Synthesis and Properties of a Chiroptically Active Oligomer from 3,4-Ethylenedioxythiophene and (–)-Myrtenal
Abstract
:1. Introduction
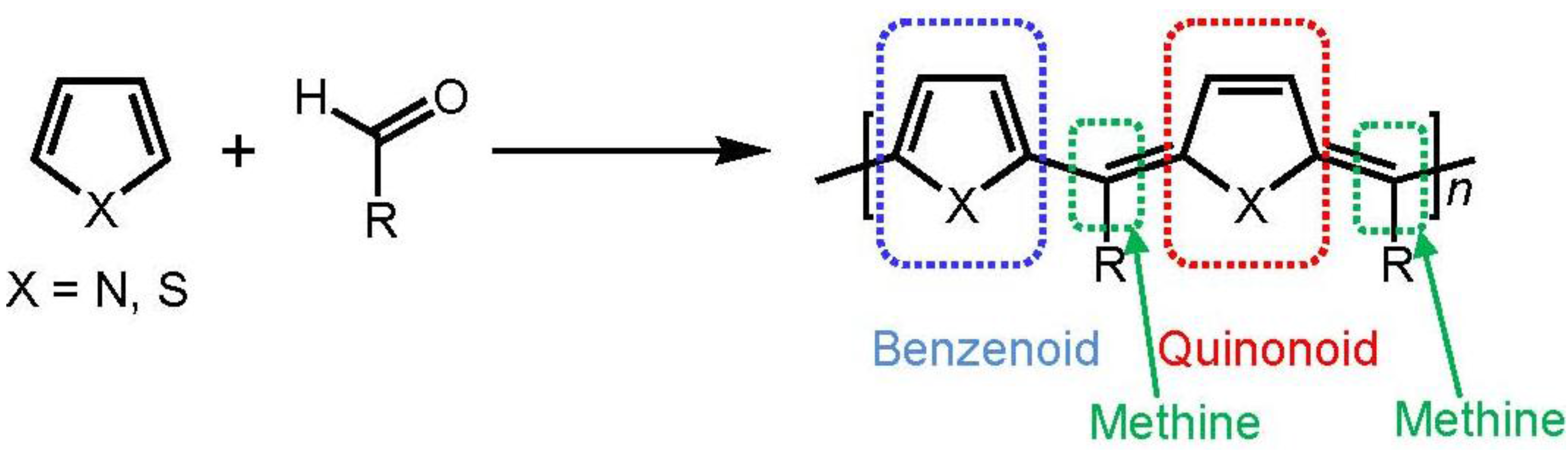
2. Experimental Section

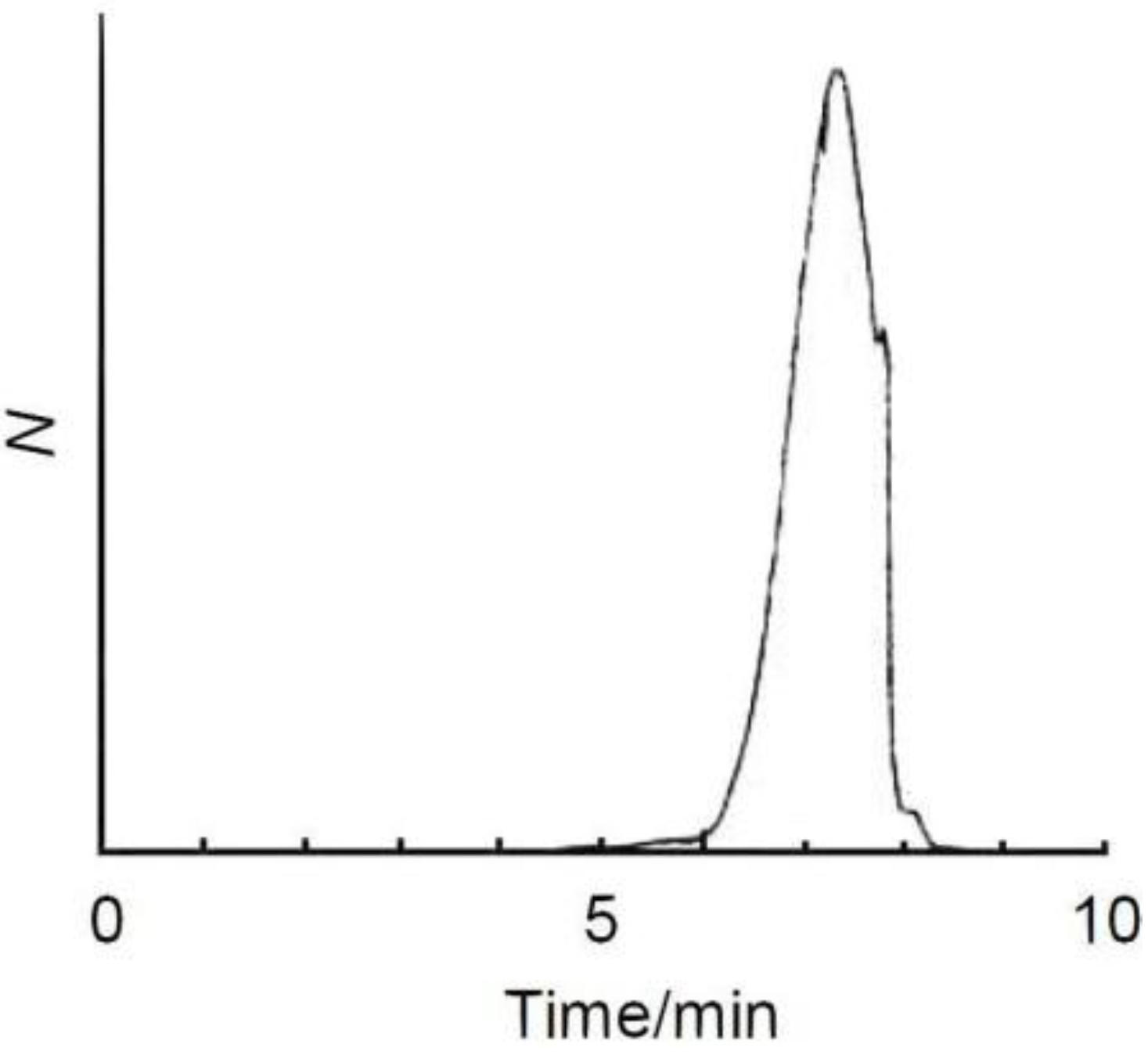
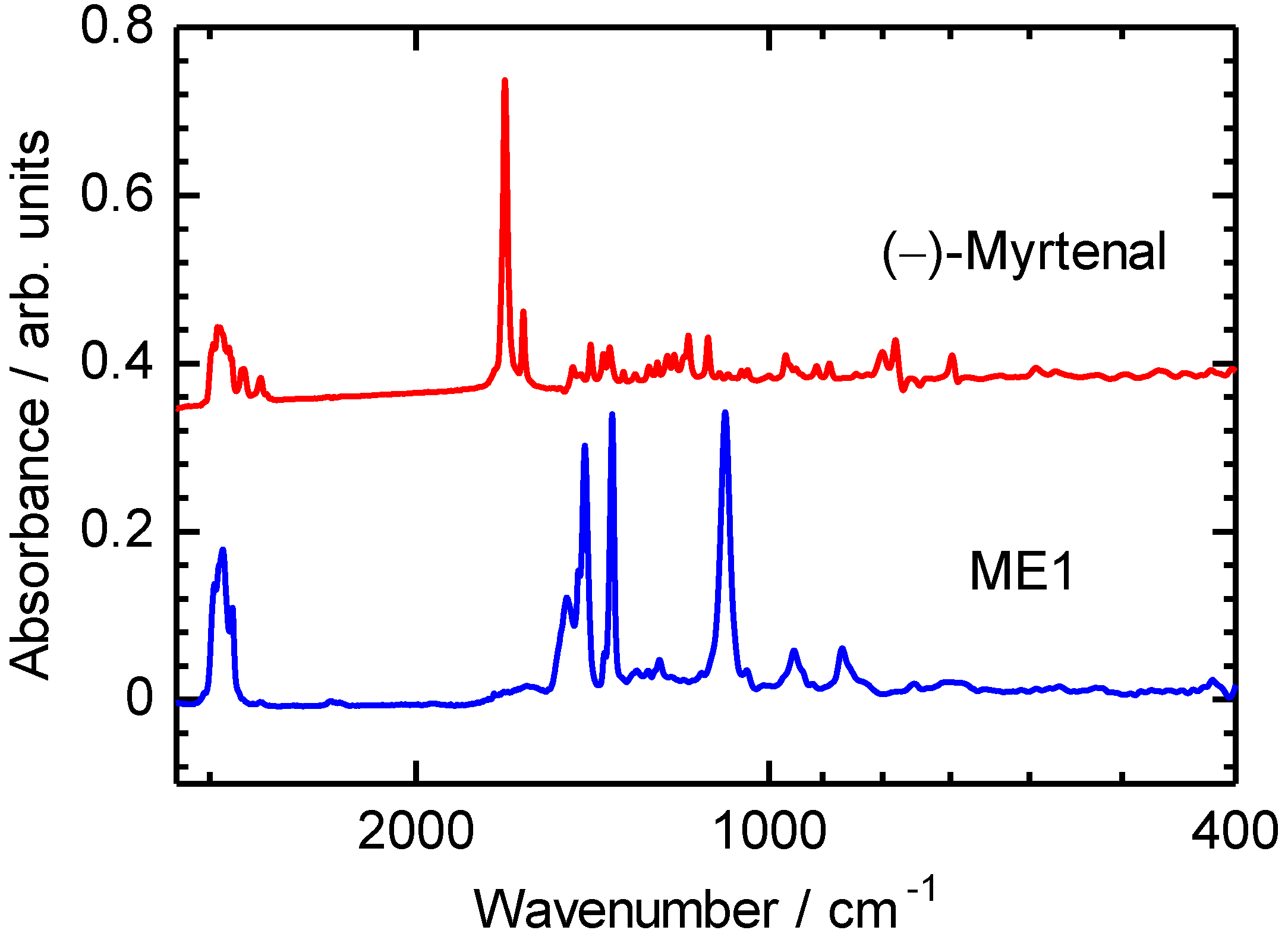
3. Results and Discussion
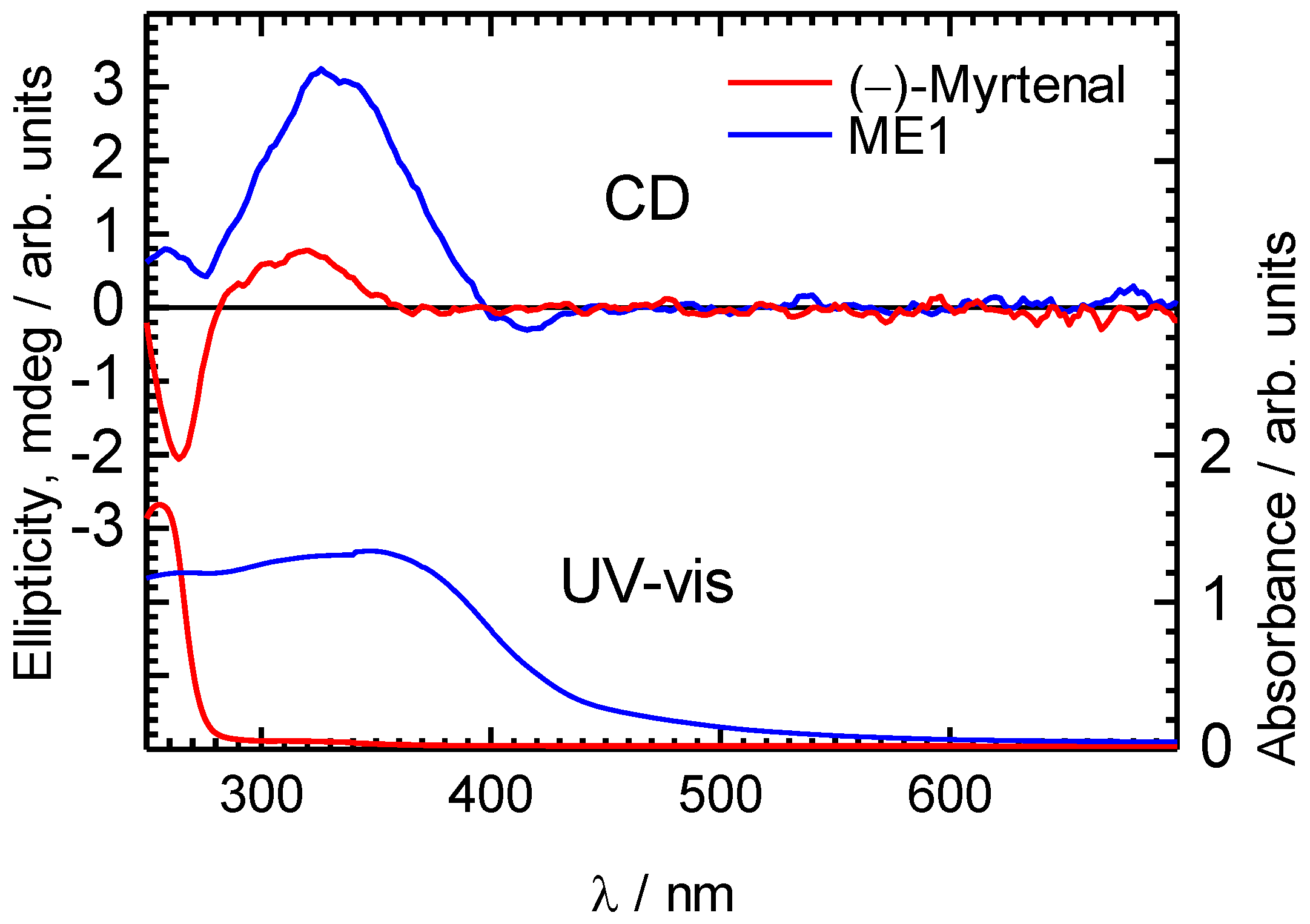
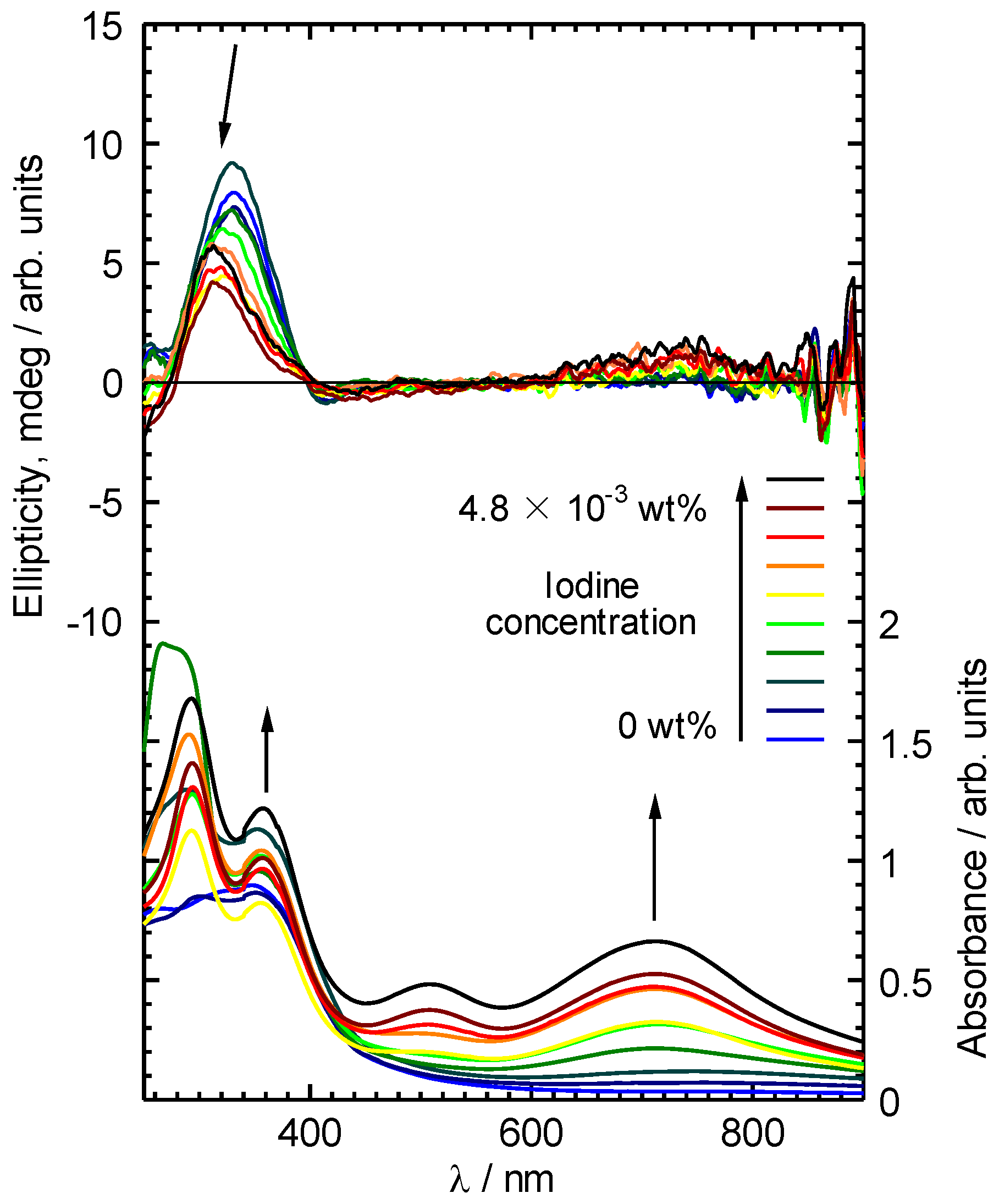
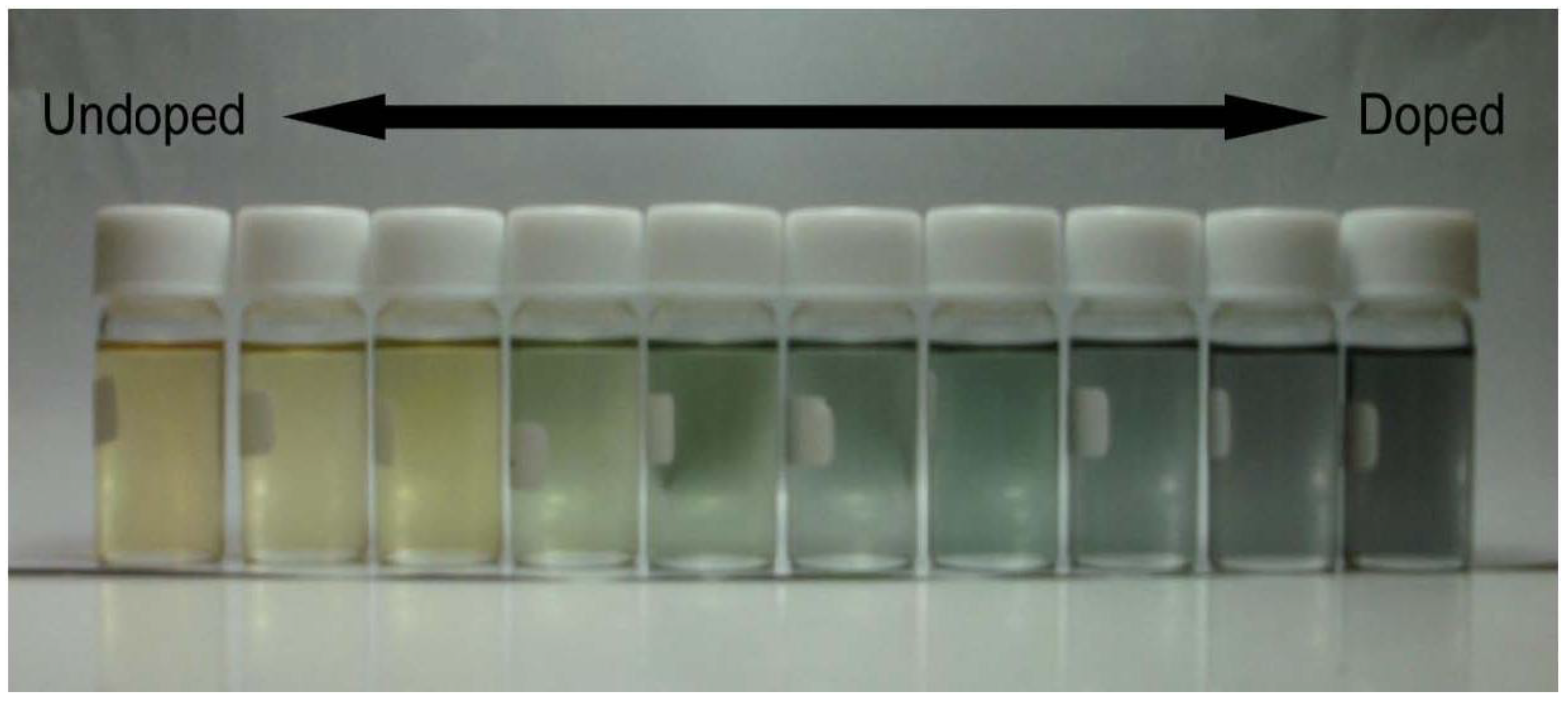
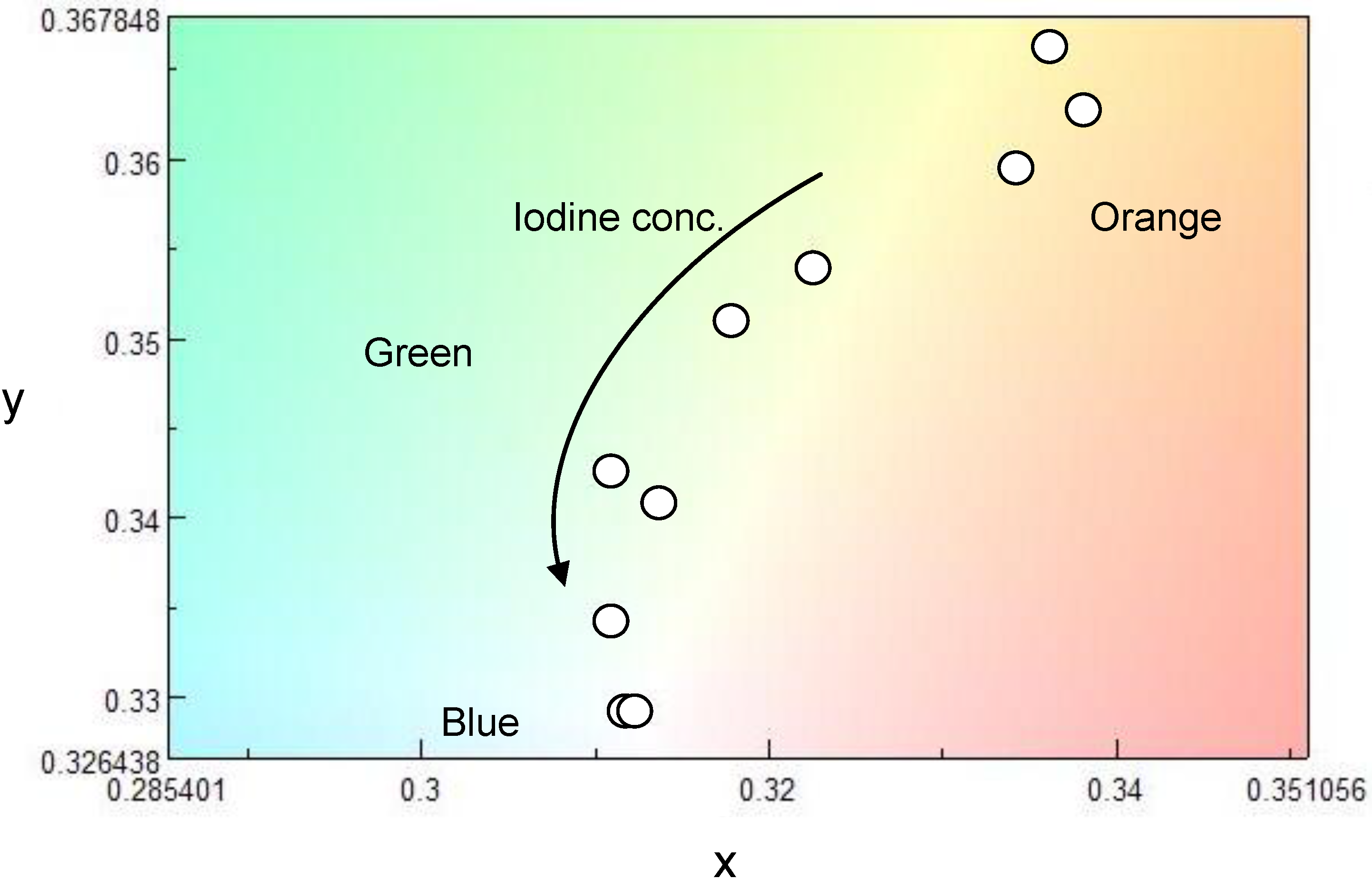
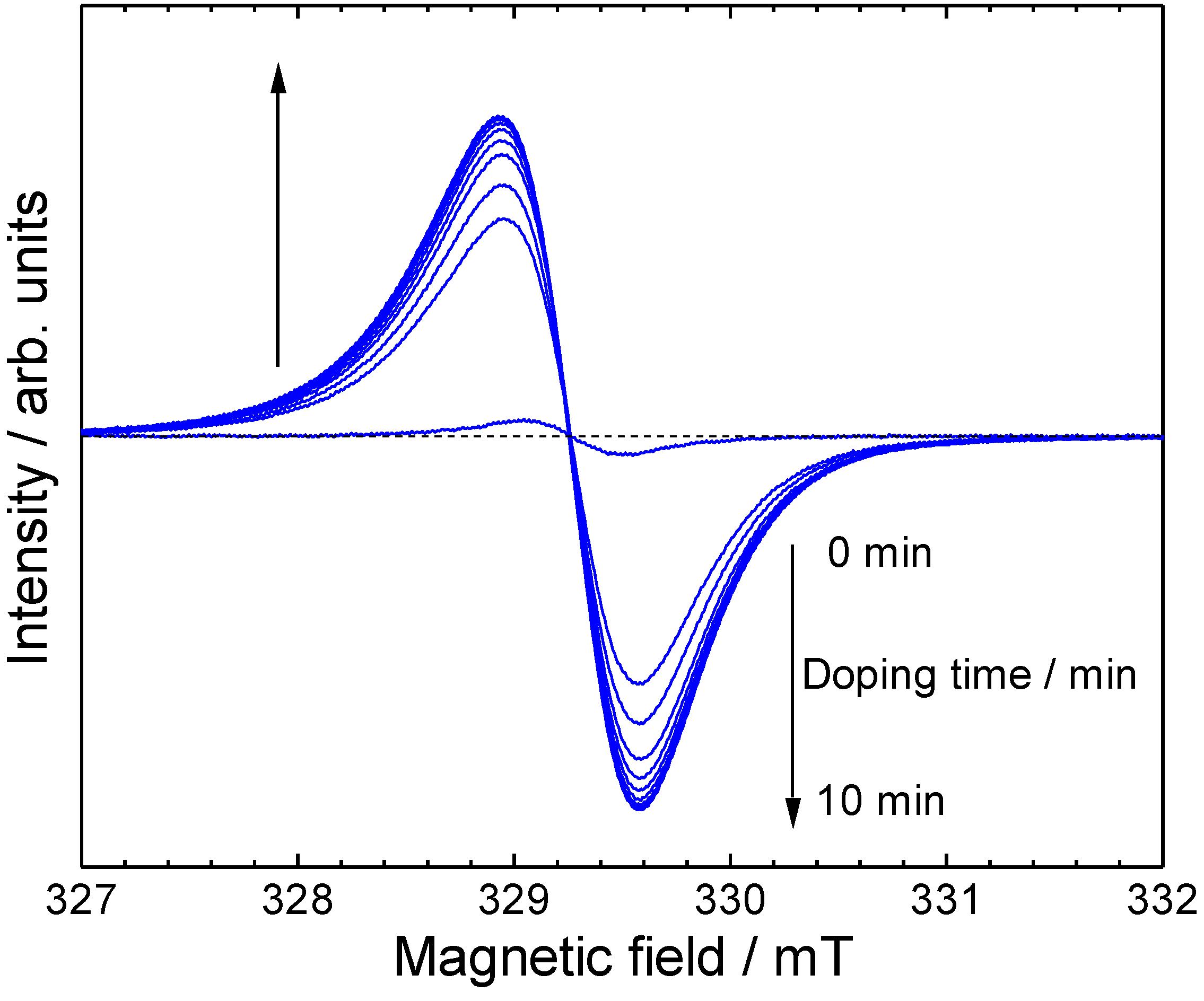
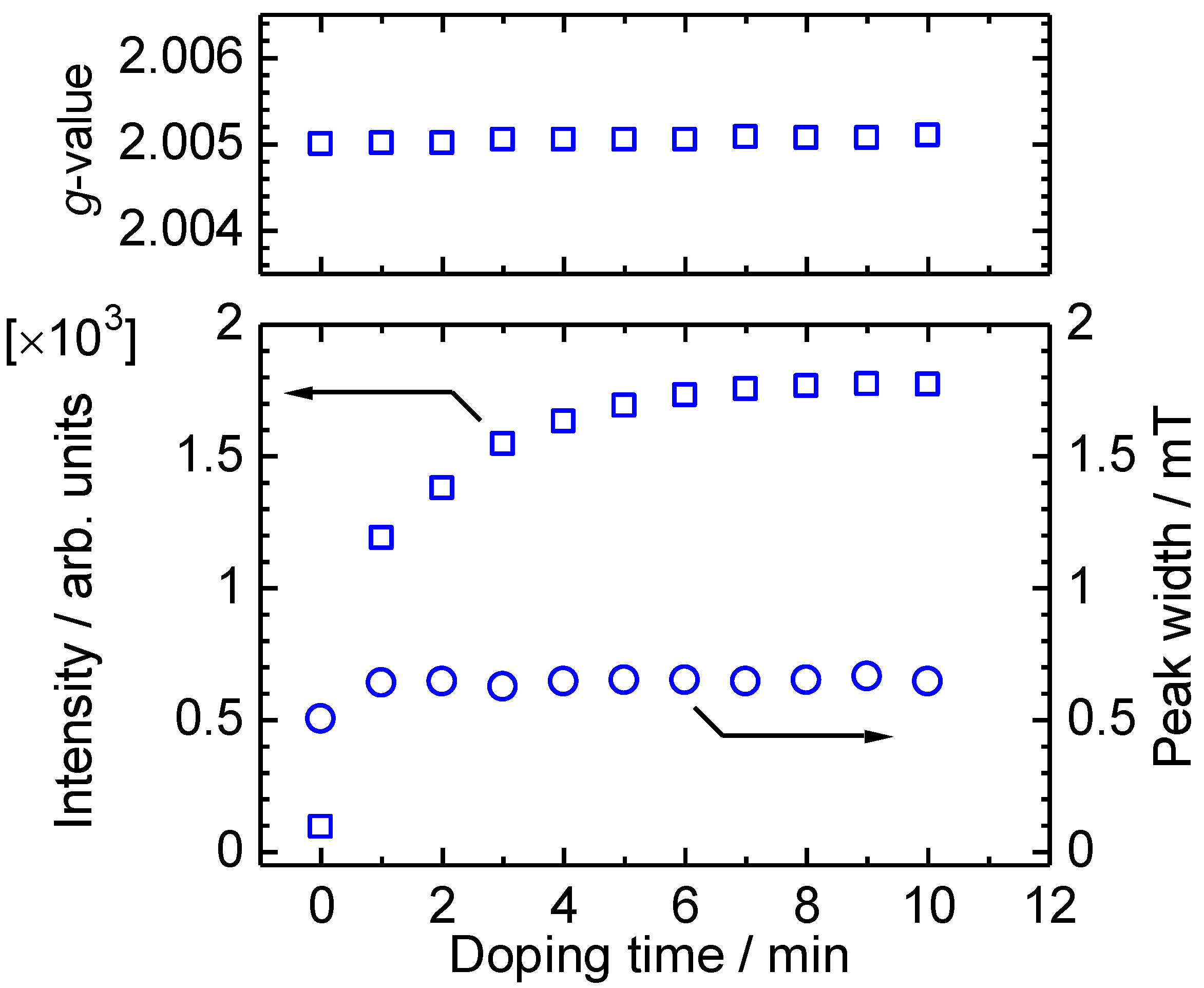
4. Conclusions
Acknowledgements
References
- Nikolou, M.; Dyer, A.L.; Steckler, T.T.; Donoghue, E.P.; Wu, Z.; Heston, N.C.; Rinzler, A.G.; Tanner, D.B.; Reynolds, J.R. Dual n- and p-type dopable electrochromic devices employing transparent carbon nanotube electrodes. Chem. Mater. 2009, 21, 5539–5547. [Google Scholar] [CrossRef]
- Kumar, A.; Bokria, J.G.; Buyukmumcu, Z.; Dey, T.; Sotzing, G.A. Poly(thieno[3,4-b]furan), a new low band gap polymer: Experiment and theory. Macromolecules 2008, 41, 7098–7108. [Google Scholar] [CrossRef]
- Zerza, G.; Röthler, B.; Sariciftci, N.S.; Gómez, R.; Segura, J.L.; Martín, N. Photophysical properties and optoelectronic device applications of a novel naphthalene-vinylene type conjugated polymer. J. Phys. Chem. B 2001, 105, 4099–4104. [Google Scholar] [CrossRef]
- Pei, J.; Yu, W.-L.; Huang, W.; Heeger, A.J. A novel series of efficient thiophene-based light-emitting conjugated polymers and application in polymer light-emitting diodes. Macromolecules 2000, 33, 2462–2471. [Google Scholar] [CrossRef]
- Rathnayake, H.P.; Cirpan, A.; Lahti, P.M.; Karasz, F.E. Optimizing LED Properties of 2,7-Bis(phenylethenyl)fluorenes. Chem. Mater. 2006, 18, 560–566. [Google Scholar] [CrossRef]
- Crouch, D.J.; Skabara, P.J.; Lohr, J.E.; McDouall, J.J.W.; Heeney, M.; McCulloch, I.; Sparrowe, D.; Shkunov, M.; Coles, S.J.; Horton, P.N.; Hursthouse, M.B. Thiophene and selenophene copolymers incorporating fluorinated phenylene units in the main chain: Synthesis, characterization, and application in organic field-effect transistors. Chem. Mater. 2005, 17, 6567–6578. [Google Scholar] [CrossRef]
- Tang, M.L.; Reichardt, A.D.; Wei, P.; Bao, Z. Correlating carrier type with frontier molecular orbital energy levels in organic thin film transistors of functionalized acene derivatives. J. Am. Chem. Soc. 2009, 131, 5264–5273. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Peng, B.; Liu, B.; Zou, Y.; Zhou, K.; He, Y.; Pan, C.; Li, Y. Synthesis and photovoltaic properties of copolymers from Benzodithiophene and Thiazole. J. Phys. Chem. C 2010, 114, 17989–17994. [Google Scholar] [CrossRef]
- Kim, J.Y.; Qin, Y.; Stevens, D.M.; Kalihari, V.; Hillmyer, M.A.; Frisbie, C.D. High open-circuit voltage photovoltaic cells with a low bandgap copolymer of Isothianaphthene, Thiophene, and Benzothiadiazole units. J. Phys. Chem. C 2009, 113, 21928–21936. [Google Scholar] [CrossRef]
- Zhen, F.Z.; Eshbaugh, A.A.; Schanze, K.S. Low-bandgap donor-acceptor conjugated polymer sensitizers for dye-sensitized solar cells. J. Am. Chem. Soc. 2011, 133, 3063–3069. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Park, M.-H.; Zhang, S.; Yao, Y.; Chen, L.-M.; Li, J.-H.; Yang, Y. Bandgap and molecular energy level control of conjugated polymer photovoltaic materials based on Benzo[1,2-b:4,5-b′] dithiophene. Macromolecules 2008, 41, 6012–6018. [Google Scholar] [CrossRef]
- Huang, X.; Shi, Q.; Chen, W.-Q.; Zhu, C.; Zhou, W.; Zhao, Z.; Duan, X.-M.; Zhan, X. Low-bandgap conjugated donor-acceptor copolymers based on porphyrin with strong two-photon absorption. Macromolecules 2010, 43, 9620–9626. [Google Scholar] [CrossRef]
- Zou, Y.; Gendron, D.; Neagu-Plesu, R.; Leclerc, M. Synthesis and characterization of new low-bandgap diketopyrrolopyrrole-based copolymers. Macromolecules 2009, 42, 6361–6365. [Google Scholar] [CrossRef]
- Kiebooms, R.H.L.; Adriaensens, P.J.A.; Vanderzande, D.J.M.; Gelan, J.M.J.V. Grignard reactions on Ortho DicarboxylicArene derivatives. Synthesis of 1,3-Dithienylisothianaphthene compounds. J. Org. Chem. 1997, 62, 1473–1480. [Google Scholar]
- Groenendaal, L.B.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Roncali, J.; Blanchard, P.; Frère, P. 3,4-Ethylenedioxythiophene (EDOT) as a versatile building block for advanced functional π-conjugated systems. J. Mater. Chem. 2005, 15, 1589–1610. [Google Scholar] [CrossRef]
- Segura, J.L.; Gómez, R.; Blanco, R.; Reinold, E.; Bäuerle, P. Synthesis and electronic properties of Anthraquinone-, Tetracyanoanthraquinodimethane-, and Perylenetetracarboxylic Diimide-functionalized Poly(3,4-ethylenedioxythiophenes). Chem. Mater. 2006, 18, 2834–2847. [Google Scholar] [CrossRef]
- Han, D.-H.; Kim, J.-W.; Park, S.-M. Electrochemistry of conductive polymers 38. Electrodeposited poly(3,4-ethylenedioxy-thiophene) studied by current sensing atomic force microscopy. J. Phys. Chem. B 2006, 110, 14874–14880. [Google Scholar]
- Chahma, M.; Myles, D.J.T.; Hicks, R.G. Synthesis and electropolymerization behavior of bis (Oligothienyl) sulfides. Generation of heteroaromatic Poly(p-phenylene sulfide) analogs. Chem. Mater. 2005, 17, 2672–2678. [Google Scholar]
- Pai, C.-L.; Liu, C.-L.; Chen, W.-C.; Jenekhe, S.A. Electronic structure and properties of alternating donor-acceptor conjugated copolymers: 3,4-Ethylenedioxythiophene (EDOT) copolymers and model compounds. Polymer 2006, 47, 699–708. [Google Scholar] [CrossRef]
- Jenekhe, S.A. Synthesis and characterization of carbon atom bridged heterocyclic polymers of specified conjugation length. 1. Novel polyterthiophenes. Macromolecules 1990, 23, 2848–2854. [Google Scholar] [CrossRef]
- Chen, W.-C.; Liu, C.-L.; Yen, C.-T.; Tsai, F.-C.; Tonzola, C.J.; Olson, N.; Jenekhe, S.A. Theoretical and experimental characterization of small band gap poly(3,4-ethylenedioxythiophene methine)s. Macromolecules 2004, 37, 5959–5964. [Google Scholar] [CrossRef]
- Chen, W.-C.; Jenekhe, S.A. New polyaniline derivatives: Poly(4,4'-diphenylamine methylenes) and Poly(4,4'-diphenylimine methines). Macromolecules 1992, 25, 5919–5926. [Google Scholar] [CrossRef]
- Tsukamoto, N.; Ha, J.; Sato, H.; Strzelec, K. Chemical doping of Triphenylaminebenzaldehyde polymers. Int. J. Polym. Mater. 2004, 53, 799–807. [Google Scholar] [CrossRef]
- Toussaint, J.M.; Brédas, J.L. Theoretical analysis of the geometric and electronic structure of small-band-gap polythiophenes: Poly(5,5'-bithiophene methine) and its derivatives. Macromolecules 1993, 26, 5240–5248. [Google Scholar] [CrossRef]
- Stagnaro, P.; Pioli, F.; Panizza, M.; Gandini, A. Acid-catalyzed polycondensation of 2-acetoxymethyl-3,4-dimethylthiophene. Access to a novel poly(thienylenemethine) with alternating aromatic- and quinoid-like structures. Macromolecules 2009, 42, 2455–2461. [Google Scholar]
- Kiebooms, R.; Wudl, F. Synthesis and characterisation of poly(isothianaphthenemethine). Synth. Met. 1999, 101, 40–43. [Google Scholar] [CrossRef]
- Chen, W.C.; Jenekhe, S.A. Small bandgap conducting polymers based on conjugated poly(heteroarylene methine). 1 Precursor poly(heteroarylene methylenes). Macromolecules 1995, 28, 454–464. [Google Scholar] [CrossRef]
- Chen, W.C.; Jenekhe, S.A. Small bandgap conducting polymers based on conjugated poly(heteroarylene methines). 2. Synthesis, structure, and properties. Macromolecules 1995, 28, 465–480. [Google Scholar]
- Caesar, P.D.; Sachanen, A.N. Thiophene-formaldehyde condensation. Ind. Eng. Chem. 1948, 40, 922–928. [Google Scholar] [CrossRef]
- Aota, H.; Itai, Y.; Matsumoto, A.; Kamachi, M. Synthesis of π-conjugated polymer containing porphyrins. Chem. Lett. 1994, 1994, 2043–2046. [Google Scholar] [CrossRef]
- Rothemund, P. Formation of porphyrines from pyrrole and aldehydes. J. Am. Chem. Soc. 1935, 57, 2010–2011. [Google Scholar] [CrossRef]
- Rothemund, P. A new porphyrin synthesis. The synthesis of porphin. J. Am. Chem. Soc. 1936, 58, 625–627. [Google Scholar] [CrossRef]
- Goto, H.; Akagi, K. Synthesis of a pyrrole-based methine bridge type liquid-crystalline conjugated polymer. J. Polym. Sci. A Polym. Chem. 2005, 43, 616–629. [Google Scholar] [CrossRef]
- Meng, L.-J.; Wu, H.-C.; Gao, C.; Yi, W.-H. Synthesis and characterization of a kind of poly(3-butylthiophene methine) with Azo side groups. J. Appl. Polym. Sci. 2005, 97, 1261–1265. [Google Scholar] [CrossRef]
- Multiplet signals at 6.0−6.5 ppm of ME1 were observed in the 1H NMR measurement. Although side reactions at the side chain might occur during the polycondensation process, the signals can be related with molecular weight dispersity.
- Zaman, M.B.; Perepichka, D.F. A new simple synthesis of poly(thiophene-methine)s. Chem. Commun. 2005, 33, 4187–4189. [Google Scholar] [CrossRef]
- Neugebauer, H.; Kvarnström, C.; Brabec, C.; Sariciftci, N.S.; Kiebooms, R.; Wudl, F.; Luzzati, S. Infrared spectroelectrochemical investigations on the doping of soluble Polyisothianaphthene Methine (PIM). J. Chem. Phys. 1999, 110, 12108–12115. [Google Scholar] [CrossRef]
- Patil, A.O.; Heeger, A.J.; Wudl, F. Optical properties of conducting polymers. Chem. Rev. 1988, 88, 183–200. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kawashima, H.; Goto, H. Synthesis and Properties of a Chiroptically Active Oligomer from 3,4-Ethylenedioxythiophene and (–)-Myrtenal. Materials 2011, 4, 1013-1022. https://doi.org/10.3390/ma4061013
Kawashima H, Goto H. Synthesis and Properties of a Chiroptically Active Oligomer from 3,4-Ethylenedioxythiophene and (–)-Myrtenal. Materials. 2011; 4(6):1013-1022. https://doi.org/10.3390/ma4061013
Chicago/Turabian StyleKawashima, Hirotsugu, and Hiromasa Goto. 2011. "Synthesis and Properties of a Chiroptically Active Oligomer from 3,4-Ethylenedioxythiophene and (–)-Myrtenal" Materials 4, no. 6: 1013-1022. https://doi.org/10.3390/ma4061013




