1. Introduction
Large-size bone defects can be the consequence of tumor, trauma or infections. Every year, there are more than 1.5 billion bone-grafting procedures worldwide [
1]. In the past as the “Golden Standard”, defects were filled up by bone auto- or allografts [
2,
3]. Disadvantages of these therapeutic strategies are limited availability, risk of infections and donor-site morbidity [
4,
5]. Therefore, it is necessary to apply appropriate synthetic, porous bone substitutes [
6] which can be adapted to the bone defect. These three-dimensional (3D) scaffolds should mimic the artificial extracellular matrix (ECM) so that cells are able to migrate, proliferate and differentiate within these structures [
7]. However, with increasing size of synthetic scaffolds, there are increasing gradients in tissue quality in terms of inhomogeneous cell proliferation and differentiation from the periphery to the core [
8,
9].
Within a 3D scaffold, cells depend on diffusion processes in order to be sufficiently supplied with oxygen and nutrients as well as to remove metabolic waste [
10]. In living tissue oxygen supply, nutrients and also waste elimination must be kept constant over 20 to 200 µm [
10,
11], whereby cells are supplied by blood vessels. Diffusion processes
in-vitro are limited to approximately 200 µm [
10]. As a consequence, gradients in environmental conditions predominate between the periphery and the core of tissue-engineered constructs [
10]. Therefore, structures or channels for vascularisation within large-scaled synthetic 3D scaffolds have to be considered.
There are many synthetic, porous materials available on the market but usually they show simple geometries such as blocks or pins. Three-dimensional printing (3D printing) is a well-suited technique for generating complex porous matrices directly from powder material [
12]. Anatomical information of the patients can be used for the design of individual implants for defect-filling [
12,
13]. Thereby, the outer shape of the implant can be closely adapted to the bone defect as well as the inner channels are optimized for cell ingrowth [
14,
15]. Tricalciumphosphate (TCP) is a suitable material for 3D printing. Scaffolds can be built up layer by layer with subsequent sintering [
13]. Consequently, the final scaffold is highly defined with precise dimensions and regular characteristics such as the pore size [
16,
17]. Besides the possibility of individual design, TCP is distinguished by its osteoconductive characteristics [
18,
19].
However, no data are shown about the oxygen supply and pH monitoring within a 3D TCP scaffold settled by human osteoblasts so far. In the present study, we designed an experimental setup to measure the oxygen content as well as acidification in the core of the TCP scaffold compared to the surrounding medium. Therefore, we placed two TCP discs on top of each other to obtain a 3D construct with a total height of 10 mm and four planes (superior, two intermediate and inferior). We seeded human osteoblasts on the superior plane and analyzed the migration capacity of cells within the scaffold. To collect information of the oxygen content and acidification, we used optical microsensors for measuring the oxygen concentration and pH value. Furthermore, we determined the ability of type I pro-collagen synthesis of the cells.
2. Materials and Methods
2.1. Isolation and Cultivation of Human Primary Osteoblasts
Human primary osteoblasts were isolated under sterile conditions from bone marrow derived from femoral heads of patients undergoing primary total hip replacement. All samples were collected after patient’s agreement and approval by the Local Ethical Committee (registration number: A 2010-10).
Cancellous bone specimens were carved from the inside of femoral heads, washed three times with PBS (PAA, Cölbe, Germany), cut into small pieces and treated with Dulbecco´s modified Eagle medium (DMEM, Gibco® Invitrogen, Darmstadt, Germany) containing collagenase A and dispase (both: Roche, Mannheim, Germany) at a ratio of 1:2:1 at 37 °C for 3 hours. Afterwards, the cell suspension was filtered through a cell strainer (pore size: 70 µm; Nunc, Wiesbaden, Germany) and centrifuged at 118 × g for 10 min. The remaining cell pellet was resuspended in complete medium containing 10% FCS, 1% Amphotericin B, 1% Penicillin-Streptomycin and 1% Hepes-Buffer (all: Gibco® Invitrogen, Darmstadt, Germany).
Freshly isolated osteoblasts from 2 to 3 g wet weight were plated in a 25 cm2 culture flask with 8 mL complete medium mentioned above containing ascorbic acid (final concentration: 50 µg/mL), β-glycerophosphate (final concentration: 10 mM) as well as dexamethasone (final concentration: 100 mM) (all: Sigma, Seelze, Germany). Cells were incubated in a humidified atmosphere of 5% CO2 and 37 °C. The cell culture medium was changed every second day to remove non-adherent cells. Cell proliferation was monitored microscopically. As cells reached 90% confluency, they were trypsinized and splitted at a ratio 1:6. To verify the osteogenic character of isolated cells, alkaline phosphatase stainings were carried out.
2.2. Cell Settlement on Tricalciumphosphate (TCP) Scaffolds
Human osteoblasts were seeded on porous cylindrical TCP scaffolds which were fabricated in a 3D printing process with loose powder and a polymer-based binder solution for local initial bonding of the powder. The CaP-based granulate TCP 4 was obtained from BioCer Entwicklungs-GmbH (Bayreuth, Germany). The printed ceramic green bodies were consolidated at a temperature of 1250 °C to improve their mechanical properties [
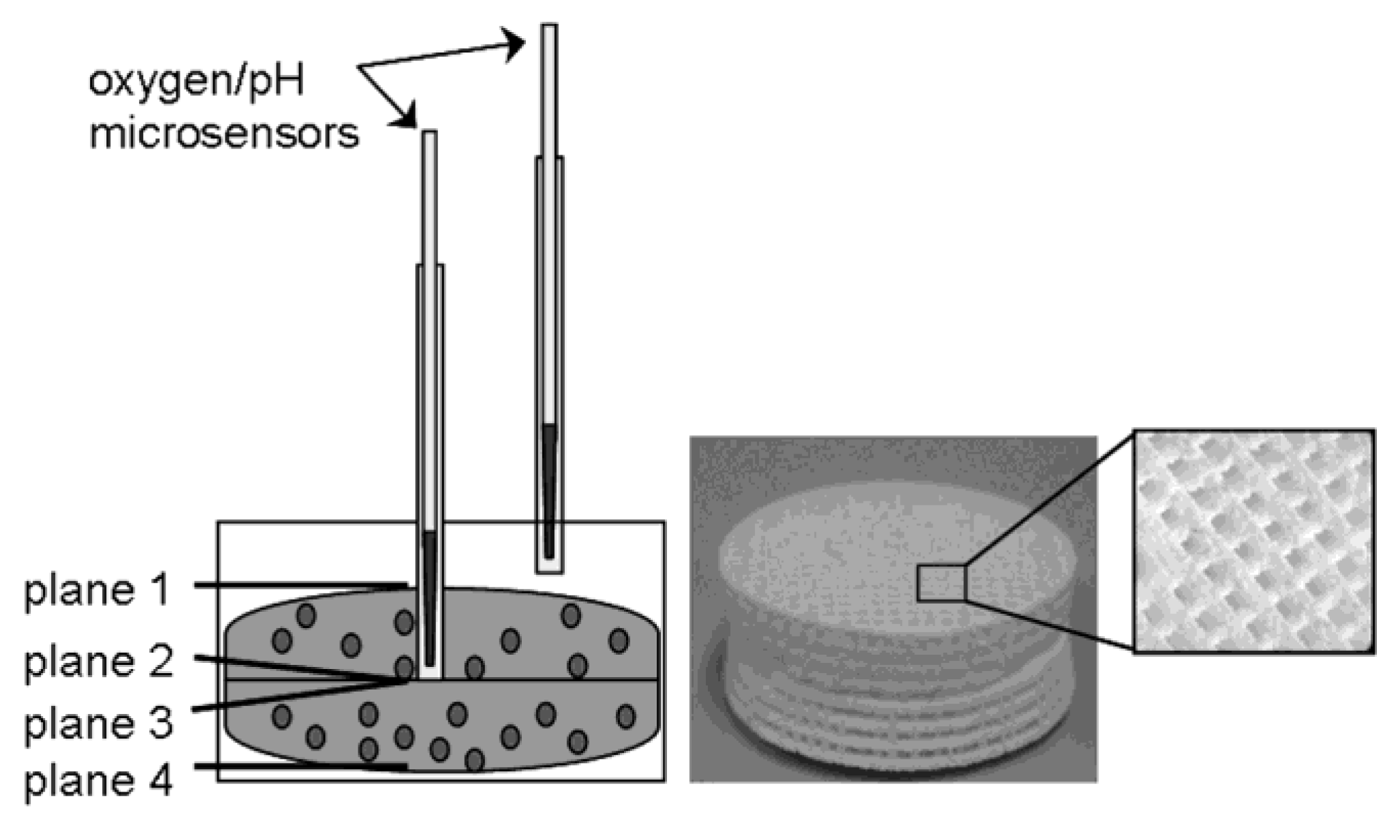
12]. The disc-shaped TCP constructs were 30 × 5 mm in size with macropores of 700 × 700 µm in all three spatial directions. To distinguish between different depths, the complete scaffold module was composed of two TCP discs which were placed on top of each other (
Figure 1). In addition, the upper TCP disc had a central hole to insert a standard hollow needle for oxygen and pH measurement (see 2.3. and 2.4.).
Before cell seeding, the TCP scaffolds were incubated in complete medium under standard culture conditions for 72 hours. Subsequently, 4 × 105 cells were seeded on the superior plane by dropping a 10 µL cell suspension point-wise on the scaffold. After 45 minutes, complete medium was refilled and the construct was incubated at 37 °C and 5% CO2 for eight days. Medium was changed completely every second day.
Figure 1.
Schematic design of the 3D-TCP scaffold which consisted of two TCP discs (30 × 10 mm in size with 700 × 700 µm macropores) and therefore four different planes (plane 1: superior; plane 2 and 3: intermediate, plane 4: inferior).
Figure 1.
Schematic design of the 3D-TCP scaffold which consisted of two TCP discs (30 × 10 mm in size with 700 × 700 µm macropores) and therefore four different planes (plane 1: superior; plane 2 and 3: intermediate, plane 4: inferior).
2.3. Oxygen Measurements
For the oxygen measurements, needle-type oxygen microsensors (Oxygen Micro-Optode, Type PSt1; Presens, Regensburg, Germany), mounted on optic fibers with a 140 µm tip, were used. For protection of the fragile sensors, they were fixed within a standard hollow needle of 0.4 mm diameter. We used standard hollow needles with a diameter of 1.02 mm to achieve higher mechanical stability during measurements (
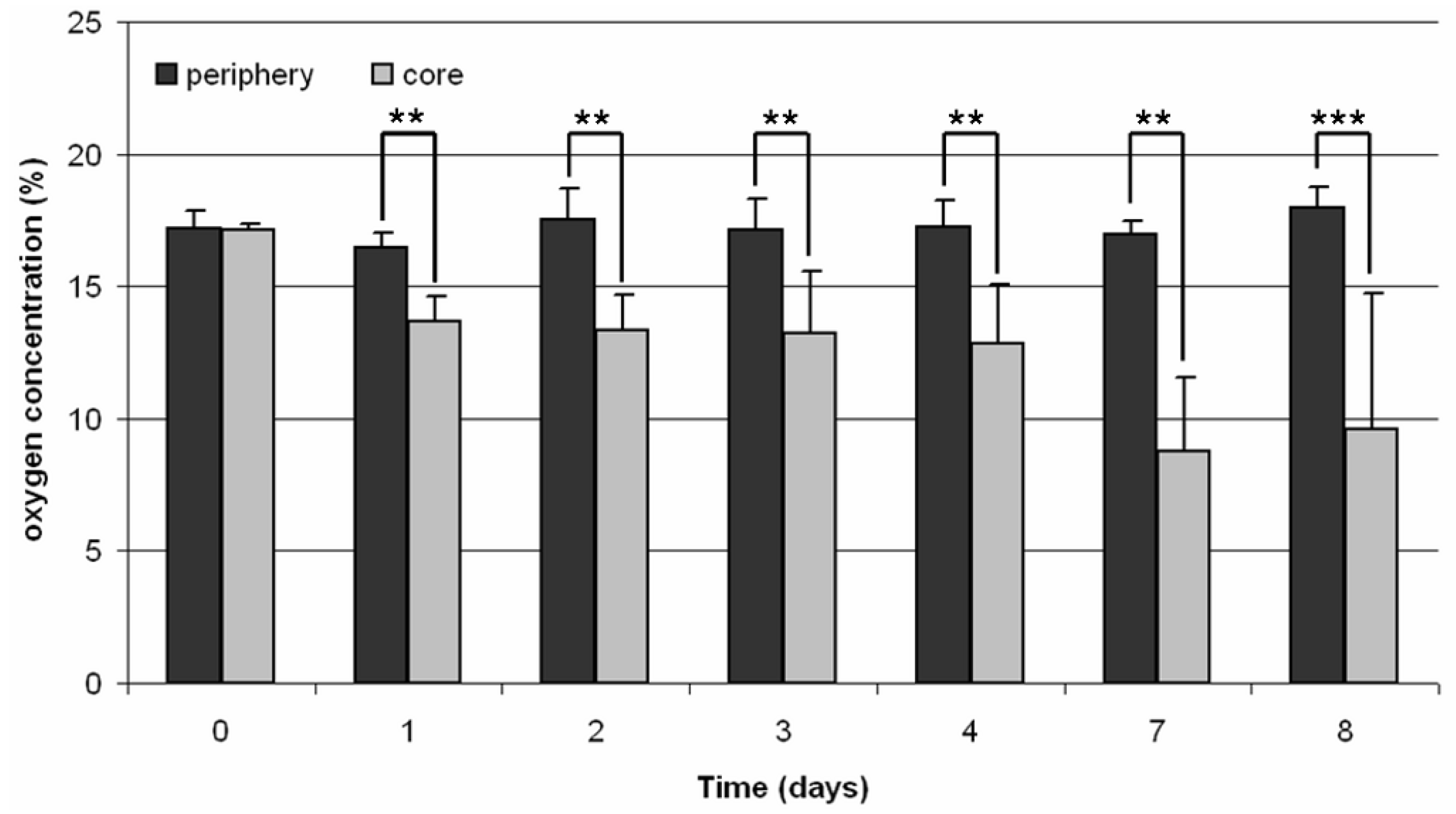
Figure 1). A two-point calibration was performed before the measurements using oxygen-free water (0% air saturation) and water vapor-saturated air (100% air saturation). Oxygen consumption was measured both in the core and the periphery of the 3D scaffold every 24 h over a period of 30 minutes for eight days.
2.4. pH Monitoring
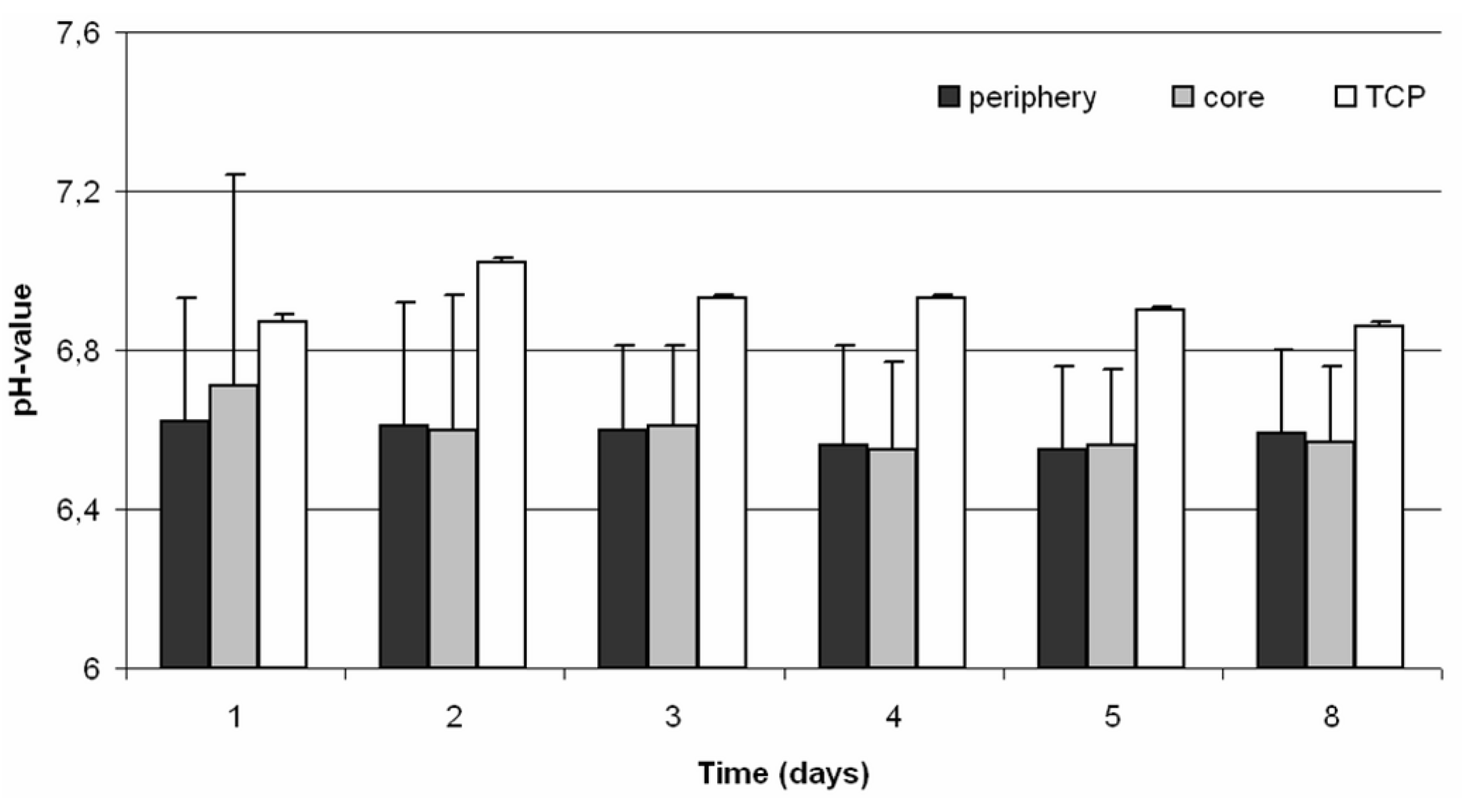
To monitor the pH values within the 3D-cell culture, needle-type pH microsensors (pH microsensor; Presens, Regensburg, Germany), mounted on optic fibers with a tip of less than 150 µm, were used. For protection of the fragile sensors, they were fixed within a standard hollow needle of 0.4 mm diameter. Analogue to the oxygen measurements we used standard hollow needles with a diameter of 1.02 mm to improve the stability during pH measurements (
Figure 1). Prior to the measurement a calibration with buffer solutions of pH 4 to 7 (all: Roth, Karlsruhe, Germany) was performed. The pH value was monitored both in the periphery and in the core of the 3D scaffold every 24 h over a period of 30 min for eight days.
2.5. WST-1 Assay
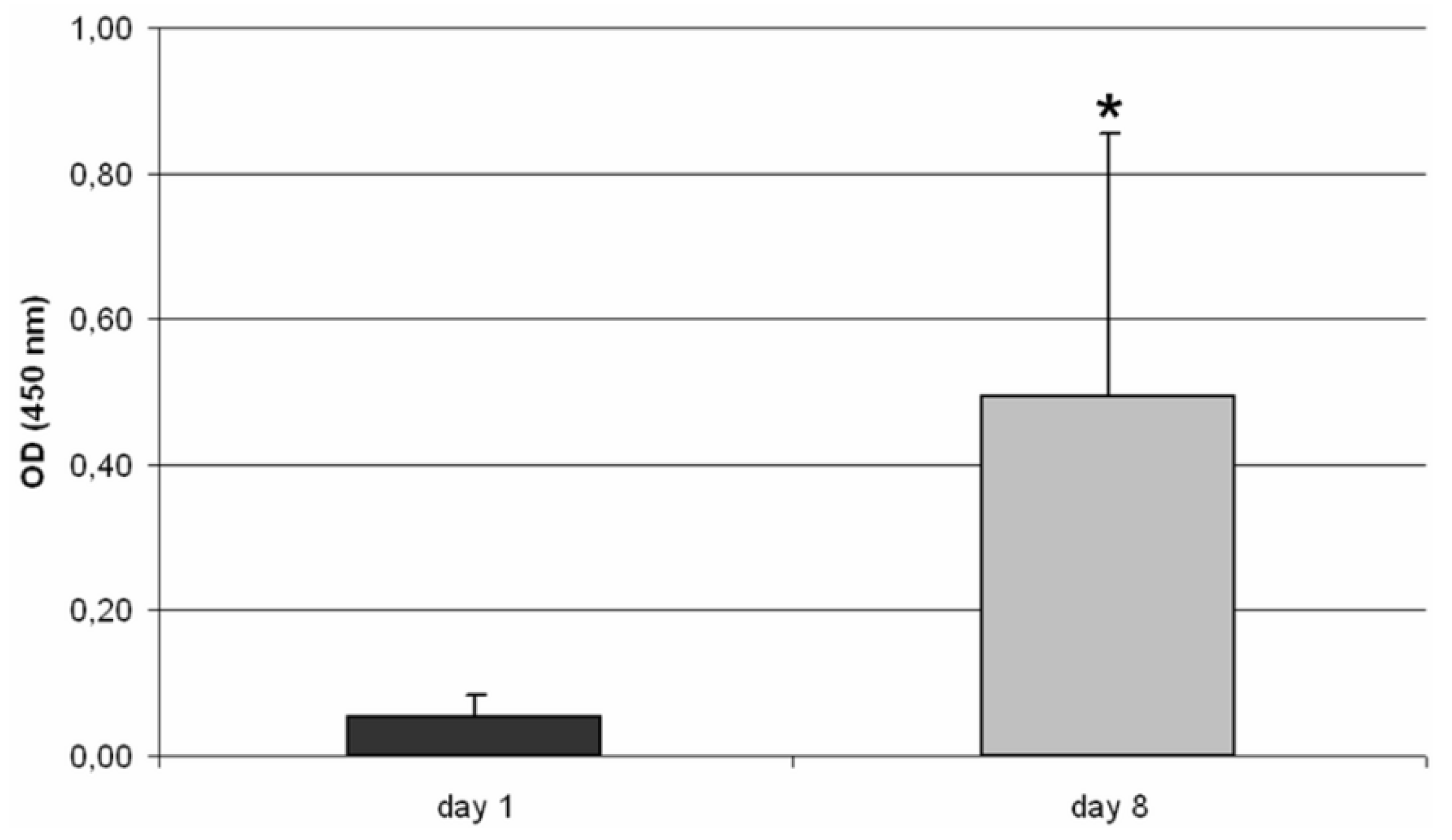
The mitochondrial activity of osteoblasts on the TCP scaffold was analyzed by using a WST-1 assay (Roche, Mannheim, Germany). The background of this assay is, that cells with a high metabolism cleave the tetrazolium salt WST-1 to formazan in direct dependency on their metabolic activity [
20]. Therefore, the TCP module was covered with assay reagent (ratio between complete medium to WST-1 reagent of 10:1). Additionally, a medium control was used. After an incubation time of 60 minutes under cell culture conditions, 200 µL aliquots of assay reagent were transferred to a 96-well format. Absorbance was measured at 450 nm (reference 630 nm) using an Opsys MR™ microplate reader (Dynex Technologies, Denkendorf, Germany).
2.6. Pro-Collagen Type I Quantification
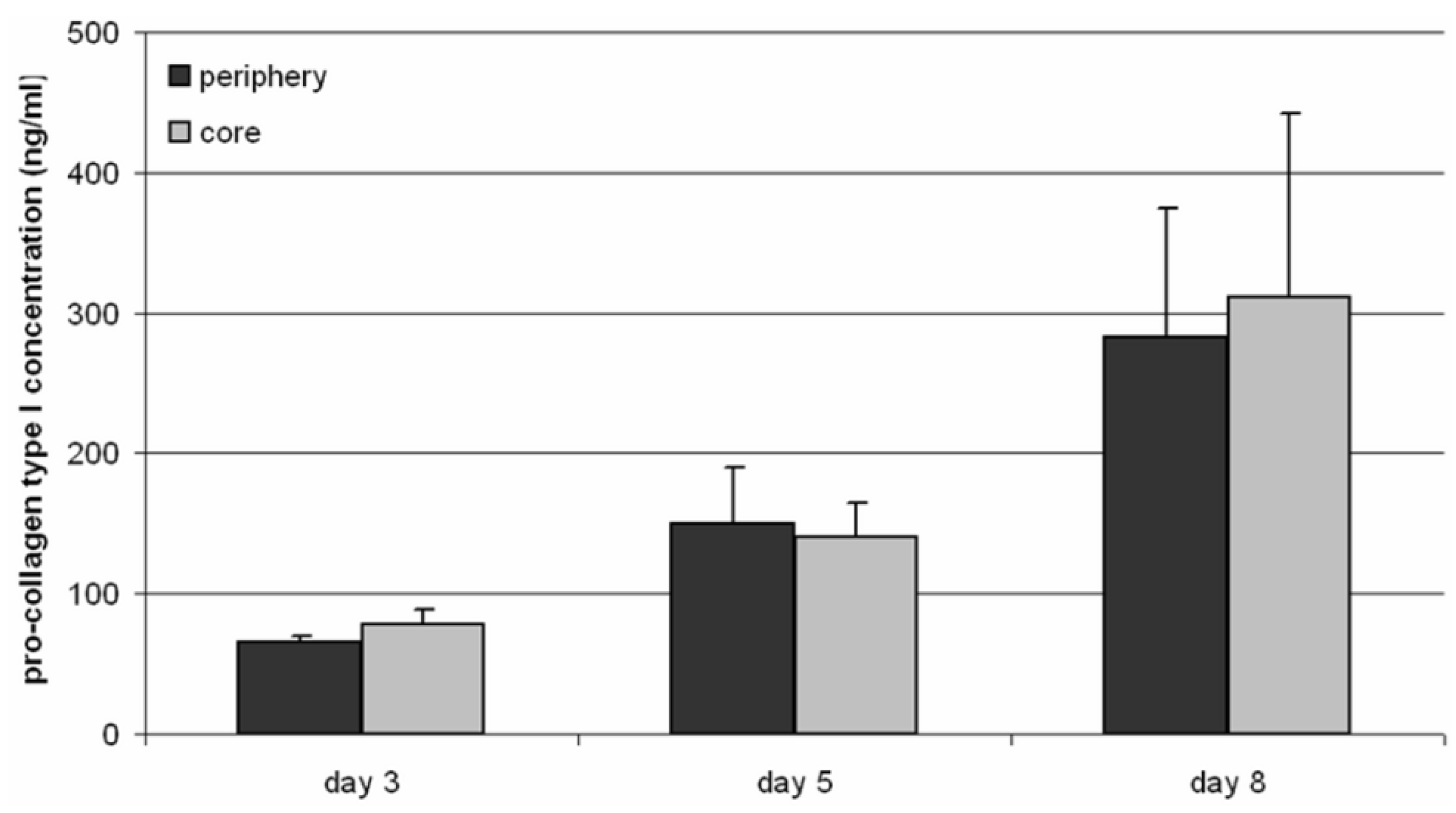
Synthesis of pro-collagen type I (Metra™ CICP EIA Kit, Quidel, Marburg, Germany) was determined by an enzyme-linked immunosorbent assay (ELISA). For the analysis, 500 µL medium were removed from the core and the periphery of the TCP scaffold. The assay was performed according to the manufacturer’s instructions. Absorbance was measured at 405 nm using the Opsys MR™ microplate reader (Dynex Technologies, Denkendorf, Germany).
2.7. LIVE/DEAD® Assay
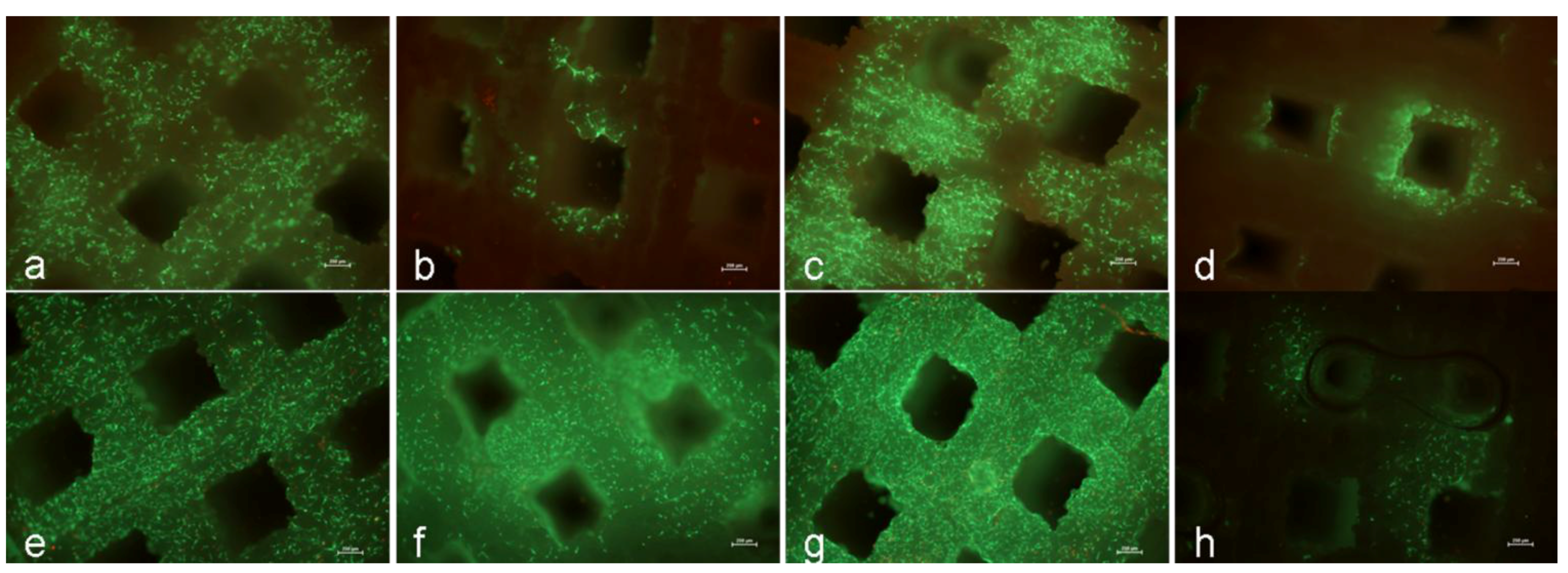
By means of the LIVE/DEAD® viability/cytotoxicity kit (Invitrogen, Darmstadt, Germany), cell viability was determined. Hence, both TCP discs were incubated in LIVE/DEAD® assay reagent containing calcein AM and ethidium homodimer. Calcein AM is the acetoxymethyl ester derivative of the fluorescent dye calcein. Calcein AM is membrane-permeant and thus is taken up by cells incubated with PBS supplemented with calcein AM. Once inside the cells, calcein AM is hydrolyzed by endogenous esterases into the polyanionic non-membrane permeant green fluorescent dye calcein. As a result, viable cells with intact membranes produce an intense uniform green fluorescence (ex/em 495 nm/515 nm). Ethidium enters cells with damaged membranes and produces a bright red fluorescence (ex/em 495 nm/635 nm) to indicate dead cells. After 30 min of incubation at room temperature with PBS containing both dyes, images of the cells were taken using a fluorescence microscope with a fourfold magnification object lens (Nikon ECLIPSE TS100; Nikon GmbH, Düsseldorf, Germany).
2.8. Statistical Analysis
Data are presented as mean values ± standard deviation. Statistical significance between groups was calculated by an ONEWAY ANOVA (Post Hoc LSD) test, the Mann-Whitney-U-Test or the student`s t-Test using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA). A value of p < 0.05 was considered significant. A minimum of three independent experimental runs were performed for all analysis.
4. Discussion
For the treatment of large bone defects, the availability of suitable substitutes, e.g., bone auto- or allografts, is limited. Therefore, alternatives such as synthetic, porous 3D scaffolds are becoming more relevant. An important aspect for the design of such constructs is the individual production of implants which was achieved by 3D printing processes [
12]. A suitable material for this application is TCP.
The objective of this study was to examine the consumption of oxygen as well as the acidification of the cell culture medium after settlement of human osteoblasts within a 3D TCP scaffold. Furthermore, the migration capacity and viability of cells was determined.
In contrast to Warnke
et al. [
13] we could show, that human osteoblasts were able to survive on the TCP construct. After one day of incubation, a lot of viable cells were detected on the surface of the scaffold which was in accordance to Becker
et al. [
19]. During incubation, cells migrated through the construct, so that the scaffold was almost fully occupied by cells. Furthermore, verification of the metabolic activity revealed a significant increase of metabolic activity by osteoblasts from day one to day eight.
An important aspect for the design of large-scaled scaffolds is the sufficient supply of oxygen and nutrients within the constructs. In engineered constructs, the transport of oxygen and nutrients is only maintained by diffusion processes [
10]. Therefore, gradients in environmental conditions predominate between the periphery and the core of 3D scaffolds [
10].
Monitoring of the oxygen concentration within the TCP scaffold showed significant differences between the core and periphery. The difference of the oxygen concentration within the scaffold compared to the surrounding medium was approximately 4% during the first five days of incubation. This difference increased further to approximately 8% on the last day of cultivation so that the remaining oxygen concentration within the scaffold was at 9.6%. This was in contrast to Volkmer
et al. [
9] where a decrease of the oxygen concentration to 0% was detected for the scaffolds used after five days of incubation.
Furthermore, the acidification of the cell culture medium within and around the TCP scaffold was monitored. It is well described that hypoxia is associated with a local decrease of the pH value which is caused by an anaerobic cell metabolism [
21]. In addition, small pH value reductions could influence the matrix deposition by osteoblasts negatively [
21]. The results showed that there were no differences between the periphery and the core of the cell-seeded scaffold, although a difference in the pH value of approximately 0.3 between the cell-seeded and untreated scaffold was demonstrated. Nevertheless, the pH value of the untreated TCP scaffold was about 6.9, which demonstrates that TCP is a material with acidic characteristics.
Hypoxia as well as acidic pH values could influence matrix deposition by cells [
21,
22]. In our experiments an increasing type I pro-collagen synthesis rate during cultivation was determined, thus collagen expression was unaffected by lower pH values and hypoxia.
Our results suggest that human osteoblasts could survive on porous TCP scaffolds in a static cell culture. Furthermore, cells migrated through the macropores to the inner structures of the scaffold. Hereby, the synthesis of pro-collagen type I was sustained during the incubation time and evidently increased. Hence, matrix production and therefore overgrowing of pores by matrix deposition, which was reported by Malda
et al. [
10], can influence and hinder the diffusion of oxygen slightly within the 3D construct. The macropores of the TCP scaffold were settled by cells but the macropore size prevented an overgrowing of cells. Thus, the used pore-size had a positive influence of cell survival and the supply of oxygen and nutrients was sufficiently guaranteed in the static cell culture. Furthermore, the monitoring of cell migration, oxygen consumption as well as acidification could be a helpful instrument for creating 3D bone implants enabling a sufficient ingrowth of human bone cells. Nevertheless, in further studies it is recommended to modify the
in-vitro conditions towards more physiological conditions. Therefore, it is necessary to analyze cell viability on 3D scaffolds in a dynamic cell culture. Moreover, vascularisation within the scaffold has to be performed to guarantee a sufficient oxygen and nutrient supply.