Osteoinductivity Assessment of BMP-2 Loaded Composite Chitosan-Nano-Hydroxyapatite Scaffolds in a Rat Muscle Pouch
Abstract
:1. Introduction
2. Results and Discussion
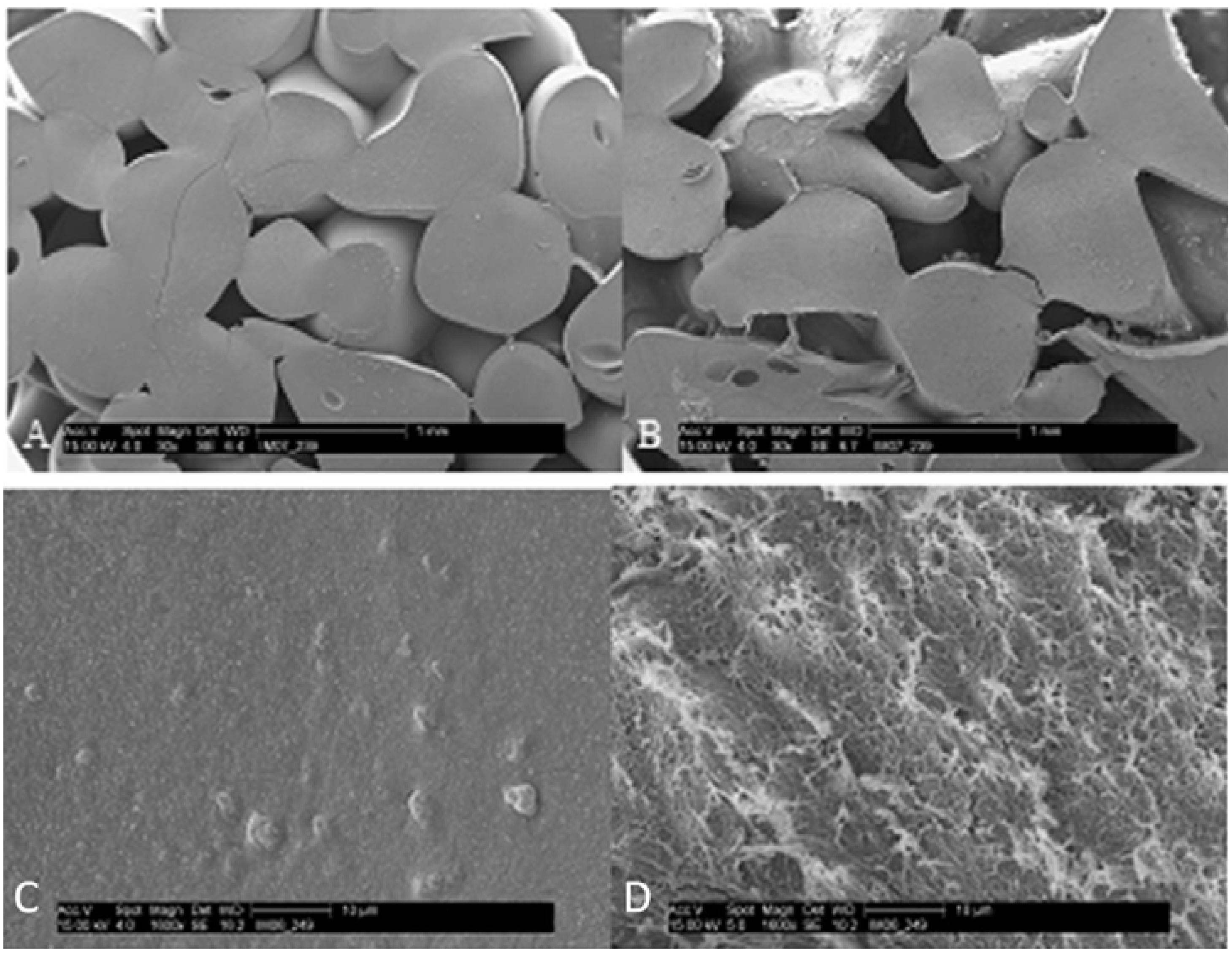
| Scaffold (%) | Osteoid (%) | Bone (%) | |
| Lyophilized (no rhBMP-2) | 65.2 ± 3.7 | 8.8 ± 2.6 | 1.8 ± 0.8 |
| Lyophilized with rhBMP-2 | 59.2 ± 6.1 | 10.4 ± 1.2 | 1.2 ± 0.3 |
| Non-lyophilized with rhBMP-2 | 71.8 ± 3.0 | 7.7 ± 2.4 | 1.0 ± 0.7 |
| Collagen Sponge with rhBMP-2 | N/A | N/A | 94.0 ± 4.4a |

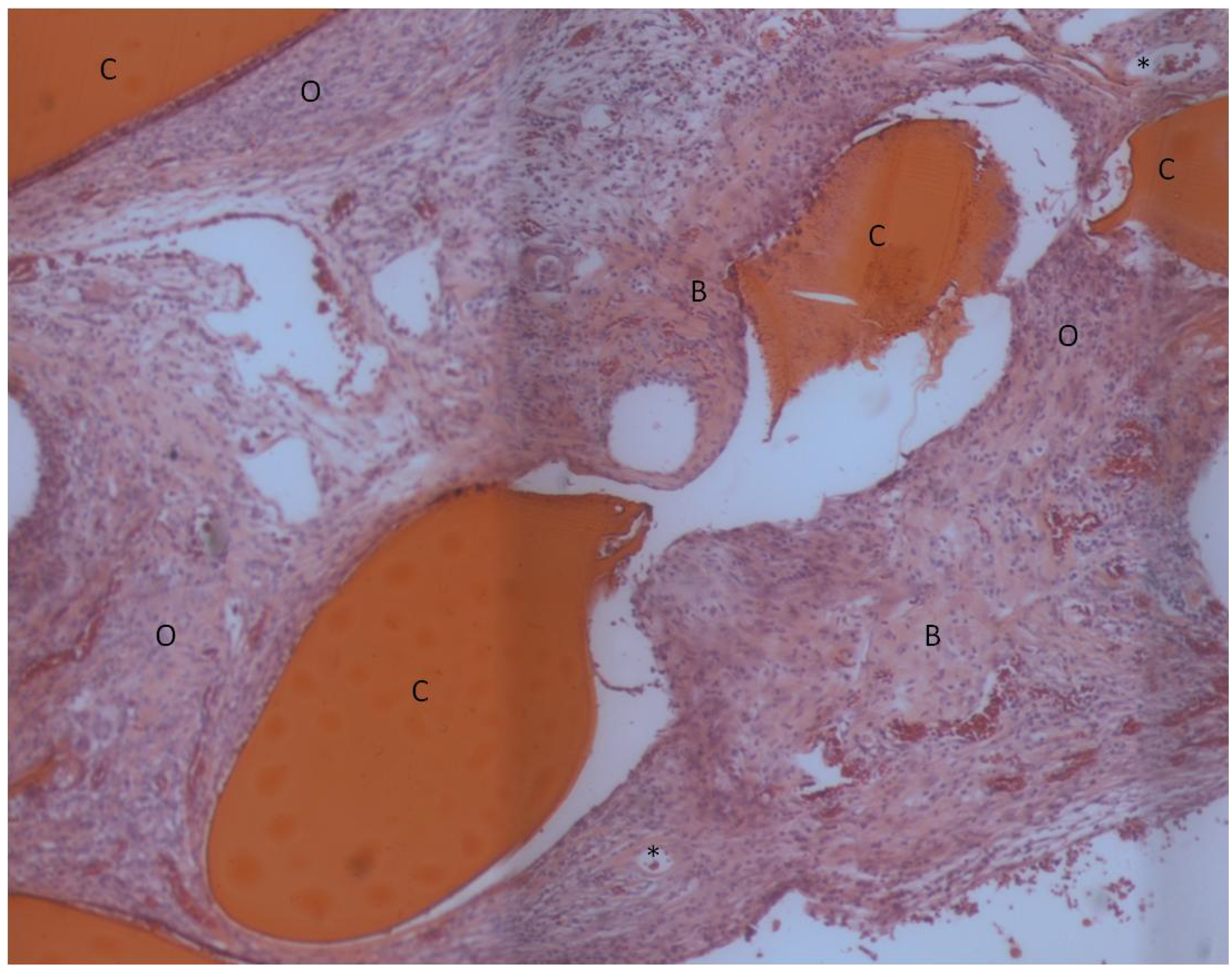
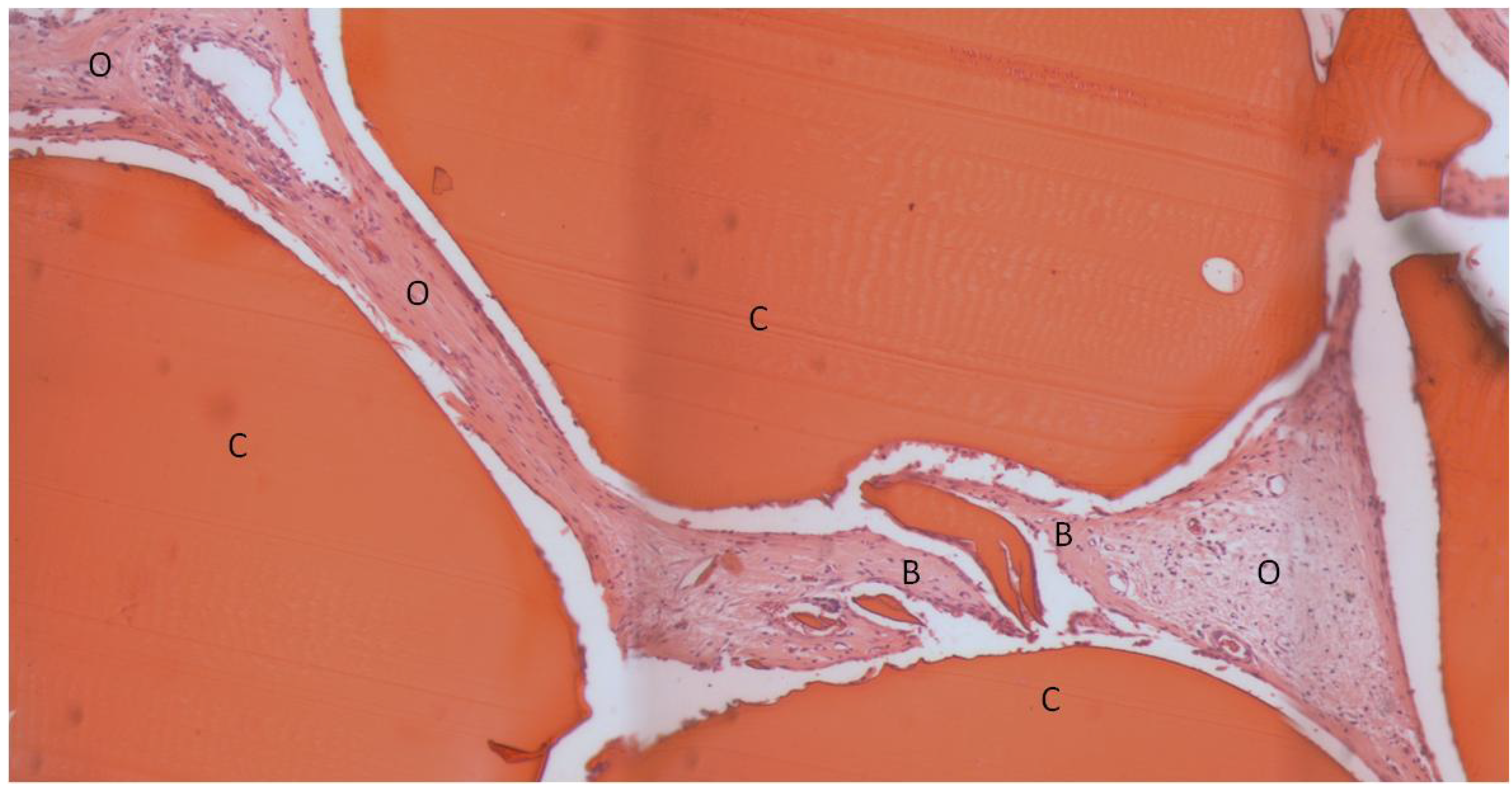
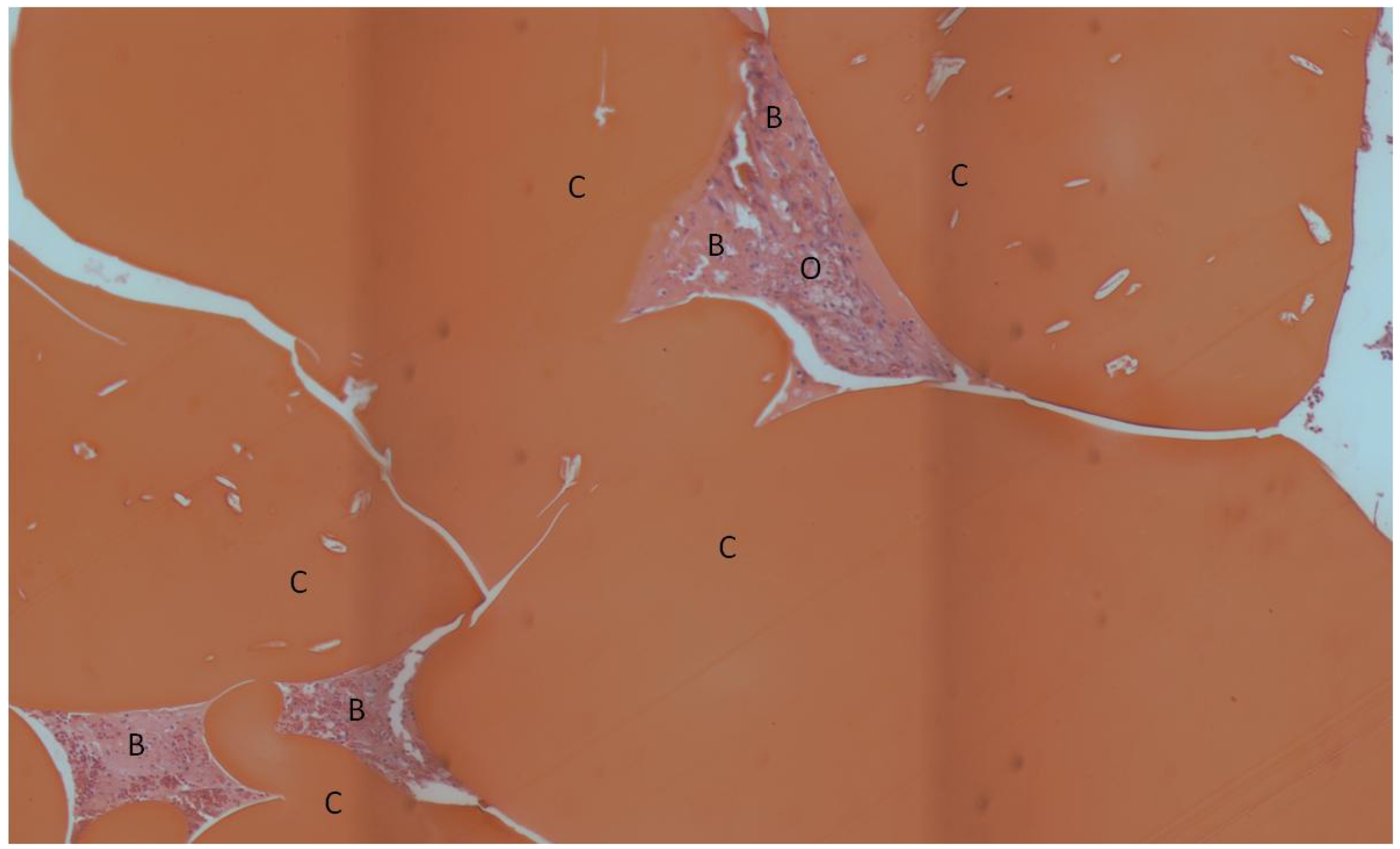
| Bone Tissue Index (%) | |
|---|---|
| Lyophilized (no rhBMP-2) | 30.6 ± 8.5 |
| Lyophilized with rhBMP-2 | 28.8 ± 5.4 |
| Air-dried with rhBMP-2 | 30.5 ± 5.7 |
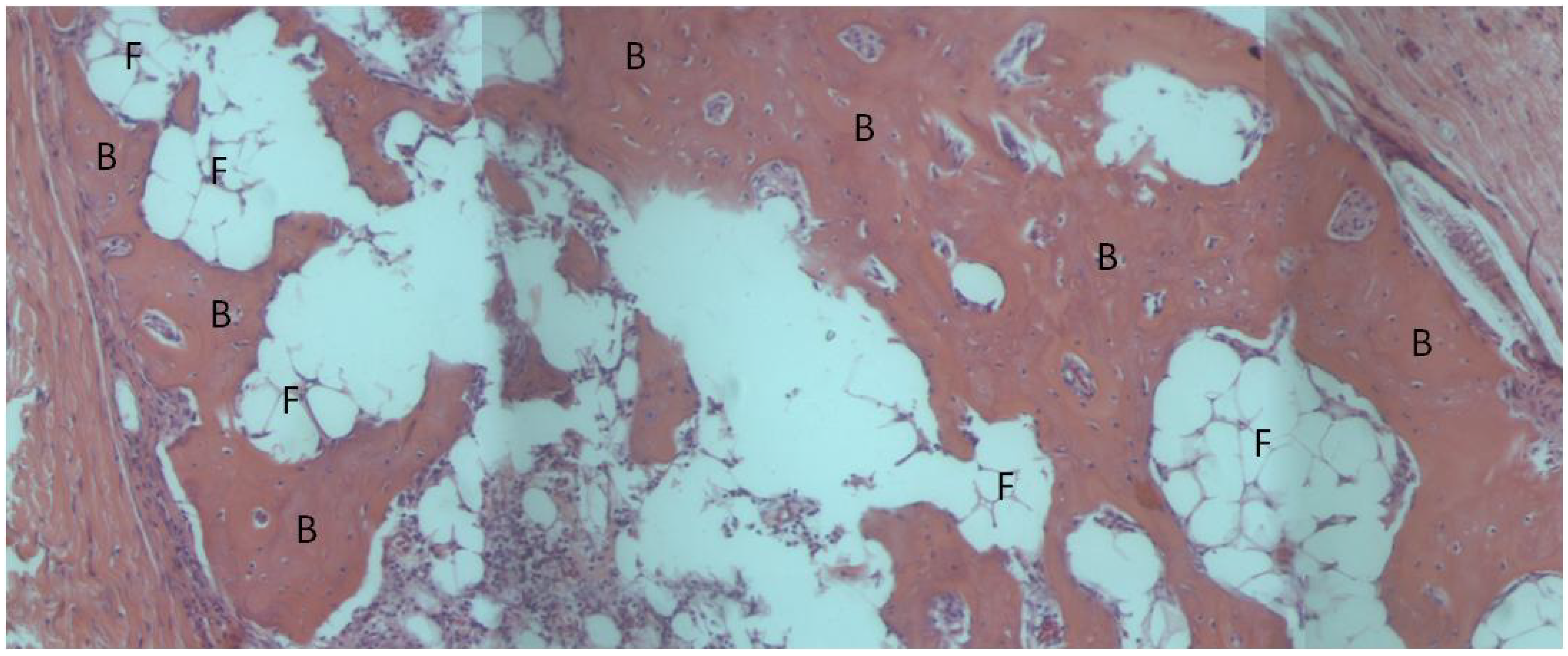
3. Experimental Section
3.1. Composite Microsphere and Scaffold Fabrication
3.2. Scaffold Preparation for Surgery
3.3. Animal Surgeries
4. Conclusions
Acknowledgments
References
- Dawson, J.I.; Oreffo, R.O. Bridging the regeneration gap: Stem cells, biomaterials and clinical translation in bone tissue engineering. Arch. Biochem. Biophys. 2008, 473, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Iqbal, S.A.; Bayat, A. Exploring the application of mesenchymal stem cells in bone repair and regeneration. J. Bone Jt. Surg. Br. 2011, 93, 427–434. [Google Scholar] [CrossRef]
- Khan, Y.; Yaszemski, M.J.; Mikos, A.G.; Laurencin, C.T. Tissue engineering of bone: Material and matrix considerations. J. Bone Jt. Surg. Am. 2008, 90, 36–42. [Google Scholar] [CrossRef]
- Nandi, S.K.; Roy, S.; Mukherjee, P.; Kundu, B.; De, D.K.; Basu, D. Orthopaedic applications of bone graft & graft substitutes: A review. Indian J. Med. Res. 2010, 132, 15–30. [Google Scholar] [PubMed]
- Kretlow, J.D.; Mikos, A.G. Review: Mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007, 13, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Zhao, L.; Zhu, D.; Kanim, L.E.; Wang, J.C.; Delamarter, R.B. Variability across ten production lots of a single demineralized bone matrix product. J. Bone Jt. Surg. Am. 2010, 92, 427–435. [Google Scholar] [CrossRef]
- Kinney, R.C.; Ziran, B.H.; Hirshorn, K.; Schlatterer, D.; Ganey, T. Demineralized bone matrix for fracture healing: Fact or fiction? J. Orthop. Trauma 2010, 24, S52–S55. [Google Scholar] [CrossRef] [PubMed]
- Logeart-Avramoglou, D.; Anagnostou, F.; Bizios, R.; Petite, H. Engineering bone: Challenges and obstacles. J. Cell. Mol. Med. 2005, 9, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Chesnutt, B.M.; Viano, A.M.; Yuan, Y.; Yang, Y.; Guda, T.; Appleford, M.R.; Ong, J.L.; Haggard, W.O.; Bumgardner, J.D. Design and characterization of a novel chitosan/nanocrystalline calcium phosphate composite scaffold for bone regeneration. J. Biomed. Mater. Res. Part A 2009, 88, 491–502. [Google Scholar] [CrossRef]
- Chesnutt, B.M.; Yuan, Y.; Buddington, K.; Haggard, W.O.; Bumgardner, J.D. Composite chitosan/nano-hydroxyapatite scaffolds induce osteocalcin production by osteoblasts in vitro and support bone formation in vivo. Tissue Eng. Part A 2009, 15, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Reves, B.T.; Bumgardner, J.D.; Cole, J.A.; Yang, Y.; Haggard, W.O. Lyophilization to improve drug delivery for chitosan-calcium phosphate bone scaffold construct: A preliminary investigation. J. Biomed. Mater. Res. Part B 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Chen, J.K.; Shen, C.R.; Liu, C.L. N-acetylglucosamine: Production and applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Kim, S.K. Chitosan composites for bone tissue engineering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2010, 47, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhu, Y.; Ran, X.; Wang, M.; Su, Y.; Cheng, T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J. Surg. Res. 2006, 133, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Peter, M.; Ganesh, N.; Selvamurugan, N.; Nair, S.V.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation and characterization of chitosan-gelatin/nanohydroxyapatite composite scaffolds for tissue engineering applications. Carbohydr. Polym. 2010, 80, 687–694. [Google Scholar] [CrossRef]
- Jiang, T.; Kumbar, S.G.; Nair, L.S.; Laurencin, C.T. Biologically active chitosan systems for tissue engineering and regenerative medicine. Curr. Top. Med. Chem. 2008, 8, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Arns, C.H.; Hutmacher, D.W.; Milthorpe, B.K.; Sheppard, A.P.; Knackstedt, M.A. The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials 2009, 30, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Hermida, J.C.; Bergula, A.; Dimaano, F.; Hawkins, M.; Colwell, C.W., Jr.; D’Lima, D.D. An in vivo evaluation of bone response to three implant surfaces using a rabbit intramedullary rod model. J. Orthop. Surg. Res. 2010, 5, 57:1–57:8. [Google Scholar] [CrossRef]
- Coelho, P.G.; Freire, J.N.; Granato, R.; Marin, C.; Bonfante, E.A.; Gil, J.N.; Chuang, S.K.; Suzuki, M. Bone mineral apposition rates at early implantation times around differently prepared titanium surfaces: A study in beagle dogs. Int. J. Oral Maxillofac. Implant. 2011, 26, 63–69. [Google Scholar]
- Xiong, J.; Li, Y.; Hodgson, P.D.; Wen, C. In vitro osteoblast-like cell proliferation on nano-hydroxyapatite coatings with different morphologies on a titanium-niobium shape memory alloy. J. Biomed. Mater. Res. Part A 2010, 95, 766–773. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Ahmad, Z.; Edirisinghe, M.J.; Jayasinghe, S.N.; Ireland, D.C.; Brooks, R.A.; Rushton, N.; Best, S.M.; Bonfield, W. Influence of nanohydroxyapatite patterns deposited by electrohydrodynamic spraying on osteoblast response. J. Biomed. Mater. Res. Part A 2008, 85, 188–194. [Google Scholar] [CrossRef]
- Lieberman, J.R.; Daluiski, A.; Einhorn, T.A. The role of growth factors in the repair of bone. Biology and clinical applications. J. Bone Jt. Surg. Am. 2002, 84-A, 1032–1044. [Google Scholar]
- Devescovi, V.; Leonardi, E.; Ciapetti, G.; Cenni, E. Growth factors in bone repair. Chir. Organi Mov. 2008, 92, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.H. Bone morphogenetic proteins: From basic science to clinical applications. J. Bone Jt. Surg. Am. 2001, 83-A, S1–S6. [Google Scholar] [CrossRef]
- Ai-Aql, Z.S.; Alagl, A.S.; Graves, D.T.; Gerstenfeld, L.C.; Einhorn, T.A. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J. Dent. Res. 2008, 87, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Roderer, G.; Gunther, K.P.; Brenner, R.E. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J. Cell. Biochem. 2002, 87, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Nelson, E.R.; Smith, R.L.; Goodman, S.B. The sequential expression profiles of growth factors from osteoprogenitors [correction of osteroprogenitors] to osteoblasts in vitro. Tissue Eng. 2007, 13, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qiu, T.; Wu, X.; Wan, C.; Shi, W.; Wang, Y.; Chen, J.G.; Wan, M.; Clemens, T.L.; Cao, X. Sustained BMP signaling in osteoblasts stimulates bone formation by promoting angiogenesis and osteoblast differentiation. J. Bone Miner. Res. 2009, 24, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.M.; Nataraj, C.; Jaw, R.; Deigl, E.; Bursac, P. Demineralized bone matrix as an osteoinductive biomaterial and in vitro predictors of its biological potential. J. Biomed. Mater. Res. Part B 2009, 89, 127–134. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, K.M.; Baek, H.R.; Jang, S.J.; Ryu, H.S. Combined effects of porous hydroxyapatite and demineralized bone matrix on bone induction: In vitro and in vivo study using a nude rat model. Biomed. Mater. 2011, 6, 015008. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.Q.; Liu, X.H.; Connor, J. Effects of e-beam radiation, storage, and hydration on osteoinductivity of DBM/AM composite. J. Biomed. Mater. Res. Part B 2009, 91, 401–408. [Google Scholar] [CrossRef]
- Borden, M.; Attawia, M.; Khan, Y.; Laurencin, C.T. Tissue engineered microsphere-based matrices for bone repair: Design and evaluation. Biomaterials 2002, 23, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Alsberg, E.; Kong, H.J.; Hirano, Y.; Smith, M.K.; Albeiruti, A.; Mooney, D.J. Regulating bone formation via controlled scaffold degradation. J. Dent. Res. 2003, 82, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Oest, M.E.; Dupont, K.M.; Kong, H.J.; Mooney, D.J.; Guldberg, R.E. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J. Orthop. Res. 2007, 25, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.W.; Yi, H.F.; Wang, W.; Ma, X.J. The enzymatic degradation and swelling properties of chitosan matrices with different degrees of N-acetylation. Carbohydr. Res. 2005, 340, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Madihally, S.V.; Matthew, H.W. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Kruyt, M.C.; Juhl, M.V.; Clyens, S.; Martinetti, R.; Dolcini, L.; Theilgaard, N.; van Blitterswijk, C.A. Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J. Orthop. Res. 2008, 26, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Danilchenko, S.N.; Kalinkevich, O.V.; Pogorelov, M.V.; Kalinkevich, A.N.; Sklyar, A.M.; Kalinichenko, T.G.; Ilyashenko, V.Y.; Starikov, V.V.; Bumeyster, V.I.; Sikora, V.Z.; et al. Characterization and in vivo evaluation of chitosan-hydroxyapatite bone scaffolds made by one step coprecipitation method. J. Biomed. Mater. Res. Part A 2011, 96, 639–647. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Misra, R.D. Biomimetic chitosan-nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.R.; Giri Dev, V.R.; Senthilram, T.; Sathiskumar, D.; Gupta, D.; Ramakrishna, S. Osteoblast mineralization with composite nanofibrous substrate for bone tissue regeneration. Cell Biol. Int. 2011, 35, 73–80. [Google Scholar] [PubMed]
- Kong, L.; Ao, Q.; Wang, A.; Gong, K.; Wang, X.; Lu, G.; Gong, Y.; Zhao, N.; Zhang, X. Preparation and characterization of a multilayer biomimetic scaffold for bone tissue engineering. J. Biomater. Appl. 2007, 22, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chow, K.L.; Leng, Y. Study of hydroxyapatite osteoinductivity with an osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2009, 89, 326–335. [Google Scholar] [CrossRef]
- Xu, Q.; Lu, H.; Zhang, J.; Lu, G.; Deng, Z.; Mo, A. Tissue engineering scaffold material of porous nanohydroxyapatite/polyamide 66. Int. J. Nanomed. 2010, 5, 331–335. [Google Scholar]
- Magan, A.; Ripamonti, U. Geometry of porous hydroxyapatite implants influences osteogenesis in baboons (Papio ursinus). J. Craniofac. Surg. 1996, 7, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Richter, P.W.; Thomas, M.E. Self-inducing shape memory geometric cues embedded within smart hydroxyapatite-based biomimetic matrices. Plast. Reconstr. Surg. 2007, 120, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Yu, B.Y.; Shao, H.J.; Chang, C.H.; Sun, Y.M.; Liu, H.C.; Hou, S.M.; Young, T.H. Effects of the surface characteristics of nano-crystalline and micro-particle calcium phosphate/chitosan composite films on the behavior of human mesenchymal stem cells in vitro. J. Biomater. Sci. Polym. Ed. 2010. [Google Scholar] [CrossRef]
- Habibovic, P.; Sees, T.M.; van den Doel, M.A.; van Blitterswijk, C.A.; de Groot, K. Osteoinduction by biomaterials—Physicochemical and structural influences. J. Biomed. Mater. Res. Part A 2006, 77, 747–762. [Google Scholar] [CrossRef]
- Bjerre, L.; Bunger, C.; Baatrup, A.; Kassem, M.; Mygind, T. Flow perfusion culture of human mesenchymal stem cells on coralline hydroxyapatite scaffolds with various pore sizes. J. Biomed. Mater. Res. Part A 2011, 97, 251–263. [Google Scholar] [CrossRef]
- Ruhe, P.Q.; Hedberg-Dirk, E.L.; Padron, N.T.; Spauwen, P.H.; Jansen, J.A.; Mikos, A.G. Porous poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. Tissue Eng. 2006, 12, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Balmayor, E.R.; Feichtinger, G.A.; Azevedo, H.S.; van Griensven, M.; Reis, R.L. Starch-poly-epsilon-caprolactone microparticles reduce the needed amount of BMP-2. Clin. Orthop. Relat. Res. 2009, 467, 3138–3148. [Google Scholar] [CrossRef] [PubMed]
- Baril, E.; Lefebvre, L.P.; Hacking, S.A. Direct visualization and quantification of bone growth into porous titanium implants using micro computed tomography. J. Mater. Sci. Mater. Med. 2011, 22, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Willie, B.M.; Yang, X.; Kelly, N.H.; Merkow, J.; Gagne, S.; Ware, R.; Wright, T.M.; Bostrom, M.P. Osseointegration into a novel titanium foam implant in the distal femur of a rabbit. J. Biomed. Mater. Res. Part B 2010, 92, 479–488. [Google Scholar]
- Wang, W.; Itoh, S.; Tanaka, Y.; Nagai, A.; Yamashita, K. Comparison of enhancement of bone ingrowth into hydroxyapatite ceramics with highly and poorly interconnected pores by electrical polarization. Acta Biomater. 2009, 5, 3132–3140. [Google Scholar] [CrossRef] [PubMed]
- Zreiqat, H.; Ramaswamy, Y.; Wu, C.; Paschalidis, A.; Lu, Z.; James, B.; Birke, O.; McDonald, M.; Little, D.; Dunstan, C.R. The incorporation of strontium and zinc into a calcium-silicon ceramic for bone tissue engineering. Biomaterials 2010, 31, 3175–3184. [Google Scholar] [CrossRef] [PubMed]
- Boerckel, J.D.; Kolambkar, Y.M.; Dupont, K.M.; Uhrig, B.A.; Phelps, E.A.; Stevens, H.Y.; Garcia, A.J.; Guldberg, R.E. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials 2011, 32, 5241–5251. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, T.; Veltheim, R.; Arnander, C.; Docherty-Skogh, A.C.; Westermark, A.; Ohlsson, C.; Adolfsson, L.; Larm, O. A novel biodegradable delivery system for bone morphogenetic protein-2. Plast. Reconstr. Surg. 2008, 121, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Takita, H.; Vehof, J.W.; Jansen, J.A.; Yamamoto, M.; Tabata, Y.; Tamura, M.; Kuboki, Y. Carrier dependent cell differentiation of bone morphogenetic protein-2 induced osteogenesis and chondrogenesis during the early implantation stage in rats. J. Biomed. Mater. Res. Part A 2004, 71, 181–189. [Google Scholar] [CrossRef]
- Brown, K.V.; Li, B.; Guda, T.; Perrien, D.S.; Guelcher, S.A.; Wenke, J.C. Improving bone formation in a rat femur segmental defect by controlling bone morphogenetic protein-2 release. Tissue Eng. Part A 2011, 17, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Sano, A.; Fujioka, K. Controlled release of rhBMP-2 from collagen minipellet and the relationship between release profile and ectopic bone formation. Int. J. Pharm. 2004, 275, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Srouji, S.; Ben-David, D.; Lotan, R.; Livne, E.; Avrahami, R.; Zussman, E. Slow-release human recombinant bone morphogenetic protein-2 embedded within electrospun scaffolds for regeneration of bone defect: In vitro and in vivo evaluation. Tissue Eng. Part A 2011, 17, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Luca, L.; Rougemont, A.L.; Walpoth, B.H.; Gurny, R.; Jordan, O. The effects of carrier nature and pH on rhBMP-2-induced ectopic bone formation. J. Control. Release 2010, 147, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.S.; Choi, K.H.; Jung, U.W.; Yun, J.H.; Choi, S.H.; Cho, K.S. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli. Biomaterials 2010, 31, 3512–3519. [Google Scholar] [CrossRef] [PubMed]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reves, B.T.; Jennings, J.A.; Bumgardner, J.D.; Haggard, W.O. Osteoinductivity Assessment of BMP-2 Loaded Composite Chitosan-Nano-Hydroxyapatite Scaffolds in a Rat Muscle Pouch. Materials 2011, 4, 1360-1374. https://doi.org/10.3390/ma4081360
Reves BT, Jennings JA, Bumgardner JD, Haggard WO. Osteoinductivity Assessment of BMP-2 Loaded Composite Chitosan-Nano-Hydroxyapatite Scaffolds in a Rat Muscle Pouch. Materials. 2011; 4(8):1360-1374. https://doi.org/10.3390/ma4081360
Chicago/Turabian StyleReves, Benjamin T., Jessica A. Jennings, Joel D. Bumgardner, and Warren O. Haggard. 2011. "Osteoinductivity Assessment of BMP-2 Loaded Composite Chitosan-Nano-Hydroxyapatite Scaffolds in a Rat Muscle Pouch" Materials 4, no. 8: 1360-1374. https://doi.org/10.3390/ma4081360




