Two Types of Inulin Fructotransferases
Abstract
:1. Introduction
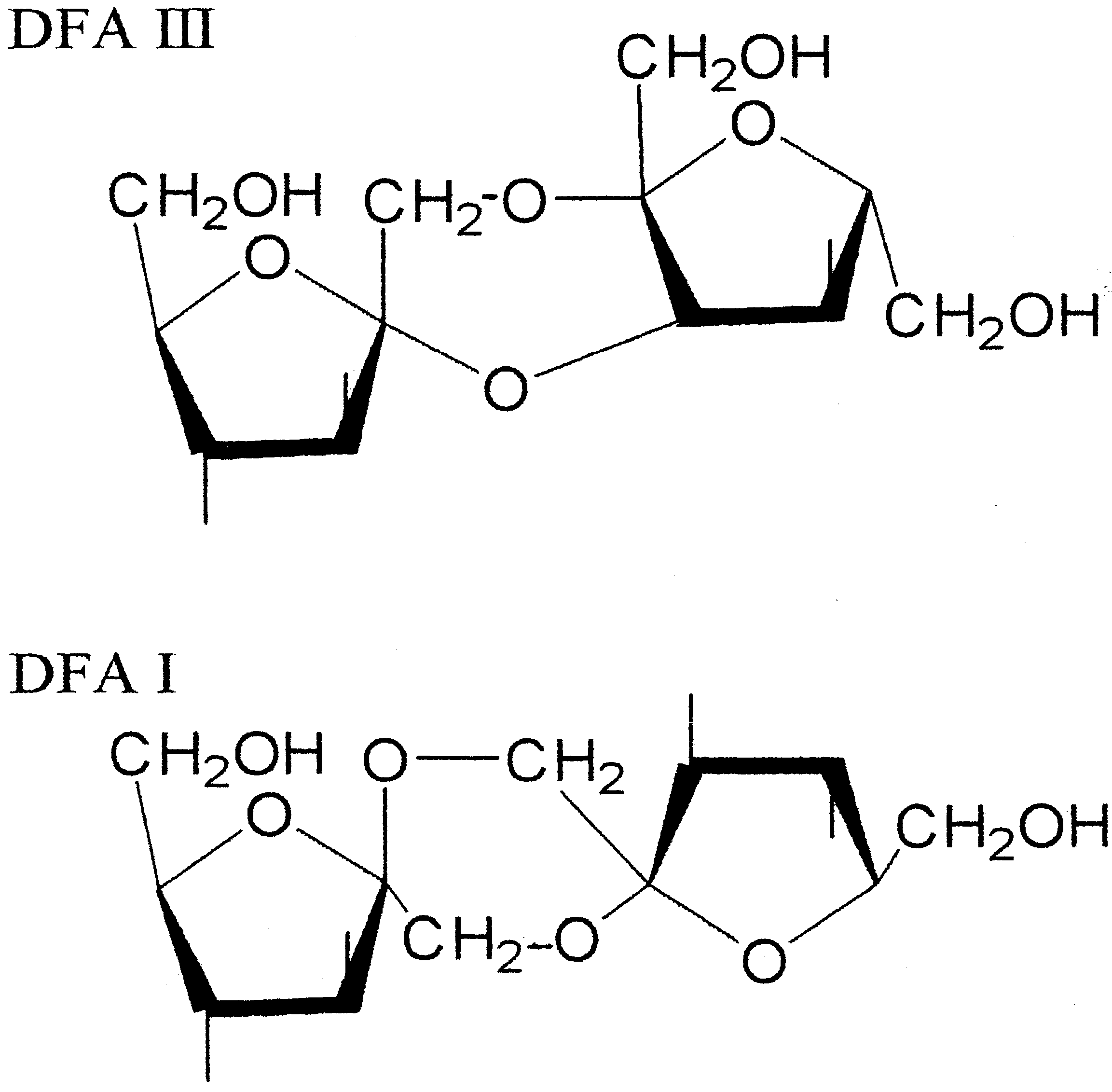
2. Inulin Fructotransferase (DFA III producing)
3. Inulin Fructotransferase (DFA I producing)
References
- Uchiyama, T.; Niwa, S.; Tanaka, K. Purification and properties of Arthrobacter ureafaciens inulase II. Biochim. Biophys. Acta 1973, 315, 412–420. [Google Scholar] [CrossRef]
- Haraguchi, K.; Kishimoto, M.; Seki, K.; Hayashi, K.; Kobayashi, S.; Kainuma, K. Purification and properties of inulin fructotransferase (depolymerizing) from Arthrobacter globiformis C11-1. Agric. Biol. Chem. 1988, 52, 291–292. [Google Scholar] [CrossRef]
- Kawamura, M.; Takahashi, S.; Uchiyama, T. Purification and some properties of inulin fructotransferase (depolymerizing) from Arthrobacter ilicis. Agric. Biol. Chem. 1988, 52, 3209–3210. [Google Scholar] [CrossRef]
- Yokota, A.; Enomoto, K.; Tomita, F. Purification and properties of an inulin fructotransferase (depolymerizing) from Arthrobacter sp. H65-7. J. Ferment. Bioeng. 1991, 72, 262–265. [Google Scholar] [CrossRef]
- Haraguchi, K.; Yoshida, M.; Ohtsubo, K. Thermostable inulin fructotransferase (DFA III-producing) from Arthrobacter sp. L68-1. Carbohydr. Polym. 2005, 59, 411–416. [Google Scholar] [CrossRef]
- Kang, S.; Kim, W.; Chang, Y.; Kim, S. Purification and properties of inulin fructotransferase (DFA III-producing) from Bacillus sp. snu-7. Biosci. Biotech. Biochem. 1998, 62, 628–631. [Google Scholar]
- Haraguchi, K.; Yoshida, M.; Ohtsubo, K. Inulin fructotransferase (DFA III-producing) from Leifsonia sp. T88-4. Carbohydr. Polym. 2006, 66, 75–80. [Google Scholar] [CrossRef]
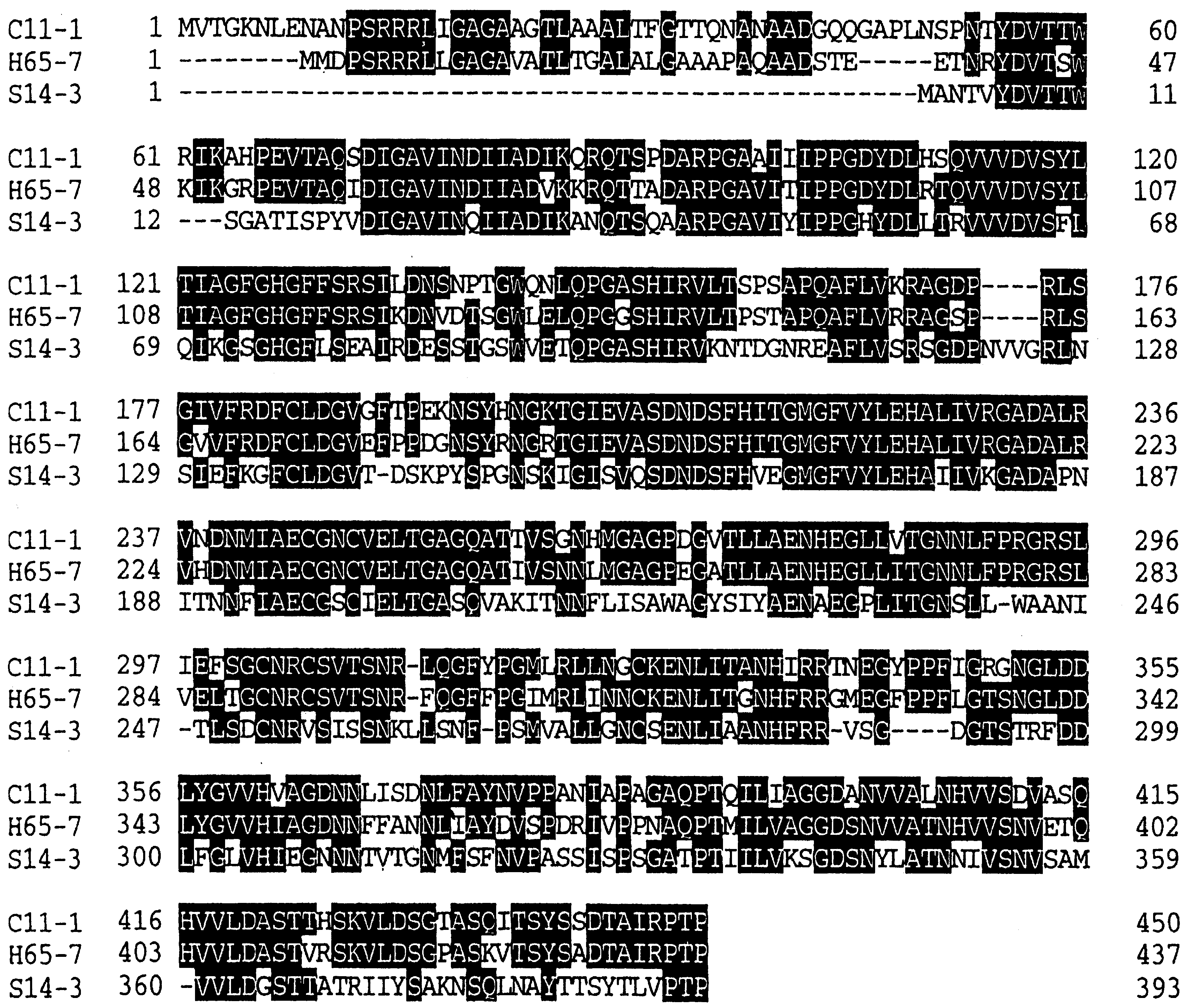
- Sakurai, H.; Yokota, A.; Tomita, F. Molecular cloning of an inulin fructotransfarase (depolymerizing) gene from Arthrobacter sp. H65-7 and its expression in Escherichia coli. Biosci. Biotech. Biochem. 1997, 61, 87–92. [Google Scholar] [CrossRef]
- Haraguchi, K.; Mori, S.; Hayashi, K. Cloning of inulin fructotransferase (DFA III-producing) gene from Arthrobacter globiformis C11-1. J. Biosci. Bioeng. 2000, 89, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Tomita, F. Difructose anhydrides: Their mass production and physiological functions. Biosci. Biotech. Biochem. 2000, 64, 1321–1327. [Google Scholar] [CrossRef]
- Seki, K.; Haraguchi, K.; Kishimoto, M.; Kobayashi, S.; Kainuma, K. Purification and properties of a novel inulin fructotransferase (DFA I-producing) from Arthrobacter globiformis S14-3. Agric. Biol. Chem. 1989, 53, 2089–2094. [Google Scholar] [CrossRef]
- Ueda, M.; Sashida, R.; Morimoto, Y.; Ohkishi, H. Purification of inulin fructotransferase (DFA I-producing) from Arthrobacter sp. MCI-2493 and production of DFA I from inulin by the enzyme. Biosci. Biotech. Biochem. 1994, 58, 574–575. [Google Scholar] [CrossRef]
- Haraguchi, K.; Yoshida, M.; Ohtsubo, K. Purification and properties of a heat-stable inulin fructotransferase from Arthrobacter ureafaciens. Biotech. Lett. 2003, 25, 1049–1053. [Google Scholar] [CrossRef]
- Kushibe, S.; Sashida, R.; Morimoto, Y.; Ohkishi, H. Purification and characterization of a di-D-fructofuranose 2’, 1: 2, 1’-dianhydride producing enzyme from Streptomyces sp. MCI-2524. Biosci. Biotech. Biochem. 1993, 57, 2054–2058. [Google Scholar] [CrossRef]
- Haraguchi, K.; Seki, K.; Kishimoto, M.; Nagata, T.; Kasumi, T.; Kainuma, K.; Kobayashi, S. Cloning and nucleotide sequence of the inulin fructotransferase (DFA I-producing) gene of Arthrobacter globiformis S14-3. Biosci. Biotech. Biochem. 1995, 59, 1809–1812. [Google Scholar] [CrossRef]
© 2011 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Haraguchi, K. Two Types of Inulin Fructotransferases. Materials 2011, 4, 1543-1547. https://doi.org/10.3390/ma4091543
Haraguchi K. Two Types of Inulin Fructotransferases. Materials. 2011; 4(9):1543-1547. https://doi.org/10.3390/ma4091543
Chicago/Turabian StyleHaraguchi, Kazutomo. 2011. "Two Types of Inulin Fructotransferases" Materials 4, no. 9: 1543-1547. https://doi.org/10.3390/ma4091543



