Non-Isothermal Kinetic Analysis of the Crystallization of Metallic Glasses Using the Master Curve Method
Abstract
:1. Introduction
2. Theoretical Basis
2.1. KJMA Constant Activation Energy Model
2.2. Master Curve Method
2.3. Transformation Rate Curves and Calorimetric Experiments
3. Case Studies
3.1. Cu-Based Metallic Glasses
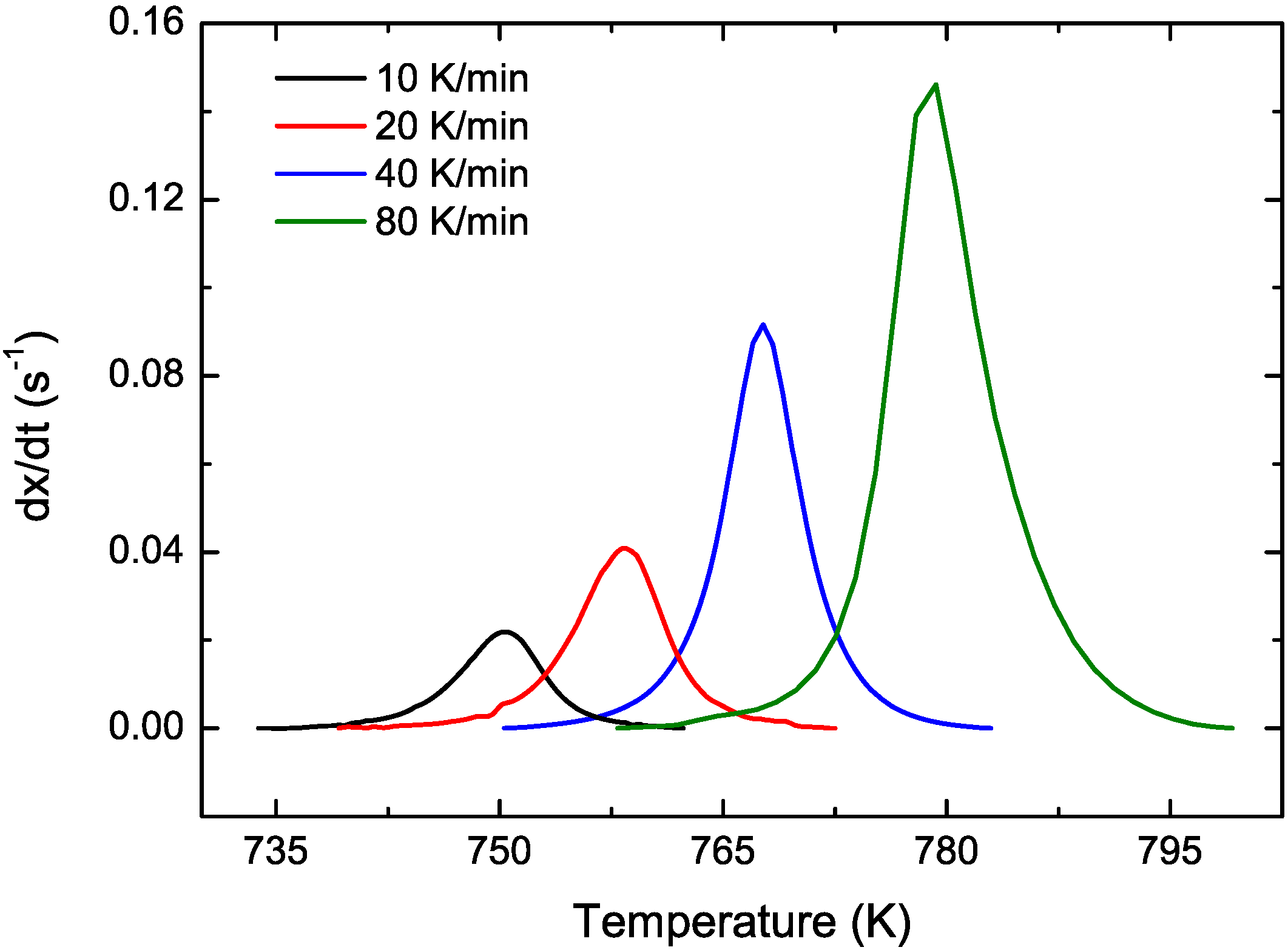
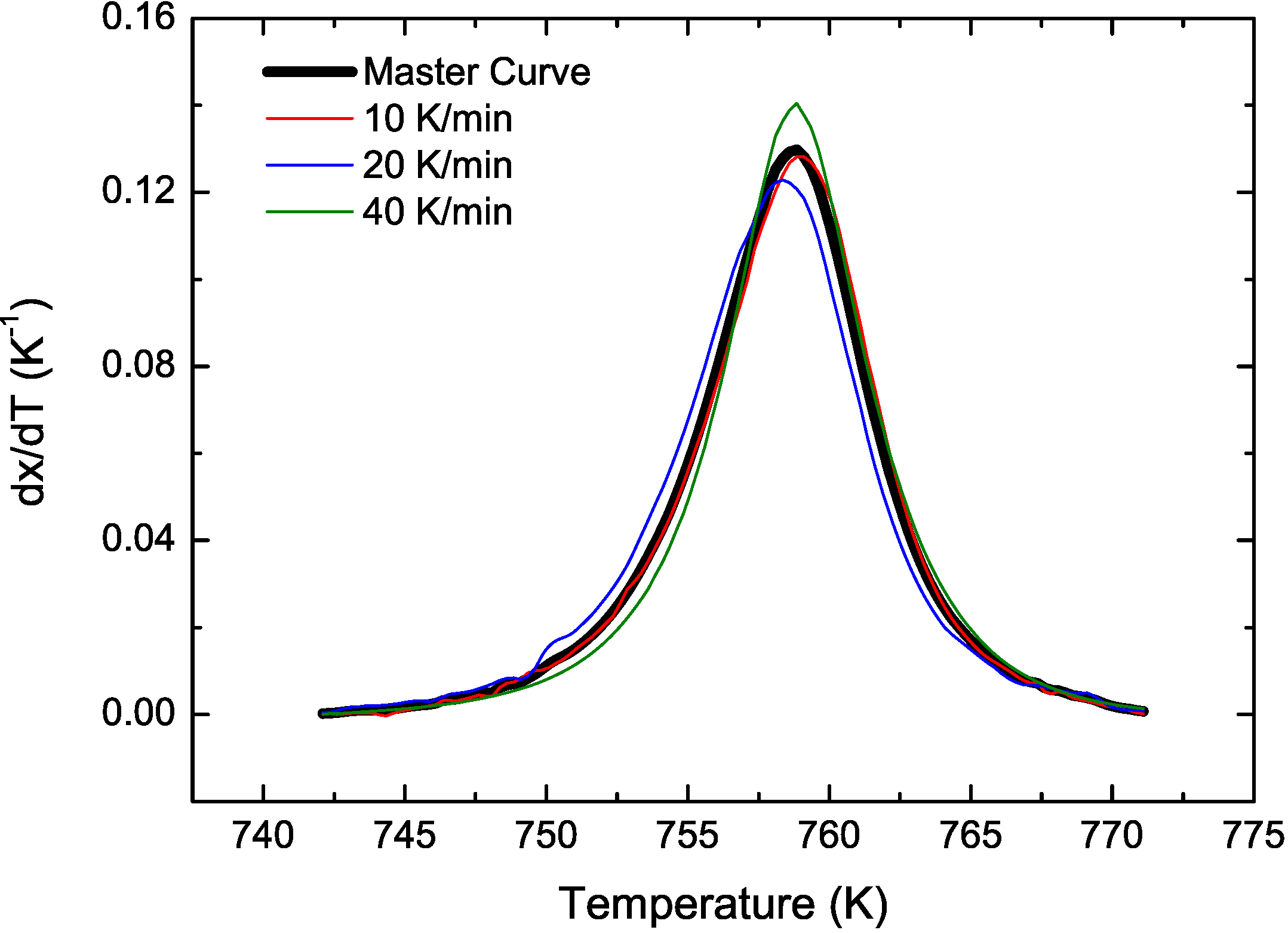
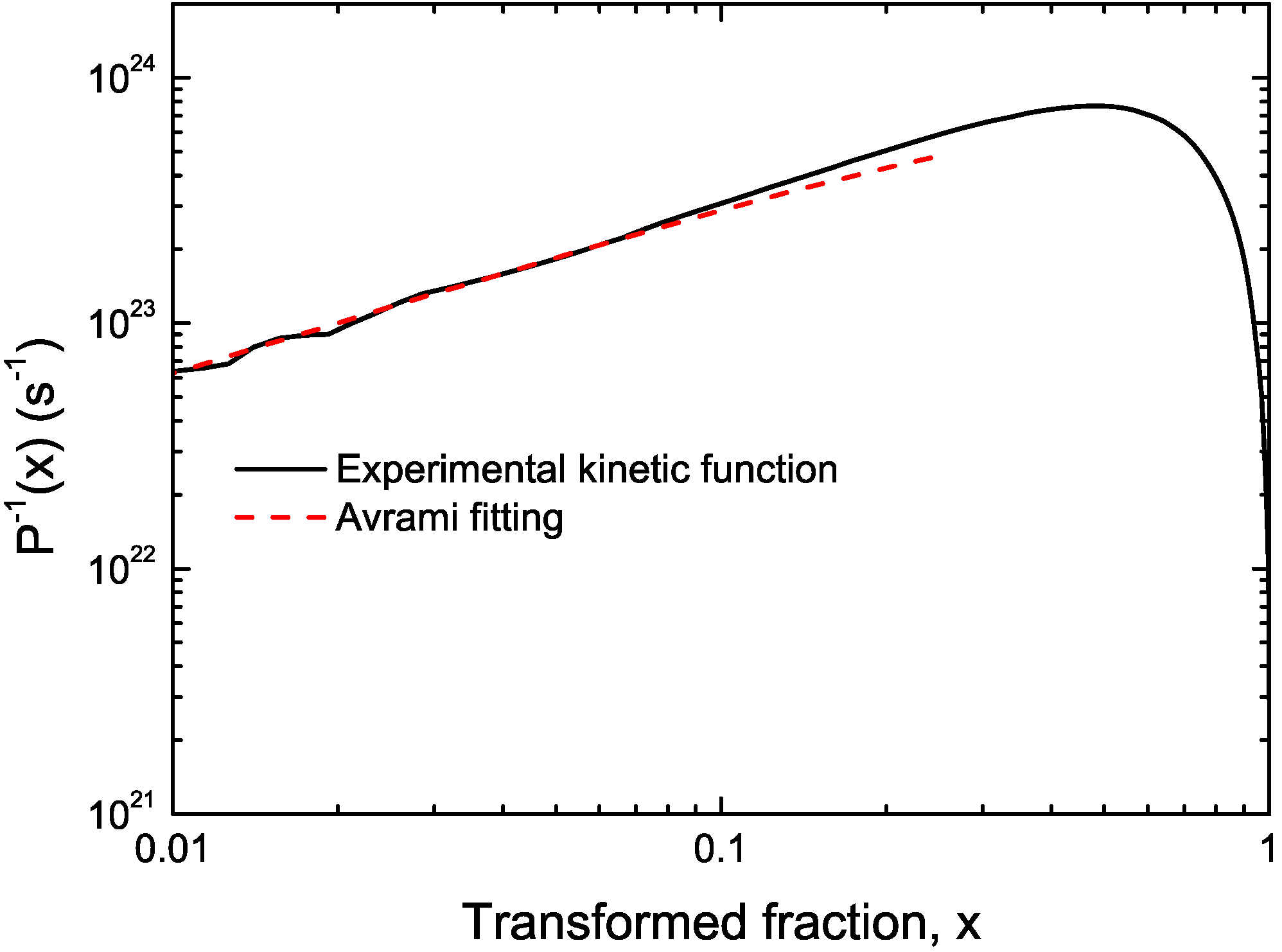
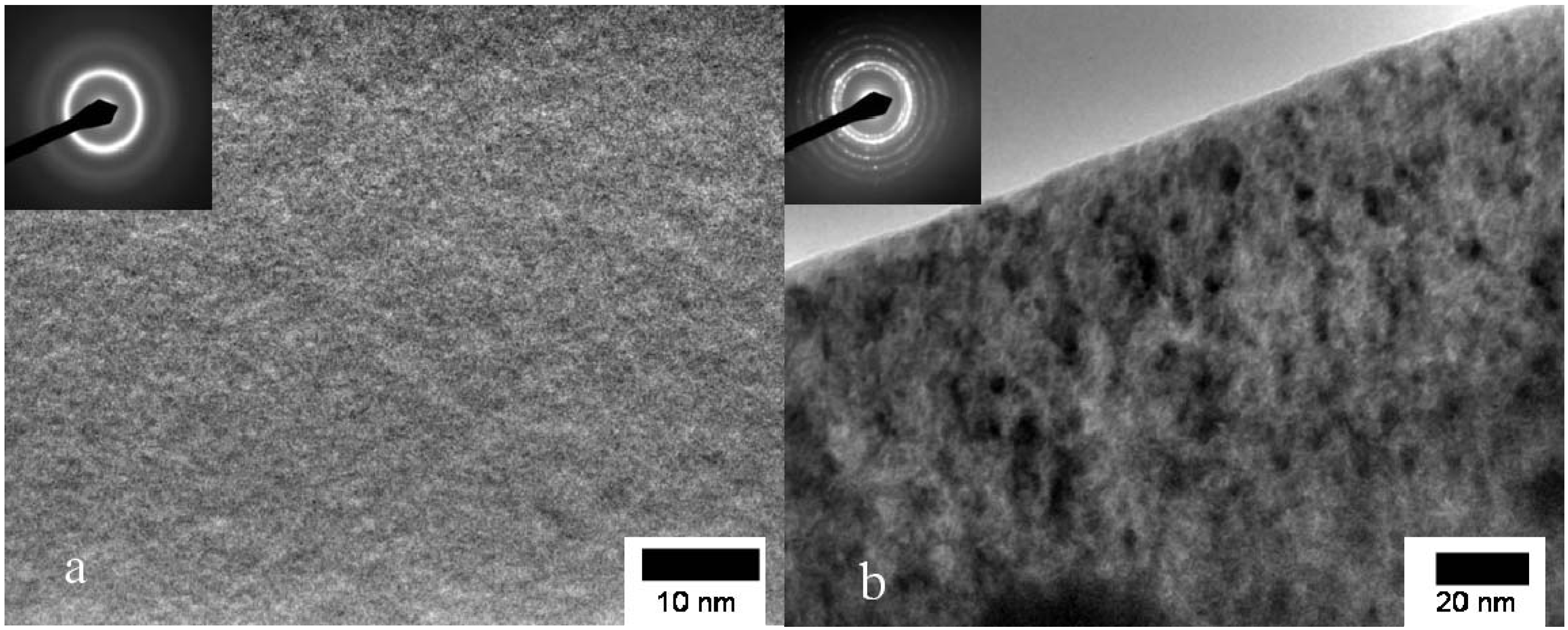
3.2. Cu Containing Fe-Based Alloy
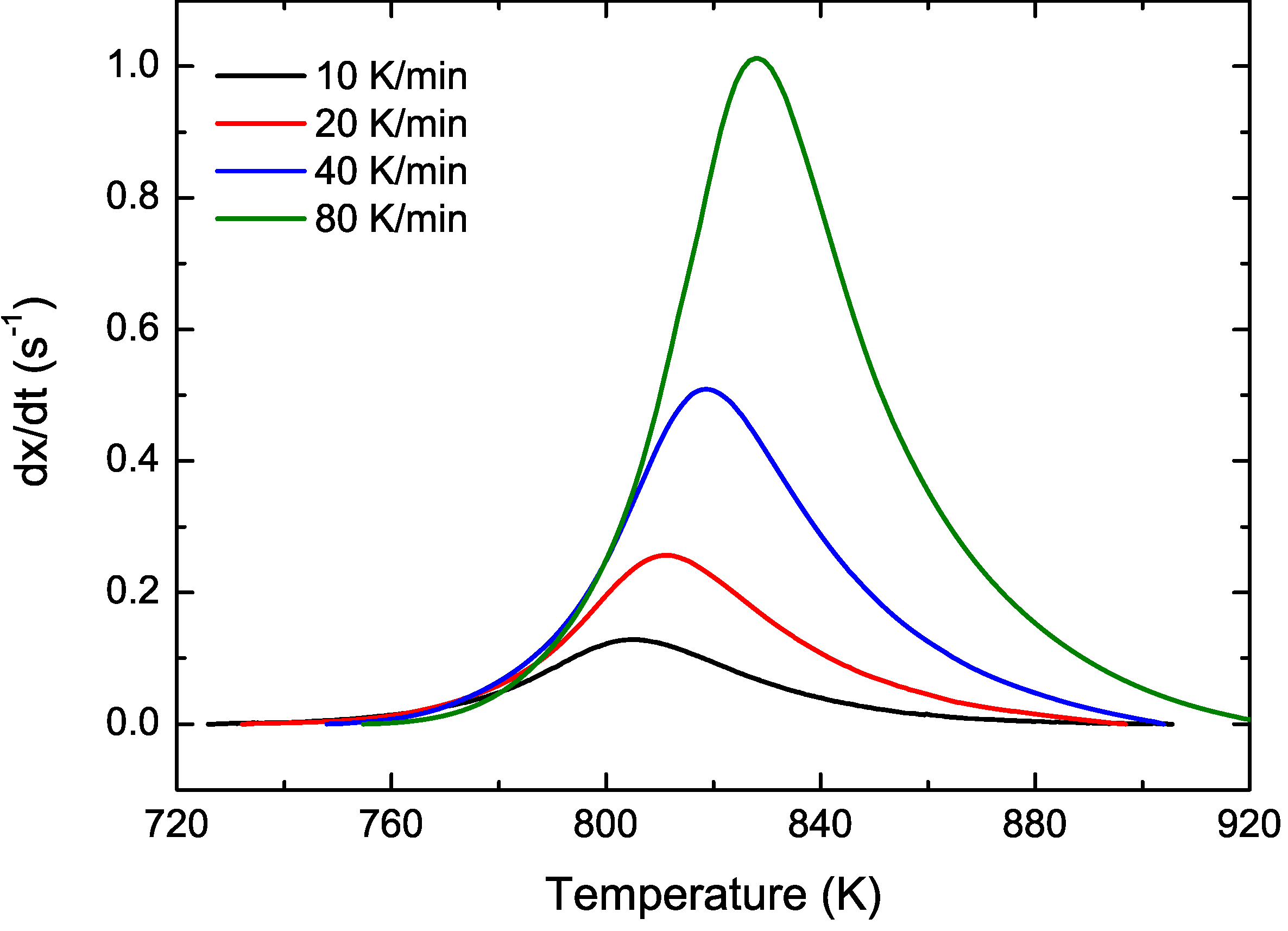
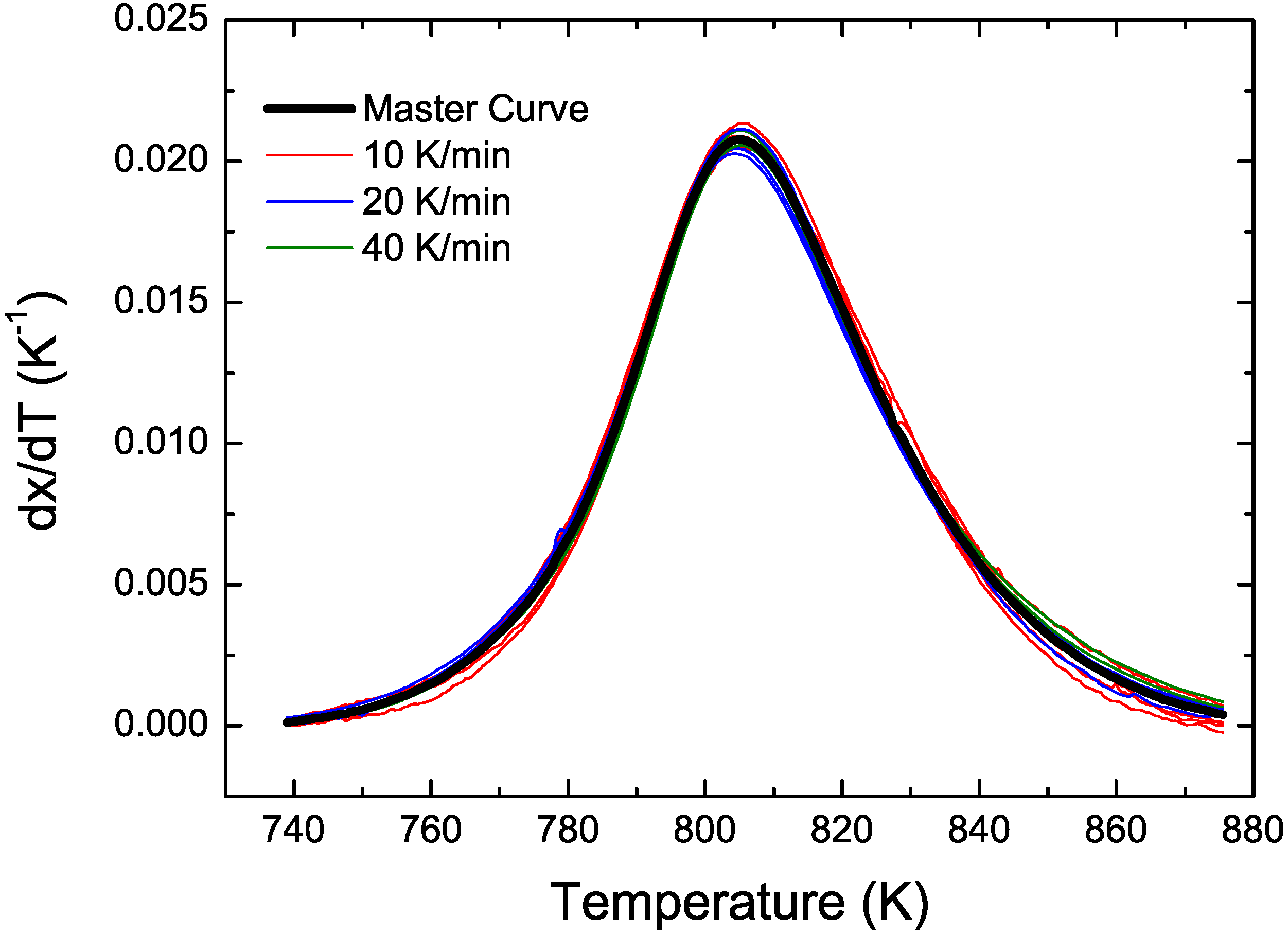
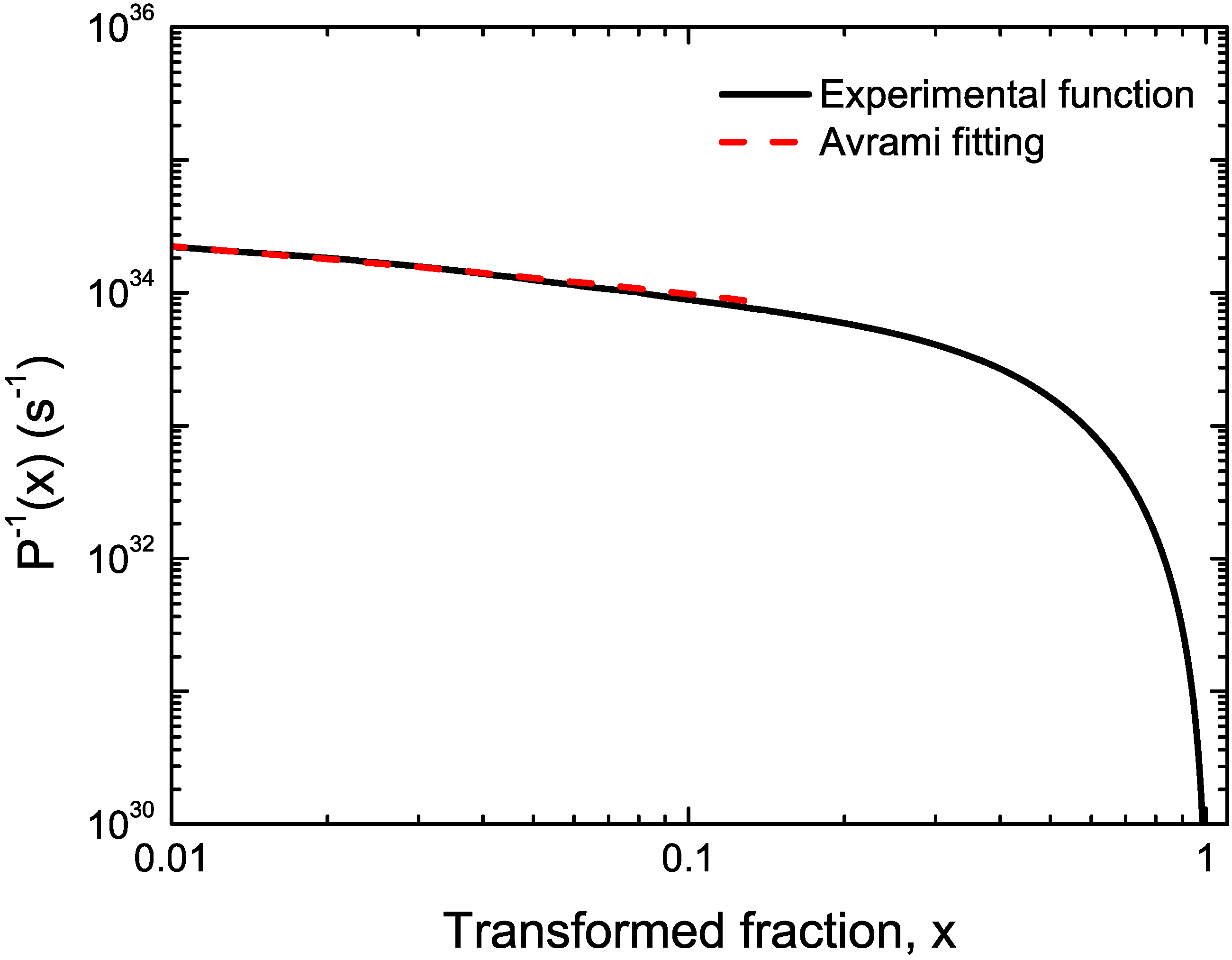
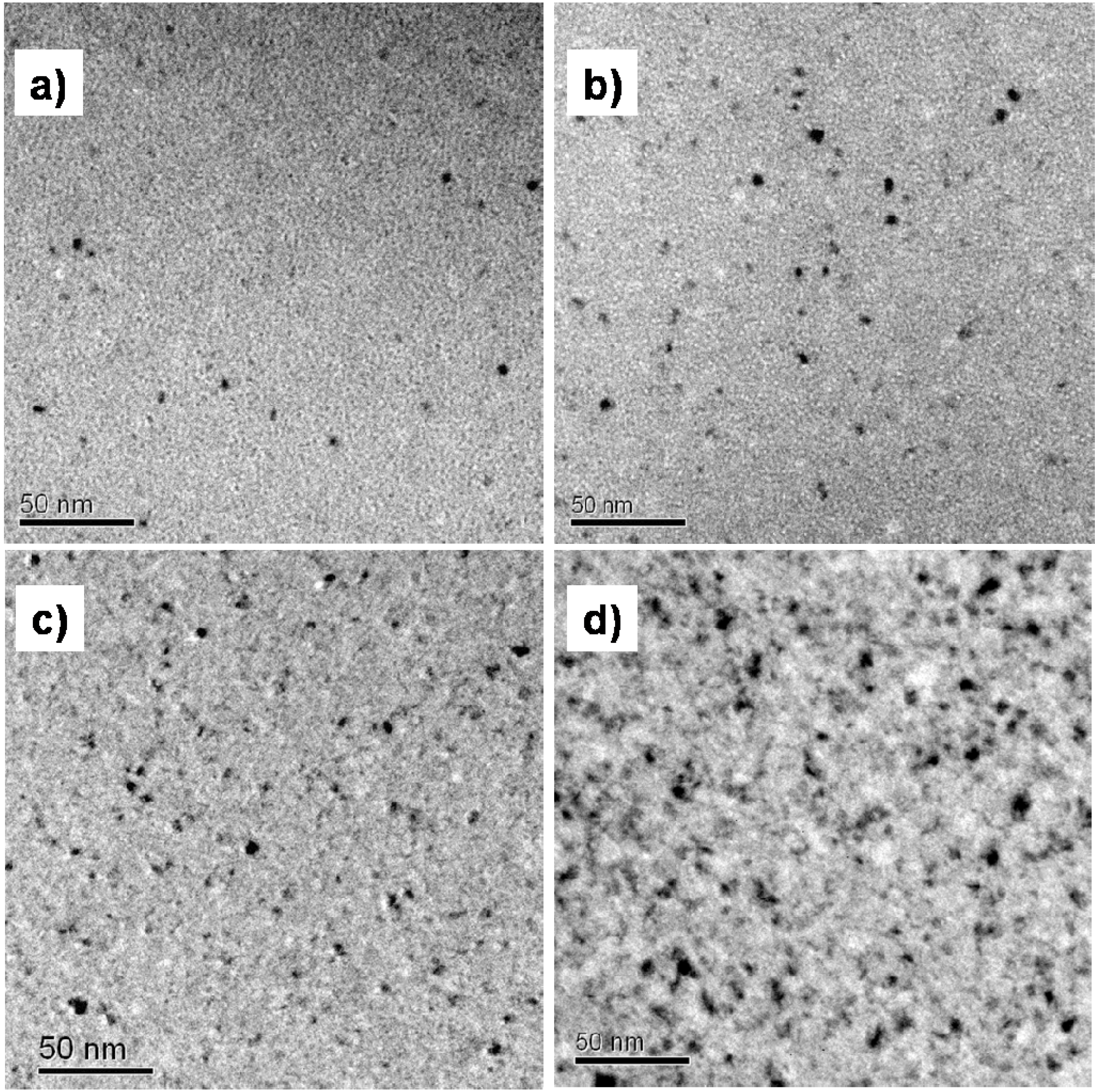
4. Conclusions
Acknowledgments
References
- Avrami, M. Kinetics of phase change. I. General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II. Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, phase change and microstructure. Kinetics of phase change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Johnson, W.; Mehl, K. Reaction kinetics in processes of nucleation and growth. Trans. Am. Inst. Min. Met. Eng. 1939, 195, 416–458. [Google Scholar]
- Kolmogorov, A. Static theory of metals crystallization. Izv. Akad. Nauk SSSR. Ser. Mater. 1937, 1, 355–359. [Google Scholar]
- Christian, J. The Theory of Transformations in Metals and Alloys, 2nd ed.; Materials Science and Technology, Pergamon Press: Oxford, UK, 1981. [Google Scholar]
- Xu, D.; Johnson, W.L. Crystallization kinetics and glass-forming ability of bulk metallic glasses Pd40Cu30Ni10P20 and Zr41.2Ti13.8Cu12.5Ni10Be22.5 from classical theory. Phys. Rev. B 2006, 74, 024207:1–024207:5. [Google Scholar]
- Turnbull, D. Under what conditions can a glass be formed? Contemp. Phys. 1969, 10, 473–488. [Google Scholar] [CrossRef]
- Uhlmann, D.R. A kinetic treatment of glass formation. J. Non Cryst. Solids 1972, 7, 337–348. [Google Scholar] [CrossRef]
- Liu, F.; Sommer, F.; Bos, C.; Mittemeijer, E.J. Analysis of solid state phase transformation kinetics: Models and recipes. Int. Mater. Rev. 2007, 25, 193–212. [Google Scholar] [CrossRef]
- Greer, A.L. Crystallisation kinetics of Fe80B20 glass. Acta Metall. 1982, 30, 171–192. [Google Scholar] [CrossRef]
- Perez-Maqueda, L.A.; Criado, J.M.; Malek, J. Combined kinetic analysis for crystallization kinetics of non-crystalline solids. J. Non Cryst. Solids 2003, 320, 84–91. [Google Scholar] [CrossRef]
- Weinberg, M.C. The use of site saturation and Arrhenius assumptions in the interpretation of non-isothermal DTA/DSC crystallization experiments. Thermochim. Acta 1992, 194, 93–107. [Google Scholar] [CrossRef]
- Clavaguera-Mora, M.T.; Clavaguera, N.; Crespo, D.; Pradell, T. Crystallisation kinetics and microstructure development in metallic systems. Progr. Mater. Sci. 2002, 47, 559–619. [Google Scholar] [CrossRef]
- Gao, X.; Chen, D.; Dollimore, D. The correlation between the value of α at the maximum reaction rate and the reaction mechanisms: A theoretical study. Thermochim. Acta 1993, 223, 75–82. [Google Scholar] [CrossRef]
- Cahn, J. Transformation kinetics during continuous cooling. Acta Metall. 1956, 4, 572–575. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Z.R.; Aust, K.T.; Erb, U. Isokinetic analysis of nanocrystalline nickel electrodeposits upon annealing. Acta Mater. 1997, 45, 1655–1669. [Google Scholar] [CrossRef]
- Chen, L.C.; Spaepen, F. Analysis of calorimetric measurements of grain growth. J. Appl. Phys. 1991, 69, 679–688. [Google Scholar] [CrossRef]
- Starink, M.J.; Zahra, A.-M. Determination of the transformation exponent s from experiments at constant heating rate. Thermochim. Acta 1997, 298, 179–189. [Google Scholar] [CrossRef]
- Kissinger, H. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Farjas, J.; Roura, P. Modification of the Kolmogorov-Johnson-Mehl-Avrami rate equation for non-isothermal experiments and its analytical solution. Acta Mater. 2006, 54, 5573–5579. [Google Scholar] [CrossRef]
- Jacovkis, D.; Xiao, Y.; Rodriguez-Viejo, J.; Clavaguera-Mora, M.T.; Clavaguera, N. Mechanisms driving primary crystallization of Al87Ni7Cu3Nd3 amorphous alloy. Acta Mater. 2004, 52, 2819–2826. [Google Scholar] [CrossRef]
- Jacovkis, D.; Rodriguez-Viejo, J.; Clavaguera-Mora, M.T. Isokinetic analysis of nanocrystallization in an Al-Nd-Ni amorphous alloy. J. Phys. Condens. Matter. 2005, 17, 4897–4910. [Google Scholar] [CrossRef]
- Fontana, M.; Arcondo, B.; Clavaguera-Mora, M.T.; Clavaguera, N. Mechanisms controlling primary crystallisation of Ga20Te80 glasses. J. Non Cryst. Solids 2007, 353, 2131–2142. [Google Scholar] [CrossRef]
- Hemminger, W.F.; Sarge, S.M. The baseline construction and its influence on the measurement of heat with differential scanning calorimeters. J. Therm. Anal. Calorim. 1991, 37, 1455–1477. [Google Scholar] [CrossRef]
- Clavaguera-Mora, M.T.; Clavaguera, N.; Rodriguez-Viejo, J. Primary transformation rate measurements through differential scanning calorimetry. Monatsch. Chem. 2005, 136, 1947–1953. [Google Scholar] [CrossRef]
- Kempen, A.T.W.; Sommer, F.; Mittemeijer, E.J. The isothermal and isochronal kinetics of the crystallisation of bulk amorphous Pd40Cu30P20Ni10. Acta Mater. 2002, 50, 1319–1329. [Google Scholar] [CrossRef]
- Clavaguera, N.; Clavaguera-Mora, M.; Fontana, M. Accuracy in the experimental calorimetric study of the crystallization kinetics and predictive transformation diagrams: Application to a Ga-Te amorphous alloy. J. Mater. Res. 1998, 13, 744–753. [Google Scholar] [CrossRef]
- Lin, X.H.; Johnson, W.L. Formation of Ti-Zr-Cu-Ni bulk metallic glasses. J. Appl. Phys. 1995, 78, 6514–6519. [Google Scholar] [CrossRef]
- Calin, M.; Stoica, M.; Eckert, J.; Yavari, A.R.; Schultz, L. Glass formation and crystallization of Cu47Ti33Zr11Ni8X1 (X = Fe, Si, Sn, Pb) alloys. Mater. Sci. Eng. A 2005, 392, 169–178. [Google Scholar] [CrossRef]
- Calin, M.; Stoica, M.; Eckert, J.; Yavari, A.R.; Schultz, L. Phase formation, thermal stability and crystallization behavior of Cu47Ti33Zr11Ni8X1 (X = Fe, Si, Sn, Pb) bulk glassy alloys. Z. Metallkd. 2004, 95, 970–977. [Google Scholar]
- Venkataraman, S.; Hermann, H.; Mickel, C.; Schultz, L.; Sordelet, D.J.; Eckert, J. Calorimetric study of the crystallization kinetics of Cu47Ti33Zr11Ni8Si1 metallic glass. Phys. Rev. B 2007, 75, 104206:1–104206:9. [Google Scholar] [CrossRef]
- Venkataraman, S.; Löser, W.; Eckert, J.; Gemming, T.; Mickel, C.; Schubert-Bischoff, P.; Wanderka, N.; Schultz, L.; Sordelet, D.J. Nanocrystal development in Cu47Ti33Zr11Ni8Si1 metallic glass powders. J. Alloys Compd. 2006, 415, 162–169. [Google Scholar] [CrossRef]
- Torrens-Serra, J.; Bruna, P.; Rodriguez-Viejo, J.; Roth, S.; Clavaguera-Mora, M.T. Effect of minor Co additions on the crystallization and magnetic properties of Fe(Co)NbBCu alloys. J. Alloys Compd. 2010, 496, 202–207. [Google Scholar] [CrossRef]
- Torrens-Serra, J.; Peral, I.; Rodriguez-Viejo, J.; Clavaguera-Mora, M.T. Microstructure evolution and grain size distribution in nanocrystalline FeNbBCu from synchrotron XRD and TEM analysis. J. Non Cryst. Solids 2012, 358, 107–113. [Google Scholar] [CrossRef]
- Blázquez, J.S.; Franco, V.; Conde, C.F.; Millan, M.; Conde, A. Instantaneous growth approximation describing the nanocrystallization process of amorphous alloys: A cellular automata model. J. Non Cryst. Solids 2008, 354, 3597–3605. [Google Scholar] [CrossRef]
- Inoue, A.; Shen, B.L.; Chang, C.T. Fe- and Co-based bulk glassy alloys with ultrahigh strength of over 4000 MPa. Intermetallics 2006, 14, 936–944. [Google Scholar] [CrossRef]
- Imafuku, M.; Sato, S.; Koshiba, H.; Matsubara, E.; Inoue, A. Structural variation of Fe-Nb-B metallic glasses during crystallization process. Scr. Mater. 2001, 44, 2369–2372. [Google Scholar] [CrossRef]
- Shapaan, M.; Lábár, J.; Lendvai, L.; Varga, L.K. Crystallization behavior of Fe62Nb8−xZrxB30 bulk amorphous alloy. Mater. Sci. Eng. A 2004, 375–377, 789–793. [Google Scholar]
- Stoica, M.; Li, R.; Yavari, A.R.; Vaughanc, G.; Eckert, J.; van Steenberge, N.; Ruiz Romera, D. Thermal stability and magnetic properties of FeCoBSiNb bulk metallic glasses. J. Alloys Compd. 2010, 504S, S123–S128. [Google Scholar] [CrossRef]
- Torrens-Serra, J.; Rodríguez-Viejo, J.; Clavaguera-Mora, M.T. Influence of composition in the crystallization process of Fe75−xNb10B15+x metallic glasses. J. Non Cryst. Solids 2007, 353, 842–844. [Google Scholar] [CrossRef]
- Torrens-Serra, J.; Rodriguez-Viejo, J.; Clavaguera-Mora, M.T. Nanocrystallization kinetics and glass forming ability of the Fe65Nb10B25 metallic alloy. Phys. Rev. B 2007, 76, 214111:1–214111:12. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Torrens-Serra, J.; Venkataraman, S.; Stoica, M.; Kuehn, U.; Roth, S.; Eckert, J. Non-Isothermal Kinetic Analysis of the Crystallization of Metallic Glasses Using the Master Curve Method. Materials 2011, 4, 2231-2243. https://doi.org/10.3390/ma4122231
Torrens-Serra J, Venkataraman S, Stoica M, Kuehn U, Roth S, Eckert J. Non-Isothermal Kinetic Analysis of the Crystallization of Metallic Glasses Using the Master Curve Method. Materials. 2011; 4(12):2231-2243. https://doi.org/10.3390/ma4122231
Chicago/Turabian StyleTorrens-Serra, Joan, Shankar Venkataraman, Mihai Stoica, Uta Kuehn, Stefan Roth, and Jürgen Eckert. 2011. "Non-Isothermal Kinetic Analysis of the Crystallization of Metallic Glasses Using the Master Curve Method" Materials 4, no. 12: 2231-2243. https://doi.org/10.3390/ma4122231



