Large-Scale Growth of Tubular Aragonite Whiskers through a MgCl2-Assisted Hydrothermal Process
Abstract
:1. Introduction
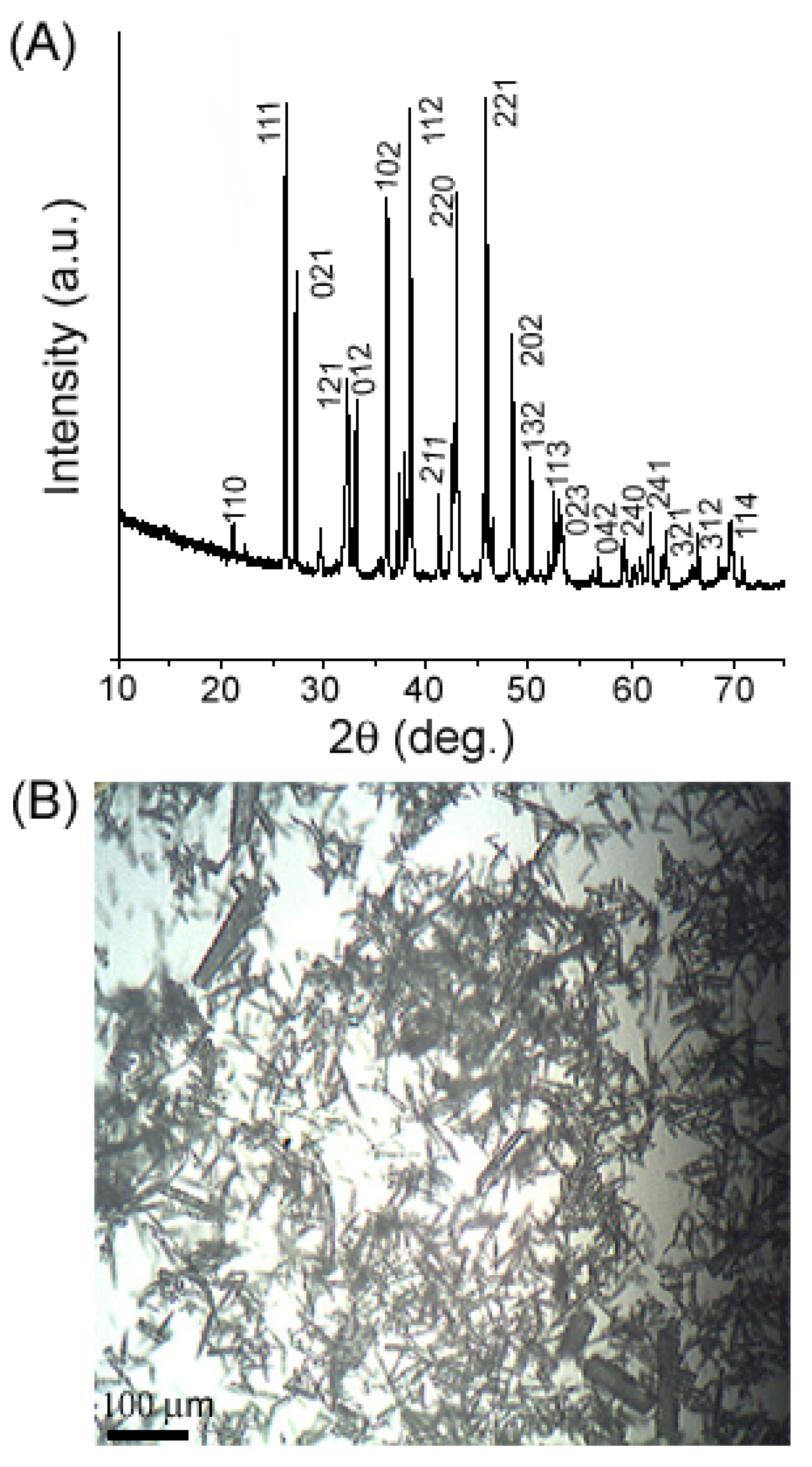
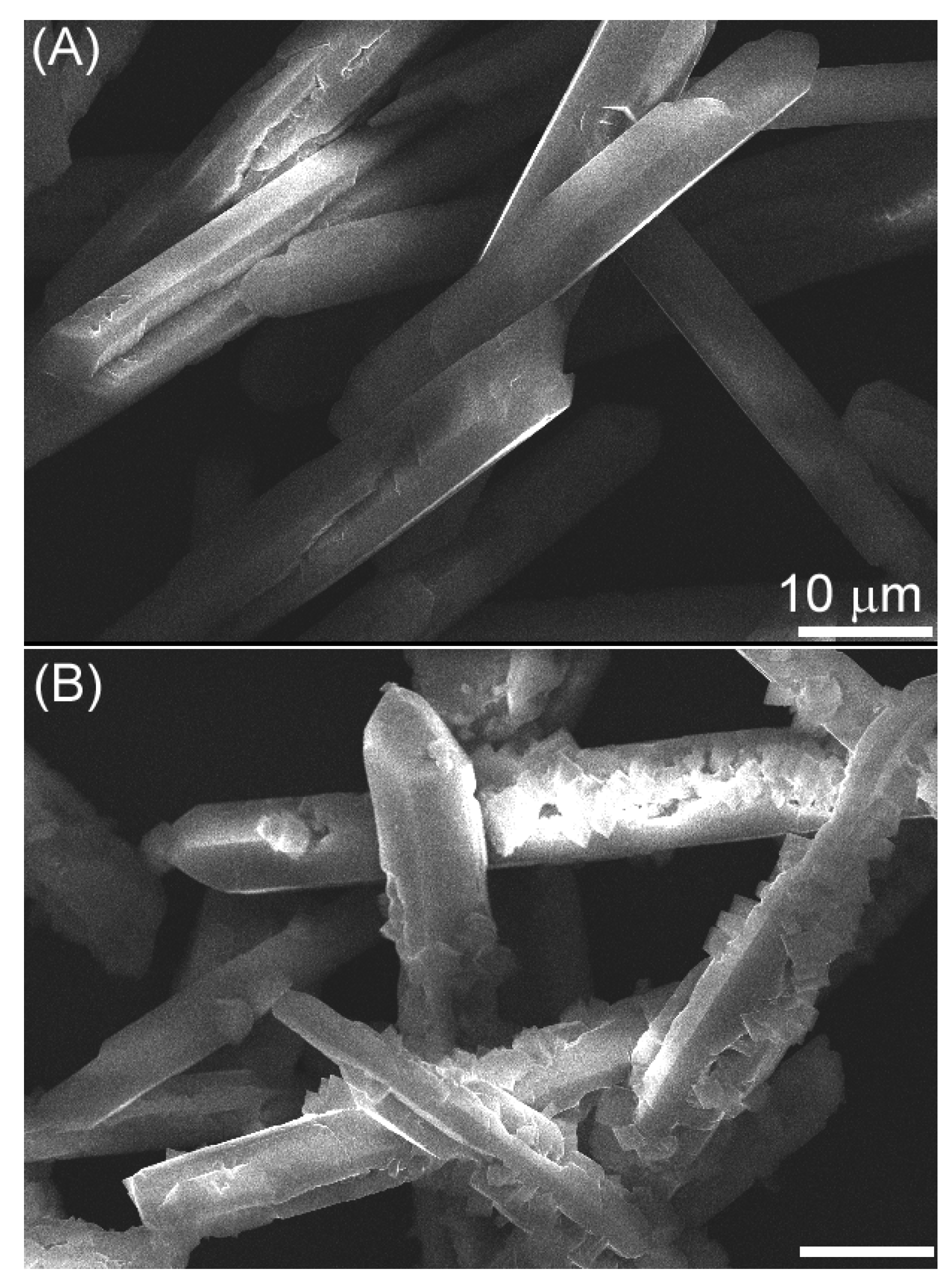
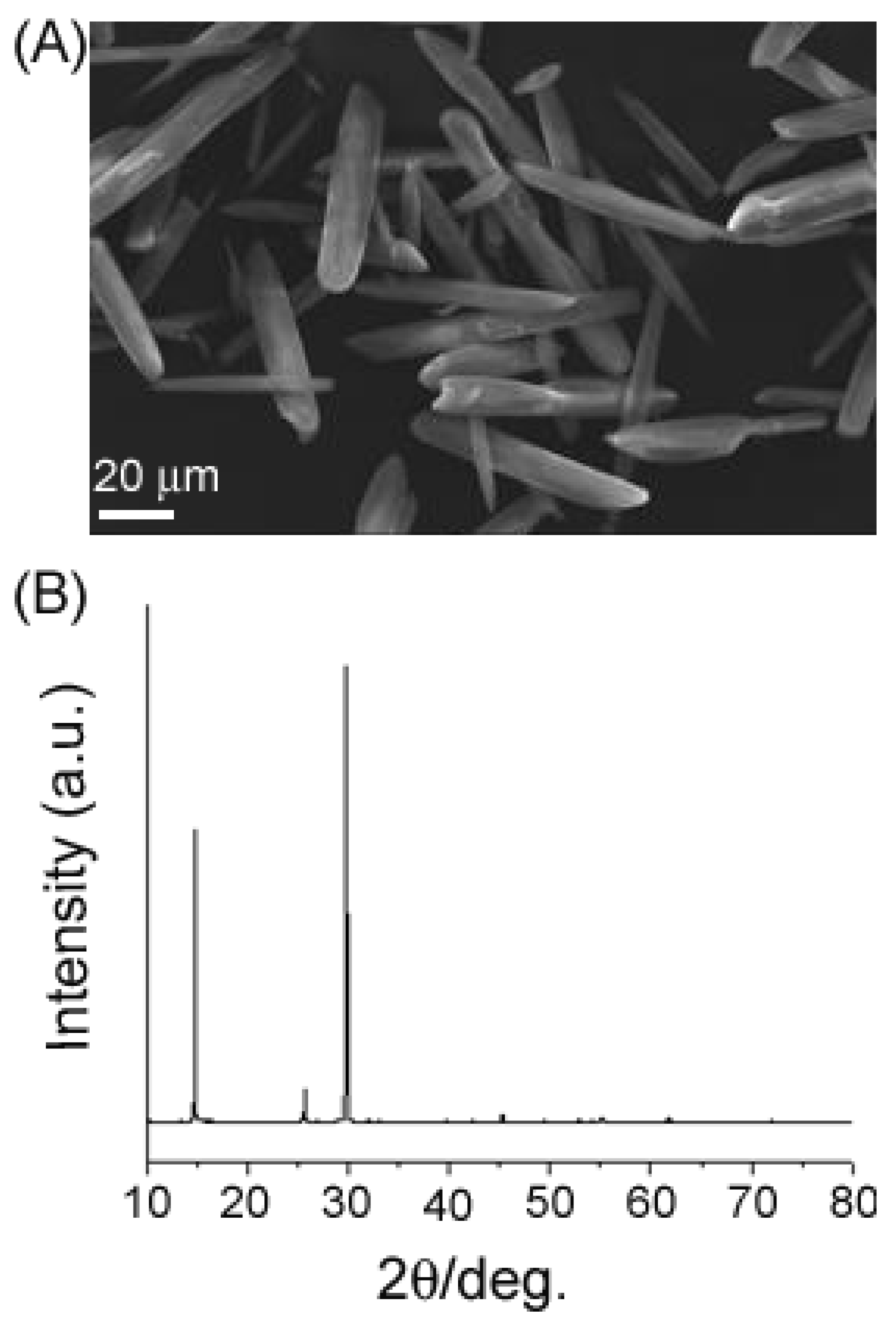
2. Results and Discussion


3. Experimental Section
4. Conclusions
Acknowledgments
References
- Dalas, E.; Klepetsanis, P.; Koutsoukos, P.G. The overgrowth of calcium carbonate on poly(vinyl chloride-co-vinyl acetate-co-maleic acid). Langmuir 1999, 15, 8322–8327. [Google Scholar] [CrossRef]
- Enomae, T. Application of hollow calcium carbonate particles to papermaking. In Proceedings of the Fifth Asian Textile Conference, Kyoto, Japan, 30 September–2 October 1999; Volume 1, pp. 464–467.
- He, E.G.; Shang, W.Y.; Chen, S.T. Effects of phosphate ion on the growth of aragonite whisker in heterogeneous precipitation from suspension of Ca(OH)2. Rare Met. Mater. Eng. 2000, 29, 398–402. [Google Scholar]
- Nassif, N.; Gehrke, N.; Pinna, N.; Shirshova, N.; Tauer, K.; Antonietti, M.; Cölfen, H. Synthesis of stable aragonite superstructures by a biomimetic crystallization pathway. Angew. Chem. Int. Ed. 2005, 44, 6004–6009. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, X.; Sheng, Y.; Wang, C.; Deng, Y.; Ma, X.; Ying, Y.; Wang, Z. Biomimetic synthesis of dendrite-shaped aragonite particles with single-crystal feature by polyacrylic acid. Colloids Surf. A 2007, 297, 198–202. [Google Scholar] [CrossRef]
- Wang, T.; Leng, B.; Che, R.; Shao, Z. Biomimetic synthesis of multilayered aragonite aggregates using alginate as crystal growth modifier. Langmuir 2010, 26, 13385–13392. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhao, X.; Cheng, B.; Zhang, Q. Controlled synthesis of calcium carbonate in a mixed aqueous solution of PSMA and CTAB. J. Solid State Chem. 2005, 178, 861–867. [Google Scholar] [CrossRef]
- Shaw, W.H.R.; Bordeaux, J.J. The decomposition of urea in aqueous media. J. Am. Chem. Soc. 1955, 77, 4729–4733. [Google Scholar] [CrossRef]
- Shen, Q.; Wei, H.; Wang, L.; Zhou, Y.; Zhao, Y.; Zhang, Z.; Wang, D.; Xu, G.; Xu, D. Crystallization and aggregation behaviors of calcium carbonate in the presence of poly(vinylpyrrolidone) and sodium dodecyl sulfate. J. Phys. Chem. B 2005, 109, 18342–18347. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Wang, L.; Huang, Y.; Sun, J.; Wang, H.; Zhou, Y.; Wang, D. Oriented aggregation and novel phase transformation of vaterite controlled by the synergistic effect of calcium dodecyl sulfate and n-pentanol. J. Phys. Chem. B 2006, 110, 23148–23153. [Google Scholar] [CrossRef] [PubMed]
- Kotachi, A.; Miura, T.; Imai, H. Morphological evaluation and film formation with iso-oriented calcite crystals using binary poly(acrylic acid). Chem. Mater. 2004, 16, 3191–3196. [Google Scholar] [CrossRef]
- Liu, D.; Yates, M.Z. Formation of rod-shaped calcite crystals by microemulsion-based synthesis. Langmuir 2006, 22, 5566–5569. [Google Scholar] [CrossRef] [PubMed]
- Juvekar, V.A.; Sharma, M.M. Absorption of CO2 in a suspension of lime. Chem. Eng. Sci. 1973, 28, 825–837. [Google Scholar] [CrossRef]
- Ahn, J.-W.; Choi, K.-S.; Yoon, S.-H.; Kim, H. Synthesis of aragonite by the carbonation process. J. Am. Ceram. Soc. 2004, 87, 286–288. [Google Scholar] [CrossRef]
- Chen, S.-F.; Yu, S.-H.; Jiang, J.; Li, F.; Liu, Y. Polymorph discrimination of CaCO3 mineral in an ethanol/water solution: Formation of complex vaterite superstructures and aragonite rods. Chem. Mater. 2005, 18, 115–122. [Google Scholar] [CrossRef]
- Nan, Z.; Yan, B.; Wang, X.; Guo, R.; Hou, W. Fabrication of calcite aggregates and aragonite rods in a water/pyridine solution. Cryst. Growth Des. 2008, 8, 4026–4030. [Google Scholar] [CrossRef]
- Beruto, D.; Giordani, M. Calcite and aragonite formation from aqueous calcium hydrogencarbonate solutions: Effect of induced electromagnetic field on the activity of CaCO3 nuclei precursors. J. Chem. Soc. Faraday Trans. 1993, 89, 2457–2461. [Google Scholar] [CrossRef]
- Henderson, P. Inorganic Geochemistry; Pergamon Press: Oxford, UK, 1982; pp. 297–353. [Google Scholar]
- Gutjahr, A.; Dabringhaus, H.; Lacmann, R. Studies of the growth and dissolution kinetics of the CaCO3 polymorphs calcite and aragonite II. The influence of divalent cation additives on the growth and dissolution rates. J. Cryst. Growth 1996, 158, 310–315. [Google Scholar] [CrossRef]
- Ota, Y.; Inui, S.; Iwashita, T.; Kasuga, T.; Abe, Y. Preparation of aragonite whiskers. J. Am. Ceram. Soc. 1995, 78, 1983–1984. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ren, M.; Dong, C.; An, C. Large-Scale Growth of Tubular Aragonite Whiskers through a MgCl2-Assisted Hydrothermal Process. Materials 2011, 4, 1375-1383. https://doi.org/10.3390/ma4081375
Ren M, Dong C, An C. Large-Scale Growth of Tubular Aragonite Whiskers through a MgCl2-Assisted Hydrothermal Process. Materials. 2011; 4(8):1375-1383. https://doi.org/10.3390/ma4081375
Chicago/Turabian StyleRen, Minyan, Changyin Dong, and Changhua An. 2011. "Large-Scale Growth of Tubular Aragonite Whiskers through a MgCl2-Assisted Hydrothermal Process" Materials 4, no. 8: 1375-1383. https://doi.org/10.3390/ma4081375



