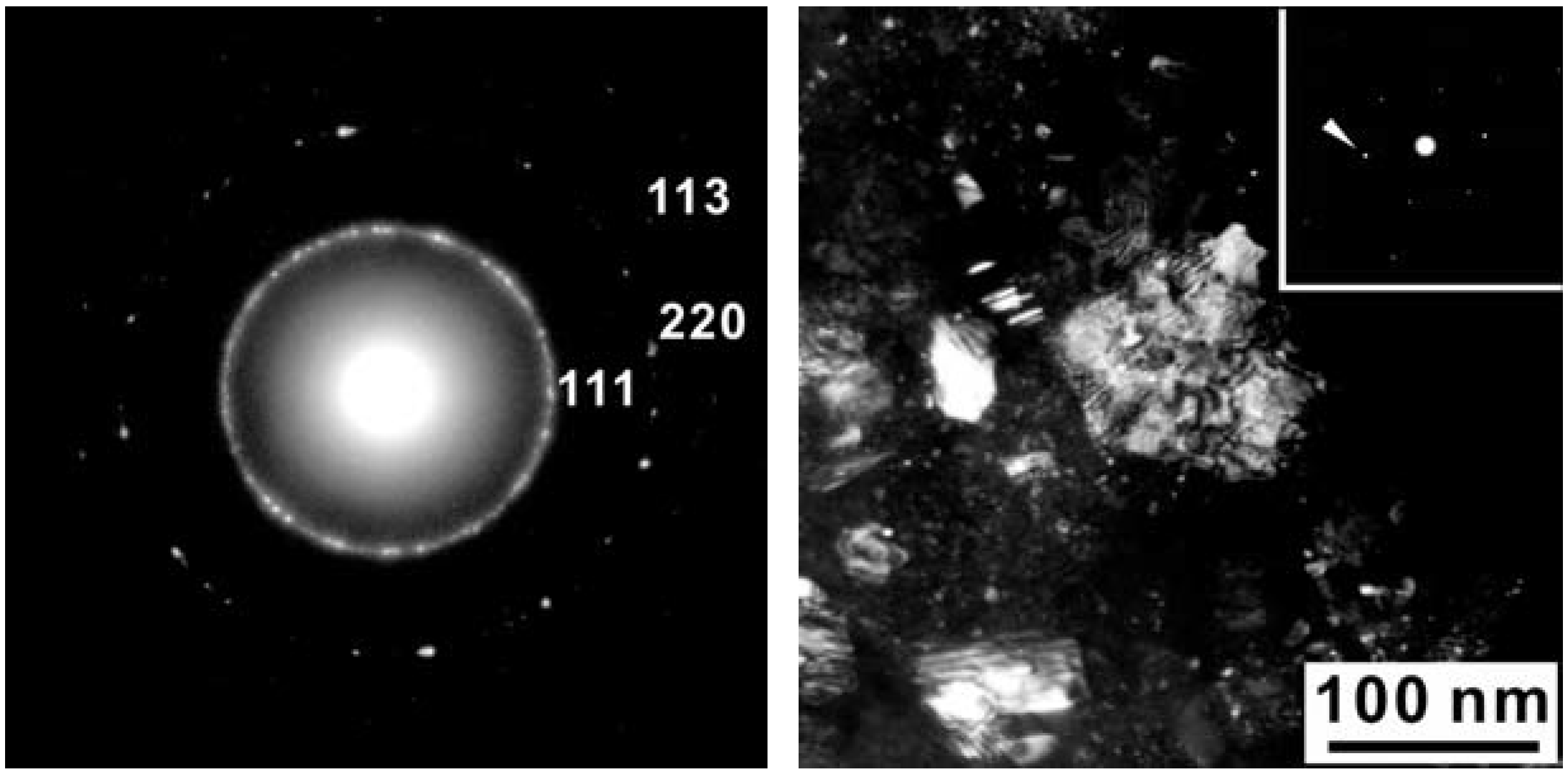
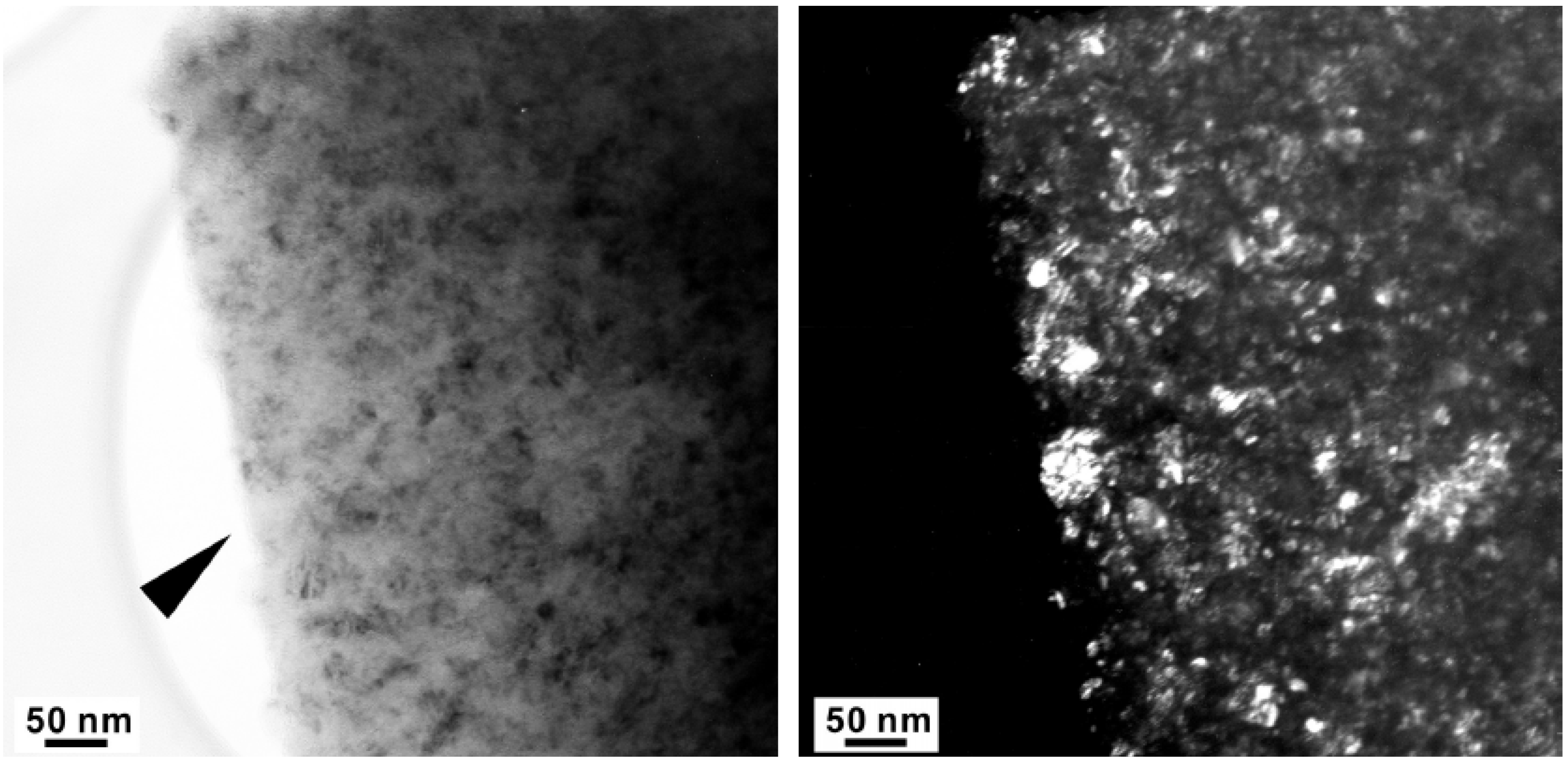

4. Discussion
5. Conclusions
Acknowledgments
References
- Solozhenko, V.L.; Gregoryanz, E. Synthesis of superhard materials. Mater. Today 2005, 8, 44–51. [Google Scholar] [CrossRef]
- Badzian, A.R. Cubic boron nitride-diamond mixed crystals. Mater. Res. Bull. 1981, 16, 1385–1393. [Google Scholar] [CrossRef]
- Nakano, S.; Akaishi, M.; Sasaki, T.; Yamaoka, S. Segregative crystallisation of several diamond-like phases from the graphitic BC2N without an additive at 7.7 GPa. Chem. Mater. 1994, 6, 2246–2251. [Google Scholar] [CrossRef]
- Knittle, E.; Kaner, R.B.; Jeanloz, R.; Cohen, M.L. High-pressure synthesis characterisation, and equation of state of cubic C-BN solid solutions. Phys. Rev. B 1995, 51, 12149–12156. [Google Scholar] [CrossRef]
- Zhao, Y.; He, D.W.; Daemen, L.L.; Shen, T.D.; Schwarz, R.B.; Zhu, Y.; Bish, D.L.; Huang, J.; Zhang, J.; Shen, G.; Qian, J.; Zerda, T.W. Superhard B-C-N materials synthesised in nanostructured bulks. J. Mater. Res. 2002, 17, 3139–3145. [Google Scholar] [CrossRef]
- Komatsu, T.; Nomura, M.; Kakudate, Y.; Fujiwara, S. Synthesis and characterisation of a shock-synthesised cubic B-C-N solid solution of composition BC2.5N. J. Mater. Chem. 1996, 6, 1799–1803. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Dub, S.N.; Novikov, N.V. Mechanical properties of cubic BC2N, a new superhard phase. Diamond Relat. Mater. 2001, 10, 2228–2231. [Google Scholar] [CrossRef]
- Badzian, A.R.; Appenheimer, S.; Niemyski, T.; Olkusnik, E. Graphite-boron nitride solid solutions by chemical vapour deposition. Proc. Int. Conf. Chem. Vap. Deposition 1972, 3, 747–753. [Google Scholar]
- Kaner, R.B.; Kouvetakis, J.; Warble, C.E.; Sattler, M.L.; Bartlett, N. Boron-carbon-nitride materials of graphite-like structure. Mater. Res. Bull. 1987, 22, 399–404. [Google Scholar] [CrossRef]
- Saugnac, F.; Teyssandier, F.; Marchand, A. New compounds obtained by LPCVD in the B-C-N chemical system. J. Phys. IV 1991, 2, 673–680. [Google Scholar]
- Moore, A.W.; Strong, S.L.; Doll, G.L.; Dresselhaus, M.S.; Spain, I.L.; Bowers, C.W.; Issi, J.P.; Piraux, L.J. Properties and characterisation of codeposited boron nitride and carbon materials. J. Appl. Phys. 1989, 65, 5109:1–5109:10. [Google Scholar] [CrossRef]
- Kosinova, M.L.; Runyantsev, Yu.M.; Golubenko, A.N.; Fainer, N.I.; Ayupov, B.M.; Dolgovesova, I.P.; Kolesov, B.A.; Kaichev, V.V.; Kuznetsov, F.A. Chemical composition of boron carbonitride films grown by plasma-enhanced chemical vapour deposition from trimethylamineborane. Inorg. Mater. 2003, 39, 366–373. [Google Scholar] [CrossRef]
- Kasinova, M.L.; Rumyantsev, Yu.M.; Fainer, N.I.; Maximovski, E.A. The structure study of boron carbonitride films obtained by use of trimethylamine borane complex. Nuclear Instr. Methods Phys. Res. A 2001, 470, 253–257. [Google Scholar] [CrossRef]
- Popov, C.; Saito, K.; Ivanov, B.; Koga, Y.; Fujiwara, S.; Shanov, V. Chemical vapour deposition of BC2N films and their laser-induced etching with SF6. Thin Solid Films 1998, 312, 99–105. [Google Scholar] [CrossRef]
- Goto, Y.; Sasaki, M.; Hashizume, M.; Suzuki, M. Synthesis of BCN ceramics from pyrolidine-borane complex. J. Eur. Ceram. Soc. 1999, 19, 2695–2700. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Tang, Y.H.; Lee, C.S.; Bello, I.; Lee, S.T. Nanocrystalline C-BN synthesised by mechanical alloying. Diamond Relat. Mater. 1999, 8, 610–613. [Google Scholar] [CrossRef]
- Luo, X.; Liu, Z.; Guo, X.-J.; He, J.-L.; Yu, D.; Tian, Y.-J.; Sun, J.; Wang, H. Synthesis of B-C-N nanocrystalline particle by mechanical alloying and particle plasma sintering. Chin. Phys. Lett. 2006, 41, 8352–8355. [Google Scholar]
- Torres, R.; Caretti, I.; Gago, R.; Martin, Z.; Jimenez, I. Bonding structure of BCN nanopowders prepared by ball milling. Diamond Relat. Mater. 2007, 16, 1450–1454. [Google Scholar] [CrossRef]
- Xiong, Y.H.; Yang, S.; Xiong, C.S.; Pi, H.L.; Zhang, J.; Ren, Z.M.; Mai, Y.T.; Xu, W.; Dai, G.H.; Song, S.J.; Xiong, J.; Zhang, L.; Xia, Z.C.; Yuan, S.L. Preparation and characterisation of CBN ternary compounds with nano-structure. Physica B 2006, 382, 151–155. [Google Scholar] [CrossRef]
- Filoneko, V.P.; Khabashesku, V.N.; Davydov, V.A.; Zibrov, I.P.; Agafonov, V.N. Synthesis of a new cubic phase in the B-C-N system. Inorg. Mater. 2008, 44, 395–400. [Google Scholar] [CrossRef]
- Riedel, R. From molecules to materials-a novel route for the synthesis of advanced ceramics. Naturwissenschaften 1995, 82, 12–20. [Google Scholar]
- Hubacek, M.; Sato, T. Preparation and properties of a compound in the B-C-N system. J. Solid State Chem. 1995, 114, 258–264. [Google Scholar] [CrossRef]
- Bill, J.; Riedel, R.; Passing, G. Amin-borane als precursoren für borcarbidnitrid. Z. Anorg. Allg. Chem. 1992, 610, 83–90. [Google Scholar] [CrossRef]
- Bill, J.; Frieβ, M.; Riedel, R. Conversion of amine-boranes to boron carbide nitride. Eur. J. Solid State Inorg. Chem. 1992, 29, 195–212. [Google Scholar]
- Riedel, R.; Bill, J.; Passing, G. A novel carbon material derived from pyridine-borane. Adv. Mater. 1991, 3, 551–552. [Google Scholar] [CrossRef]
- Goto, Y.; Sasaki, M.; Mukaida, K. Synthesis of B-N-C ceramics from piperazine-borane complex. Key Eng. Materials 1999, 159-160, 347–352. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tashiro, S.; Sekine, T.; Sato, T. Phase transformation of turbostratic BN by shock compression. Chem. Mater. 1997, 9, 233–236. [Google Scholar] [CrossRef]
- Hammersley, A. Program FIT2D; ESRF: Grenoble, France, 1995. [Google Scholar]
- Larson, A.C.; von Dreele, R.B. GSAS, General Structure Analysis System; LANL: Los Alamos, NM, USA, 1986. [Google Scholar]
- Langenhorst, F.; Solozhenko, V.L. A TEM-EELS study of new diamond-like phases in the B-C-N system. Phys. Chem. Chem. Phys. 2002, 4, 5183–5188. [Google Scholar] [CrossRef]
- Bando, Y.; Nakano, S.; Kurashima, K. A new cubic B-C-N compound revealed by high resolution analytical electron microscopy. J. Electron Microscopy 1996, 45, 135–142. [Google Scholar] [CrossRef]
- Dubrovinskaia, N.; Solozhenko, V.L.; Miyajima, N.; Dmitriev, V.; Kurakevych, O.; Dubrovinsky, L. Superhard nanocomposite of dense polymorphs of boron nitride: Noncarbon material has reached diamond hardness. Appl. Phys. Lett. 2007, 90, 1912–1912. [Google Scholar] [CrossRef]
- Irifune, T.; Kurio, A.; Sakamoto, S.; Inoue, T.; Sumiya, H. Ultrahard polycrystalline diamond from graphite. Nature 2003, 421, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Dubrovinskaia, N.; Dubrovinsky, L.; Crichton, W.; Langenhorst, F.; Richter, A. Aggregated diamond nanorods, the densest and least compressible form of carbon. Appl. Phys. Lett. 2005, 87, 083106:1–083106:3. [Google Scholar] [CrossRef]
- Dubrovinskaia, N.; Wirth, R.; Wosnitza, J.; Papageorgiou, T.; Braun, H.F.; Miyajima, N.; Dubrovinsky, L. An insight into what superconducts in polycrystalline boron-doped diamonds based on investigations of microstructure. Proc. Natl. Acad. Sci. USA 2008, 105, 11619–11622. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Matizamhuka, W.R.; Sigalas, I.; Herrmann, M.; Dubronvinsky, L.; Dubrovinskaia, N.; Miyajima, N.; Mera, G.; Riedel, R. Characterization of the Materials Synthesized by High Pressure-High Temperature Treatment of a Polymer Derived t-BC2N Ceramic. Materials 2011, 4, 2061-2072. https://doi.org/10.3390/ma4122061
Matizamhuka WR, Sigalas I, Herrmann M, Dubronvinsky L, Dubrovinskaia N, Miyajima N, Mera G, Riedel R. Characterization of the Materials Synthesized by High Pressure-High Temperature Treatment of a Polymer Derived t-BC2N Ceramic. Materials. 2011; 4(12):2061-2072. https://doi.org/10.3390/ma4122061
Chicago/Turabian StyleMatizamhuka, Wallace R., Iakovos Sigalas, Mathias Herrmann, Leonid Dubronvinsky, Natalia Dubrovinskaia, Nobuyoshi Miyajima, Gabriela Mera, and Ralf Riedel. 2011. "Characterization of the Materials Synthesized by High Pressure-High Temperature Treatment of a Polymer Derived t-BC2N Ceramic" Materials 4, no. 12: 2061-2072. https://doi.org/10.3390/ma4122061





