2.1. Measurement Types
Two types of measurements are generally employed to report the ability of materials to inhibit cell growth. The first is the growth inhibition, GI, value. Generally the concentration at which 50% growth inhibition occurs, GI50, is reported. The second value is the chemotherapeutic index (CI) value. The CI is employed to compare the toxicity of a drug on normal cell lines (or other “base-line” cell line) to its toxicity to a cancer (or second) cell line. The CI50 is the GI50 drug concentration for the normal (or base line) cell line divided by the GI50 drug concentration for the cancer cell line. Values in excess of two are generally considered significant.
To gain a better picture of the relationship between polymer and possible preference for one cell line over the other one additional column have been added to each table. For the GI50 values, a column noting the ratio of the GI50 for the MDA cell line divided by the GI50 for the MCF-7 cell line, MDA/MCF-7, has been added. Here, values greater than one are consistent with lower concentrations of drug inhibiting the growth of the MCF-7 cells compared to the MDA cells. Values less than one are consistent with inhibition of the MDA cells occurring at lower concentrations than for the MCF-7 cells. When describing the CI some researchers employ the similar terms ED or effective dose in place of the GI value for the cancer cells and LD or lethal dose in place of GI for the healthy cell line, here the WI-38 cell line (strain line ATCC CCL-75). Thus, CI50 = LD50/ED50. For the CI50 values the added column contains the CI50 for the MDA cell line divided by the CI50 for the MCF-7 cell line, CI-MDA/CI-MCF-7. Here, values greater than one are consistent with inhibition of the MDA cells occurring at lower concentrations than for the MCF-7 cells. And, values less than one are consistent with lower concentrations of drug inhibiting the growth of the MCF-7 cells compared to the MDA cells. The values contained in the added columns should be related since the CI values are “normalized” with the same WI-38 cell line values.
2.2. Aliphatic-Derived Organotin Polyethers
Table 1.
GI50 concentrations (micrograms/mL) for organotin polyethers from ethylene glycols and methylene diols for MDA and MCF-7 cell lines.
Table 1.
GI50 concentrations (micrograms/mL) for organotin polyethers from ethylene glycols and methylene diols for MDA and MCF-7 cell lines.
| Sample | Cell Line |
|---|
| WI-38 | MDA | MCF-7 | MDA/MCF-7 |
|---|
| Bu2Sn/Ethylene glycol | 0.90(.10) | 0.30(.023) | 0.60(.05) | 0.50 |
| Bu2Sn/Diethylene glycol | 1.20(.10) | 1.20(.10) | 1.20(.10) | 1.0 |
| Bu2Sn/Triethylene glycol | 1.10(.10) | 1.20(.10) | 1.20(.11) | 1.0 |
| Bu2Sn/Pentaethylene glycol | 0.05(.01) | 0.90(.01) | 1.20(.01) | 0.75 |
| Bu2Sn/PEG(400),DMSO | 3.50(.29) | 1.90(.16) | 2.80(.22) | 0.68 |
| Bu2Sn/PEG(400),H2O | 0.28(.03) | 2.40(.22) | 1.40(.10) | 1.7 |
| Bu2Sn/PEG(8000),DMSO | 0.11(.01) | 3.20(.29) | 3.20(.30) | 1.0 |
| Bu2Sn/PEG(8000),H2O | 1.00(.10) | 10.00(.93) | 10.00(.96) | 1.0 |
| Bu2Sn/PEG(10000),DMSO | 4.20(.33) | 10.00(.89) | 5.80(.47) | 1.7 |
| Bu2Sn/PEG(10000),H2O | 1.00(.10) | 10.00(.97) | 10.00(1.0) | 1.0 |
| Bu2Sn/Ethylene glycol | 0.90(.10) | 0.30(.02) | 0.60(.05) | 0.50 |
| Bu2Sn/1,3-Propanediol | 0.05(.01) | 0.90(.10) | 1.1(0.10) | 0.82 |
| Bu2Sn/1,4-Butanediol | 0.06(.01) | 0.22(.02) | 0.15(.02) | 1.5 |
| Bu2Sn/1,5-Pentanediol | 0.05(.01) | 0.09(.04) | 0.20(.01) | 0.45 |
| Bu2Sn/1,6-Hexanediol | 0.05(.01) | 0.35(.04) | 0.22(.02) | 1.6 |
| Bu2Sn/1,7-Heptanediol | 0.04(.01) | 0.10(.01) | 0.20(.01) | 0.50 |
| Bu2Sn/1,8-Octanediol | 0.02(.01) | 0.09(.01) | 0.22(.03) | 0.41 |
Table 2.
Chemotherapeutic Index-50% for the organotin polyethers from ethylene glycols and methylene diols for MDA and MCF-7 cell lines.
Table 2.
Chemotherapeutic Index-50% for the organotin polyethers from ethylene glycols and methylene diols for MDA and MCF-7 cell lines.
| Sample | Cell Line |
|---|
| WI-38/ | WI-38/ | WI-38/ | CI-MDA/ |
|---|
| WI-38 | MDA | MCF-7 | CI-MCF-7 |
|---|
| Bu2Sn/Ethylene glycol | 1.0 | 3.0 | 1.5 | 2.0 |
| Bu2Sn/Diethylene glycol | 1.0 | 1.0 | 1.0 | 1.0 |
| Bu2Sn/Triethylene glycol | 1.0 | 0.92 | 0.92 | 1.0 |
| Bu2Sn/Pentaethylene glycol | 1.0 | 0.06 | 0.04 | 1.5 |
| Bu2Sn/PEG(400),DMSO | 1.0 | 1.8 | 1.3 | 1.4 |
| Bu2Sn/PEG(400),H2O | 1.0 | 0.12 | 0.20 | 0.60 |
| Bu2Sn/PEG(8000),DMSO | 1.0 | 0.03 | 0.03 | 1.0 |
| Bu2Sn/PEG(8000),H2O | 1.0 | 0.10 | 0.10 | 1.0 |
| Bu2Sn/PEG(10000),DMSO | 1.0 | 0.42 | 0.72 | 0.58 |
| Bu2Sn/PEG(10000),H2O | 1.0 | 0.10 | 0.10 | 1.0 |
| Bu2Sn/Ethylene glycol | 1.0 | 21 | 5.2 | 4.0 |
| Bu2Sn/1,3-Propanediol | 1.0 | 0.43 | 0.17 | 2.5 |
| Bu2Sn/1,4-Butanediol | 1.0 | 1.6 | 1.1 | 1.5 |
| Bu2Sn/1,5-Pentanediol | 1.0 | 4.0 | 0.86 | 4.7 |
| Bu2Sn/1,6-Hexanediol | 1.0 | 1.0 | 0.79 | 1.3 |
| Bu2Sn/1,7-Heptanediol | 1.0 | 3.6 | 0.86 | 4.2 |
| Bu2Sn/1,8-Octanediol | 1.0 | 1.6 | 0.31 | 5.2 |
Figure 1.
Repeat unit for the polymers derived from the reaction of organotin dihalides with various ethylene glycols (left) and with methylene oxide diols (right) where R is butyl and R1 represents simple chain extension.
Figure 1.
Repeat unit for the polymers derived from the reaction of organotin dihalides with various ethylene glycols (left) and with methylene oxide diols (right) where R is butyl and R1 represents simple chain extension.
Characterization of these materials has been described [
11,
33]. The values of GI
50 MDA/ GI
50 MCF-7 as well as the corresponding CI
50 MDA/ CI
50 MCF-7 values are around one consistent with the organotin polyethers not having a marked preference for either cell line. The aliphatic diols themselves do not exhibit cancer cell inhibition. The values of GI
50 MDA/ GI
50 MCF-7 are about one (average = 0.95) and are consistent with the organotin polyethers not having a preference for either cell line. The corresponding CI
50 MDA/ CI
50 MCF-7 values are around two (average = 2.0) consistent with the organotin polyethers having some preference for inhibiting the non-estrogen MDA cell line at lower concentration of drug than the estrogen-sensitive MCF-7 cells.
2.3. Hydroquinone and Hydroquinone-Derived Organotin Polyethers
Characterization of these products has been described [
34].
Table 3 and
Table 4 contain similar GI
50 and CI
50 data for organotin polyethers derived from hydroquinone and hydroquinone derivatives (
Figure 2).
The GI50 MDA/ GI50 MCF-7 values are much less than one (average = 0.068) while the CI50 MDA/ CI50 MCF-7 values are much greater than one (average = 20). These are markedly different than for the organotin polyethers derived from aliphatic diols. Here, inhibition occurs at a much lower concentration for the MDA cell line in comparison to the MCF-7 cell line. The diols in this study showed little or no inhibition of the cancer cell lines.
Table 3.
GI50 concentrations (micrograms/mL) for dibutyltin polyethers from hydroquinone and hydroquinone derivatives for MDA and MCF-7 cell lines.
Table 3.
GI50 concentrations (micrograms/mL) for dibutyltin polyethers from hydroquinone and hydroquinone derivatives for MDA and MCF-7 cell lines.
| Sample | Cell Line |
|---|
| WI-38 | MDA | MCF-7 | MDA/MCF-7 |
|---|
| Methoxyhydroquinone | 2.0(0.3) | 0.09(0.01) | 1.7(0.4) | 0.053 |
| Tert-Butylhydroquinone | 1.8(0.5) | 0.23(0.01) | 2.4(0.5) | 0.096 |
| 2,5-Di-tert-Butylhydroquinone | 2.2(0.5) | 0.22(0.01) | 1.8(0.5) | 0.12 |
| Methylhydroquinone | 2.6(0.5) | 0.036(0.1) | 1.7(0.4) | 0.021 |
| Phenylhydroquinone | 0.21(.03) | 0.11(0.01) | 1.7(0.4) | 0.065 |
| Hydroquinone | 2.0(0.5) | 0.045(0.01) | 1.7(0.5) | 0.026 |
| 2,3-Dicyanohydroquinone | 2.4(0.5) | 0.22(0.09) | 1.9(0.5) | 0.12 |
| Bromphydroquinone | 0.25(0.1) | 0.085(0.01) | 2.7(0.4) | 0.031 |
| Chlorohydroquinone | 1.8(0.3) | 0.086(0.01) | 1.7(0.4) | 0.051 |
| 2,5-Dichlorohydroquinone | 1.9(0.3) | 0.38(0.09) | 2.6(0.5) | 0.15 |
| Tetrachlorohydroquinone | 2.2(0.5) | 0.12(0.01) | 3.9(0.5) | 0.031 |
| 2,5-Dihydroxybenzaldehyde | 2.0(0.5) | 0.13(0.01) | 2.4(0.5) | 0.054 |
Table 4.
Chemotherapeutic Index-50% for the polymers formed from reaction of dibutyltin dichloride and hydroquinone and hydroquinone derivatives for MDA and MCF-7 cell lines.
Table 4.
Chemotherapeutic Index-50% for the polymers formed from reaction of dibutyltin dichloride and hydroquinone and hydroquinone derivatives for MDA and MCF-7 cell lines.
| Sample | Cell Line |
|---|
| WI-38/ | WI-38/ | WI-38/ | CI-MDA/ |
|---|
| WI-38 | MDA | MCF-7 | CI-MCF-7 |
|---|
| Methoxyhydroquinone | 1.0 | 21 | 1.2 | 1.8 |
| Tert-Butylhydroquinone | 1.0 | 7.6 | 0.72 | 11 |
| 2,5-Di-tert-Butylhydroquinone | 1.0 | 9.8 | 1.2 | 8.2 |
| Methylhydroquinone | 1.0 | 71 | 1.5 | 47 |
| Phenylhydroquinone | 1.0 | 1.9 | 0.12 | 16 |
| Hydroquinone | 1.0 | 43 | 1.1 | 39 |
| 2,3-Dicyanohydroquinone | 1.0 | 11 | 1.2 | 9.2 |
| Bromphydroquinone | 1.0 | 2.9 | 0.090 | 32 |
| Chlorohydroquinone | 1.0 | 21 | 1.1 | 19 |
| 2,5-Dichlorohydroquinone | 1.0 | 4.9 | 0.72 | 6.8 |
| Tetrachlorohydroquinone | 1.0 | 19 | 0.57 | 33 |
| 2,5-Dihydroxybenzaldehyde | 1.0 | 15 | 0.84 | 18 |
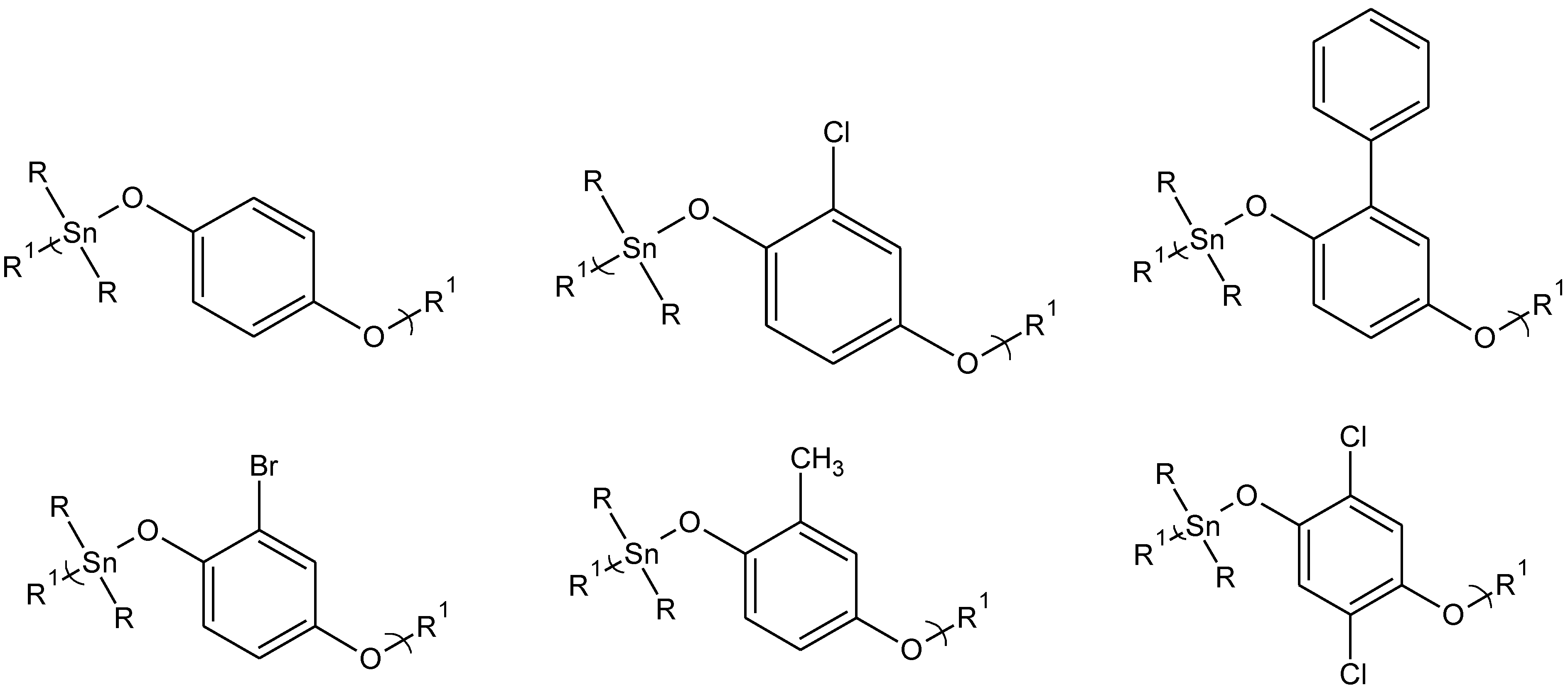
Figure 2.
Representative structures for some of the hydroquinone and hydroquinone-derived polyethers. The diols are from left to right, top: hydroquinone itself, chlorohydroquinone, phenylhydroquinone, and left to right, bottom: bromohydroquinone, methylhydroquinone, and 2,5-dichlorohydroquinone where R is butyl and R1 represents simple chain extension.
Figure 2.
Representative structures for some of the hydroquinone and hydroquinone-derived polyethers. The diols are from left to right, top: hydroquinone itself, chlorohydroquinone, phenylhydroquinone, and left to right, bottom: bromohydroquinone, methylhydroquinone, and 2,5-dichlorohydroquinone where R is butyl and R1 represents simple chain extension.
2.4. Hormone-Derived Organotin Polyethers
In our study of a variety of organotin polyethers we included the study of two related hormone-containing products. Characterization of these products has been reported [
10,
35,
36].
The first is diethylstilbestrol (DES). Diethylstilbestrol (4,4’[(1E)-1,2-ethenediyl]bisphenol), is a synthetic estrogen that mimics estrogen, one of the primary ovarian hormones. It is known by a number of common names including stilbesterol and stilboestrol and sold under a number of names including Apstil, cyren A, distilbene, and stilbetin.
DES was first used in 1938 for women in an effort to prevent miscarriage or premature deliveries. In 1953, a double-blind study showed that DES did little to improve premature deliveries or miscarriage. Even so, it was still widely marketed until the early 1970s for this use. By 1971 it was estimated that 5 to 10 million people were exposed to DES. In 1971 the Food and Drug Administration issued a Drug Bulletin advising physicians to halt prescribing DES. DES was linked to a rare vaginal cancer in female offspring. Further research has shown that DES is a teratogen that can cause malformation of an embryo or fetus.
DES is currently used with animals. Its primary use is to treat urinary incontinence in spayed female cats and dogs. It has also been used to prevent unwanted pregnancy in dogs and cats. DES has been used to treat breast and prostate cancer but its use is limited because of relatively poor water solubility and a wide range of dose-related toxicities that includes nausea and vomiting, venous and arterial thrombosis, and fluid retention. The use of estrogens as potent antiandrogens in hormonal therapy of metastatic prostate cancer has also been described. Thus, there exist several studies that indicate the potential usefulness of DES as a positive drug in the treatment of specific cancers.
The second hormone was dienestrol. Dienestrol,(4-[4-(hydroxyphenyl)hexa-2,4-dien-3-yl]phenol, is one of the most widely used sex hormones. It was initially synthesized by Dodd and others in 1938 and initially patented by both Boots and Hoffman-La Roche in 1949. In the popular literature it is often confused with DES, diethylstilbestrol, but it is a distinct hormone with its own chemical and biological properties. It is sold under a variety of trade names including Farmacyrol, Lipamone, and Retalon-Oral.
Dienestrol is widely used in hormone therapy, mainly hormone replace therapy or more precisely, estrogen replacement therapy.
Table 5 and
Table 6 contain data for organotin polyethers derived from the hormones diethylstilbestrol, DES, and dienestrol (
Figure 3).
Table 5.
GI50 concentrations (micrograms/mL) for organotin polyethers derived from diethylstilbestrol (DES) and dienestrol for MDA and MCF-7 cell lines.
Table 5.
GI50 concentrations (micrograms/mL) for organotin polyethers derived from diethylstilbestrol (DES) and dienestrol for MDA and MCF-7 cell lines.
| Sample | Cell Line |
|---|
| WI-38 | MDA | MCF-7 | MDA/MCF-7 |
|---|
| DES | 0.25(0.2) | 0.05(0.01) | 0.64(0.05) | 0.078 |
| Me2Sn/DES | 1.60(0.5) | 0.47(0.04) | 0.66(0.05) | 0.71 |
| Et2Sn/DES | 0.05(0.01) | 0.16(0.01) | 0.55(0.05) | 0.29 |
| Pr2Sn/DES | 2.30(0.5) | 0.09(0.01) | 0.66(0.05) | 0.14 |
| Bu2Sn/DES | 2.50(0.5) | 0.05(0.01) | 0.62(0.05) | 0.081 |
| Cy2Sn/DES | 0.22(0.02) | 0.21((0.02) | 0.50(0.05) | 0.42 |
| Ph2Sn/DES | 2.30(0.5) | 0.11(0.02) | 0.65(0.05) | 0.17 |
| Dienestrol | 0.25(0.2) | 0.11(.02) | 0.44(0.05) | 0.25 |
| Me2Sn/Dienestrol | 1.5(.5) | 0.13(.06) | 0.76(0.06) | 0.17 |
| Et2Sn/Dienestrol | 1.4(.5) | 0.04(.01) | 0.81(0.06) | 0.049 |
| Pr2Sn/Dienestrol | 0.31(.2) | 0.21(.02) | 0.69(0.05) | 0.30 |
| Bu2Sn/Dienestrol | 0.06(.01) | 0.03(.01) | 0.76(0.05) | 0.039 |
| Cy2Sn/Dienestrol | 0.26(.2) | 0.24(.02) | 0.70(0.05) | 0.34 |
| Ph2Sn/Dienestrol | 0.19(.2) | 0.31(.04) | 0.70(0.05) | 0.44 |
Table 6.
Chemotherapeutic Index-50% for the organotin polyethers derived from diethylstilbestrol (DES) and dienestrol for MDA and MCF-7 cell lines.
Table 6.
Chemotherapeutic Index-50% for the organotin polyethers derived from diethylstilbestrol (DES) and dienestrol for MDA and MCF-7 cell lines.
| Sample | Cell Line |
|---|
| WI-38/ | WI-38/ | WI-38/ | CI-MDA/ |
|---|
| WI-38 | MDA | MCF-7 | CI-MCF-7 |
|---|
| DES | 1.0 | 5.0 | 0.39 | 13 |
| Me2Sn/DES | 1.0 | 3.4 | 2.5 | 1.4 |
| Et2Sn/DES | 1.0 | 0.31 | 0.09 | 3.4 |
| Pr2Sn/DES | 1.0 | 2.6 | 3.5 | 0.74 |
| Bu2Sn/DES | 1.0 | 50 | 4.0 | 13 |
| Cy2Sn/DES | 1.0 | 1.0 | 4.4 | 0.23 |
| Ph2Sn/DES | 1.0 | 21 | 3.5 | 6.0 |
| Dienestrol | 1.0 | 2.2 | 0.6 | 3.7 |
| Me2Sn/Dienestrol | 1.0 | 12 | 2 | 6.0 |
| Et2Sn/Dienestrol | 1.0 | 35 | 1.7 | 20 |
| Pr2Sn/Dienestrol | 1.0 | 1.6 | 0.5 | 3.2 |
| Bu2Sn/Dienestrol | 1.0 | 2 | 0.1 | 20 |
| Cy2Sn/Dienestrol | 1.0 | 1.1 | 0.4 | 2.8 |
| Ph2Sn/Dienestrol | 1.0 | 0.6 | 0.3 | 2.0 |
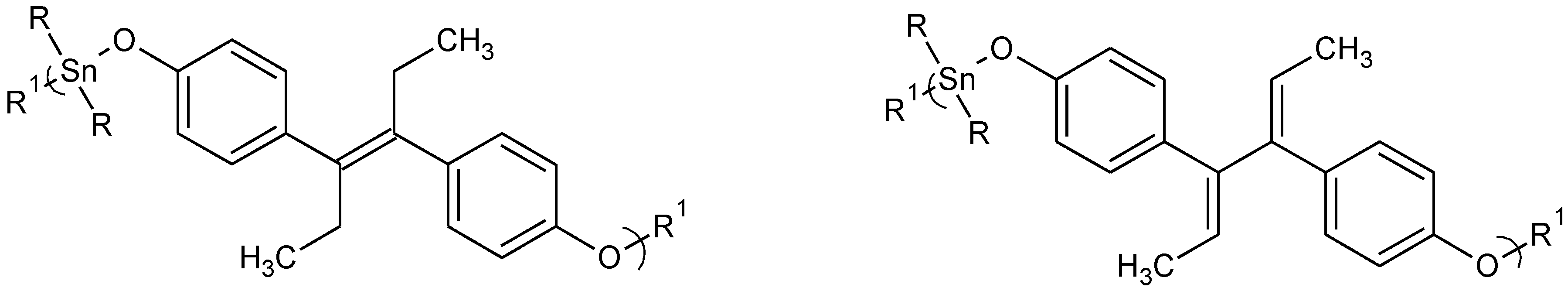
Figure 3.
Repeat unit for organotin polymers from the reaction of organotin dihalides with diethylstilbestrol (DES), left, and with dienestrol, right.
Figure 3.
Repeat unit for organotin polymers from the reaction of organotin dihalides with diethylstilbestrol (DES), left, and with dienestrol, right.
Here, the GI
50 MDA/ GI
50 MCF-7 are generally much smaller than one (average = 0.30 for DES and 0.22 for dienestrol) while the CI
50 MDA/ CI
50 MCF-7 values are generally much larger than one (average = 4.0 for DES and 8.5 for dienestrol). The values are consistent with these organotin polyethers preferentially inhibiting the MDA non-estrogen sensitive cancer cell line. Further, the values for DES and dienestrol themselves are consistent with a preferential inhibition of the MDA non-estrogen cancer cell line. DES is effective against estrogen receptor positive (ER+) tumors such as the MCF-7 cell line [
37,
38,
39,
40,
41,
42,
43,
44,
45,
46]. It is possible that some of the drug is bound to the estrogen receptors in the MCF-7 cells making the drug unavailable to act within the cell.
Thus, the results for the two hormones are similar to those found for the hydroquinone and hydroquinone-derived products described in
Table 3 and
Table 4 but different from those derived from simple aliphatic diols
Table 1 and
Table 2. DES and dienestrol have a structural similarity to the hydroquinone products in that all possess an O-phenylene linkage to the organotin. The organotin polyethers derived from aliphatic diols do not possess this linkage.