The Effect of a Small Amount of Water on the Structure and Electrochemical Properties of Solid-State Synthesized Polyaniline
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fourier Transform Infrared (FT-IR) Spectra
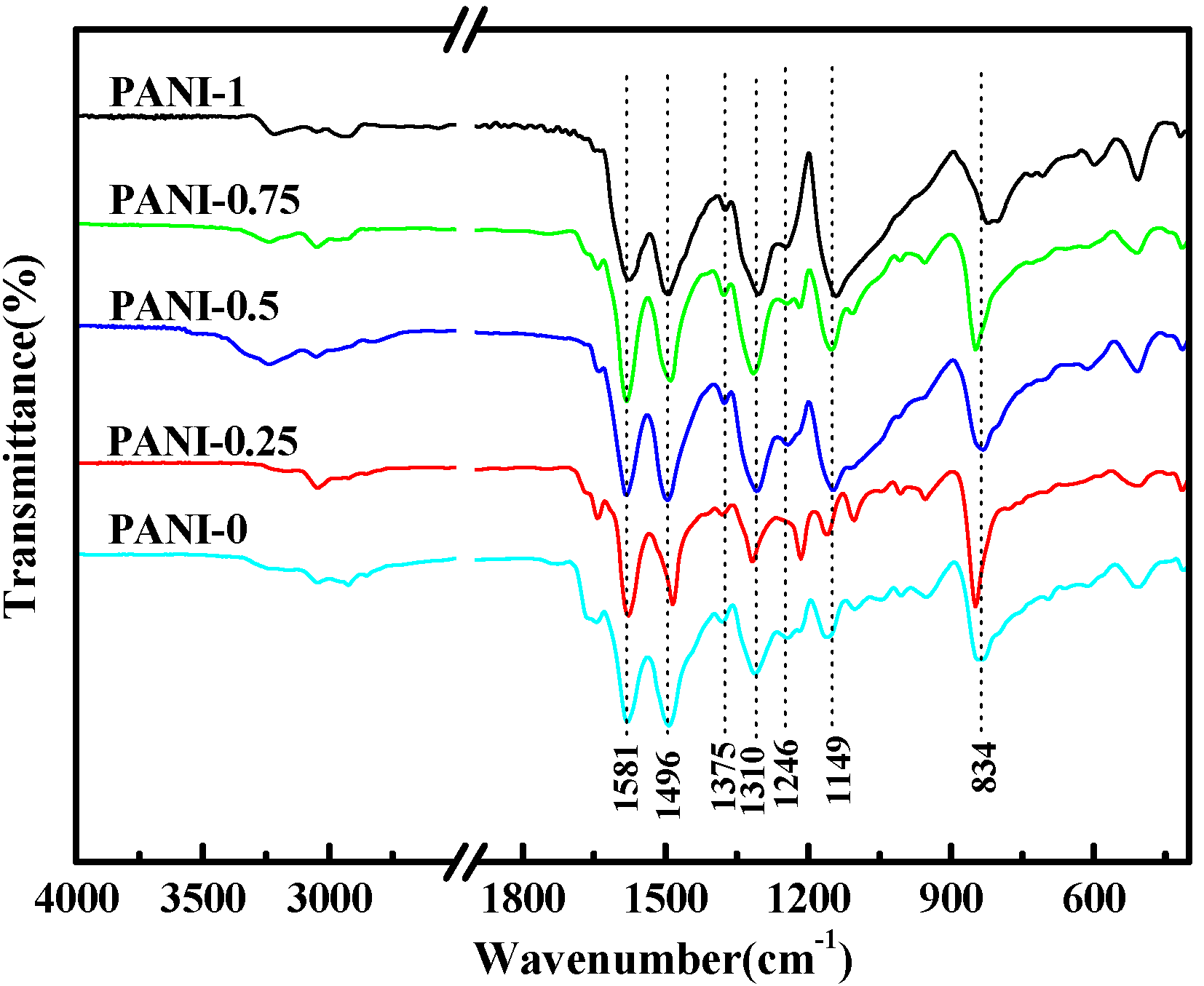
| Sample | Aniline (mL) | p-TSA (g) | APS (g) | H2O (mL) | Yield (%) |
|---|---|---|---|---|---|
| PANI-0 | 1 | 1.9 | 2.2 | 0 | 5.0 |
| PANI-0.25 | 1 | 1.9 | 2.2 | 0.25 | 7.8 |
| PANI-0.5 | 1 | 1.9 | 2.2 | 0.5 | 23.7 |
| PANI-0.75 | 1 | 1.9 | 2.2 | 0.75 | 55.0 |
| PANI-1 | 1 | 1.9 | 2.2 | 1 | 73.2 |
2.2 Ultraviolet-Visible(UV-Vis) Spectra
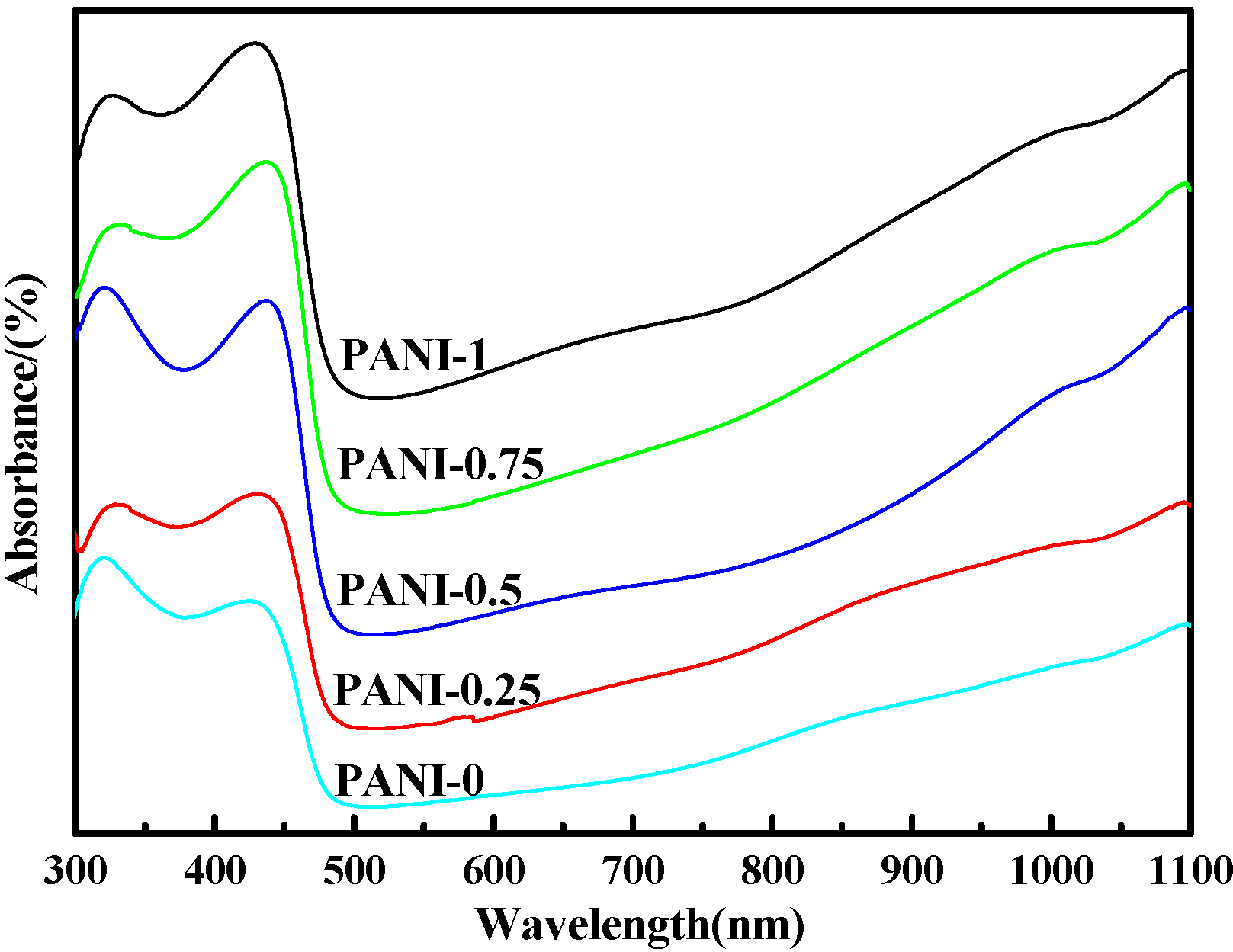
2.3. X-ray Diffraction (XRD) Analysis
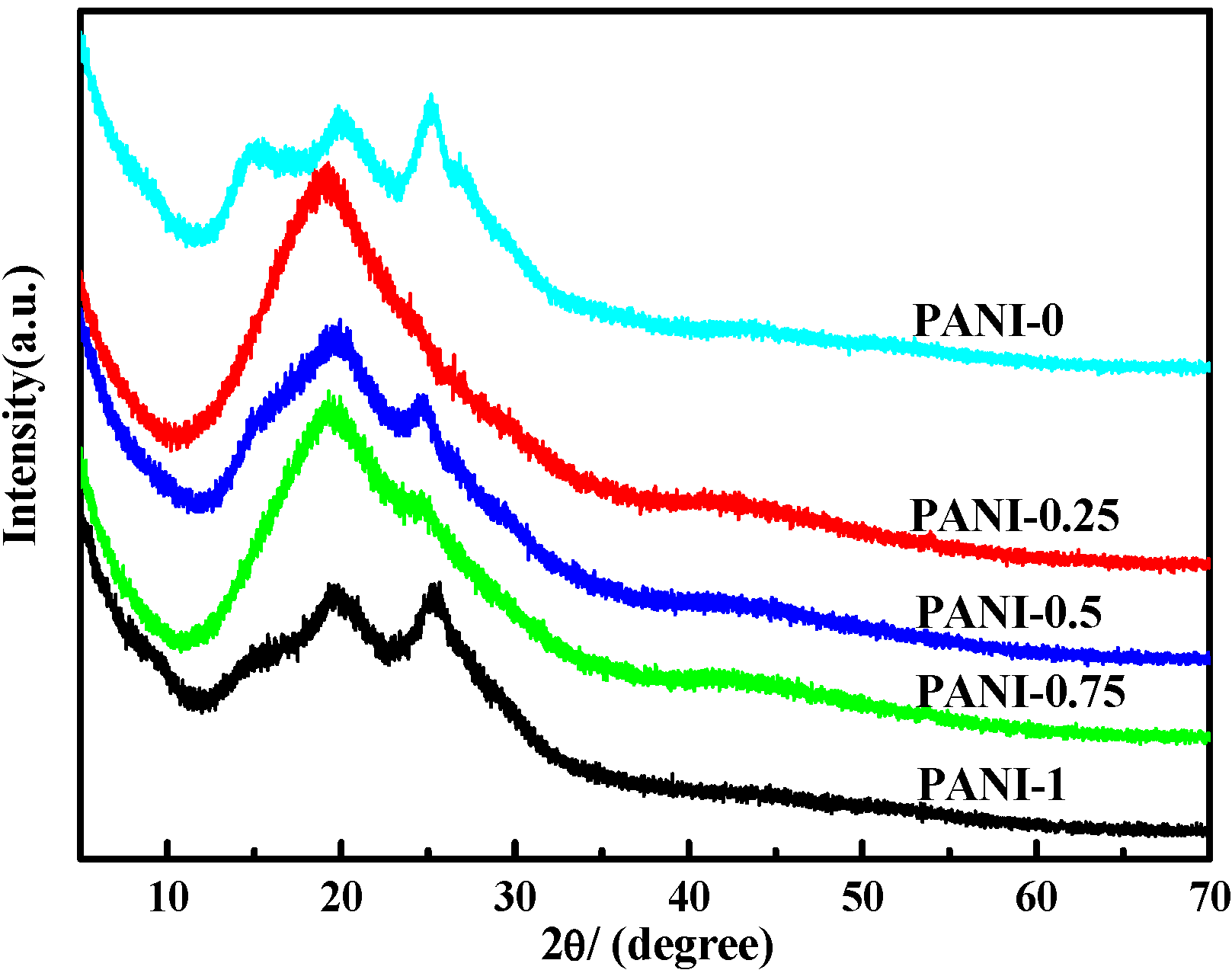
2.4. Morphology

2.5. Electrochemical Properties
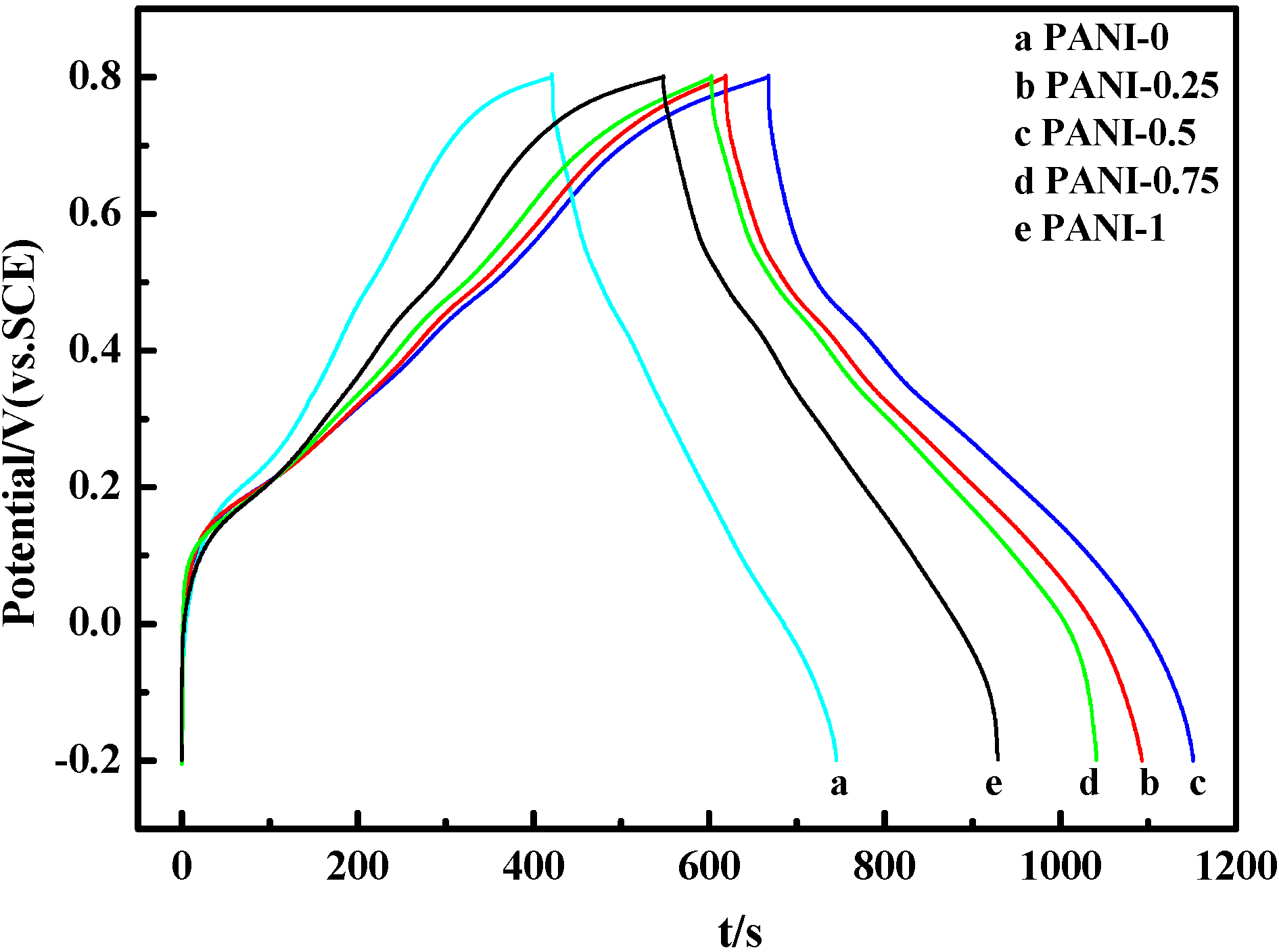
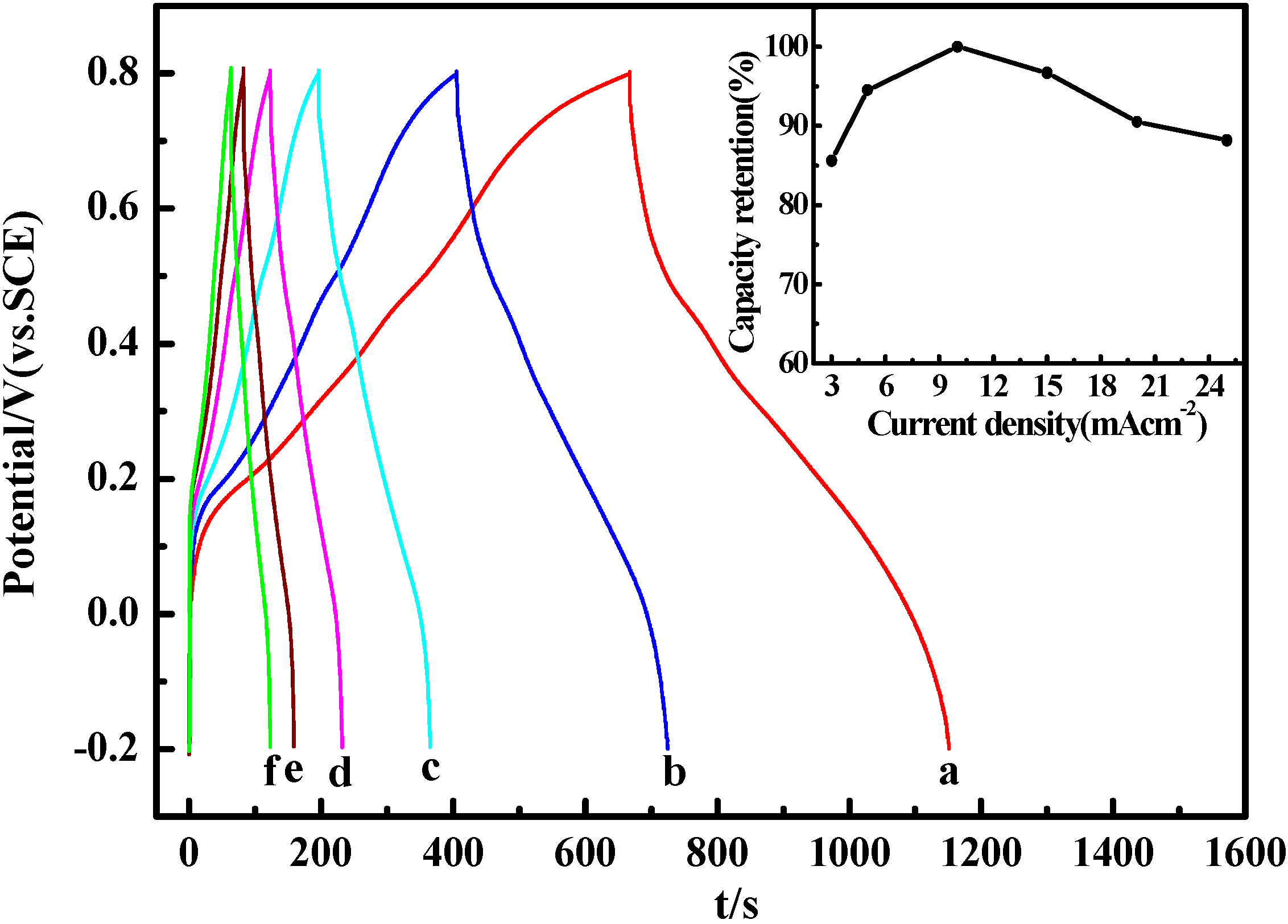
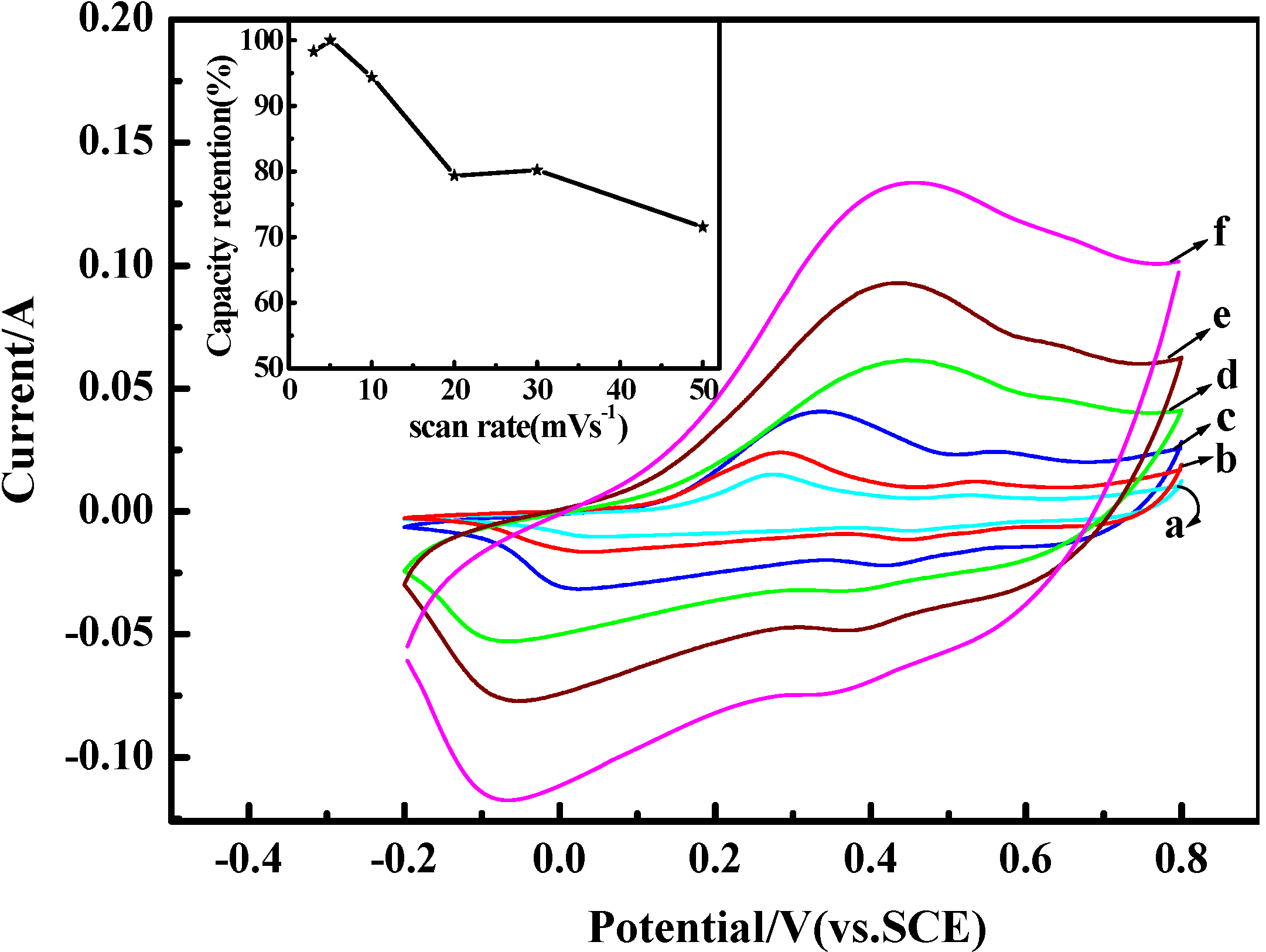
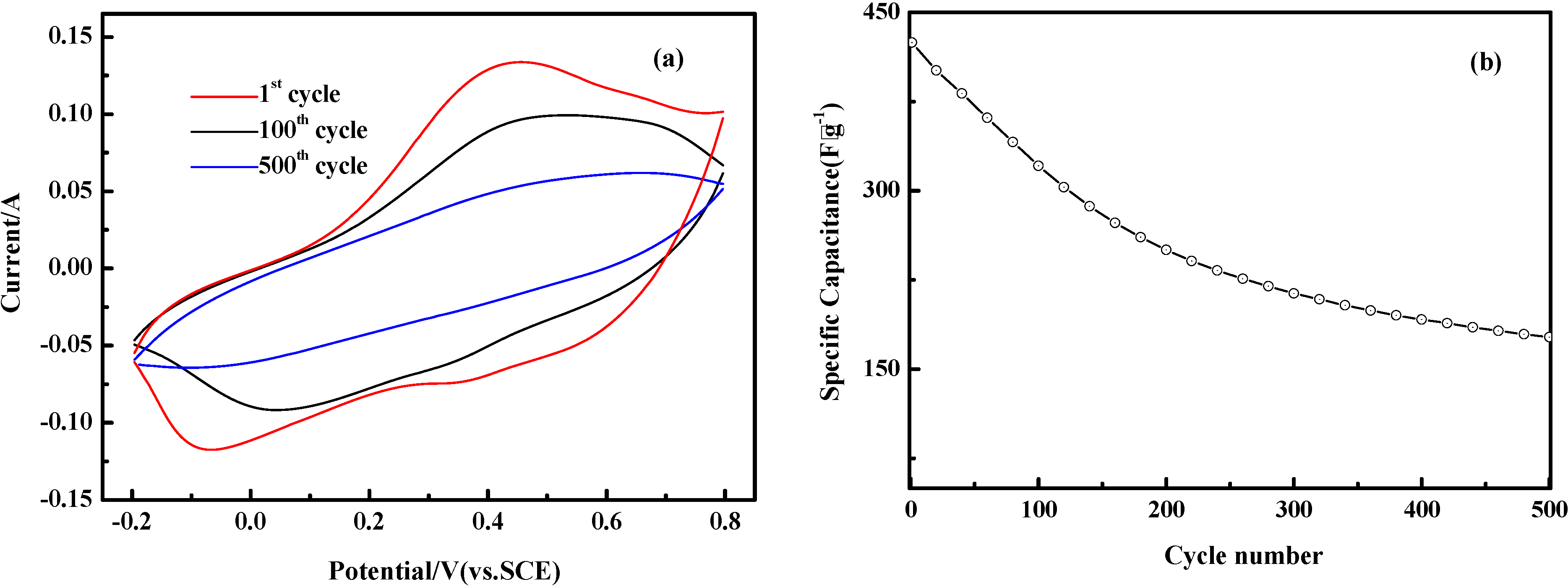
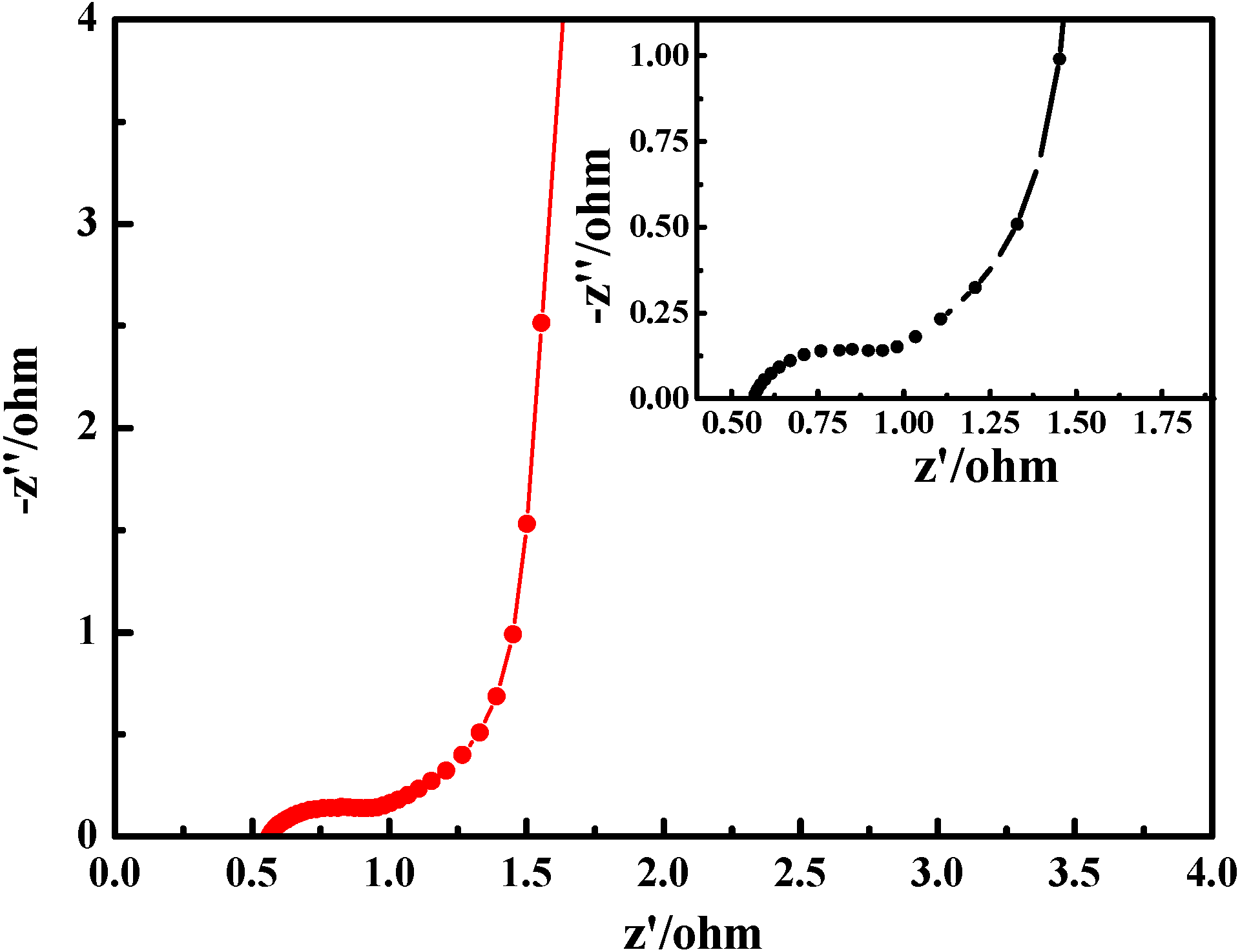
3. Experimental Section
3.1. Materials
3.2. Synthesis of Polyaniline Salts
3.3. Structure Characterization
3.4. Electrochemical Tests
4. Conclusions
Acknowledgments
References
- Novák, P.; Müller, K.; Santhanam, K.S.V.; Haas, O. Electrochemically active polymers for rechargeable batteries. Chem. Rev. 1997, 97, 207–281. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.B.; Han, Z.J.; Yang, Y.; Tay, B.K. NO2 gas sensing with polyaniline nanofibers synthesized by a facile aqueous/organic interfacial polymerization. Sensor. Actuator. B Chem. 2007, 123, 107–113. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Vinu, A.; Lokhande, C.D. Stable nanostructured polyaniline electrode for supercapacitor application. Electrochim. Acta 2011, 56, 9482–9487. [Google Scholar] [CrossRef]
- Bowman, D.; Mattes, B.R. Conductive fibre prepared from ultra-high molecular weight polyaniline for smart fabric and interactive textile applications. Synth. Met. 2005, 154, 29–32. [Google Scholar] [CrossRef]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Semiconductive composites from ethylene 1-octene copolymer and polyaniline coated Nylon 6: Studies on mechanical, thermal, processability, electrical, and EMI shielding properties. Polym. Eng. Sci. 2008, 48, 995–1006. [Google Scholar] [CrossRef]
- Jamadade, V.S.; Dhawale, D.S.; Lokhande, C.D. Studies on electrosynthesized leucoemeraldine, emeraldine and pernigraniline forms of polyaniline films and their supercapacitive behavior. Synth. Met. 2010, 160, 955–960. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Paoli, M.-A.D.; Mastragostino, M. Electrochromic properties of chemically prepared polyaniline. Electrochim. Acta 1991, 36, 2143–2146. [Google Scholar] [CrossRef]
- Gupta, V.; Miura, N. Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance supercapacitors. Electrochim. Acta 2006, 52, 1721–1726. [Google Scholar] [CrossRef]
- Saini, P.; Choudhary, V.; Dhawan, S.K. Electrical properties and EMI shielding behavior of highly thermally stable polyaniline/colloidal graphite composites. Polym. Adv. Technol. 2009, 20, 355–361. [Google Scholar] [CrossRef]
- Palaniappan, S. Chemical and electrochemical polymerization of aniline using tartaric acid. Eur. Polym. J. 2001, 37, 975–981. [Google Scholar] [CrossRef]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Polyaniline by new miniemulsion polymerization and the effect of reducing agent on conductivity. Synth. Met. 2006, 156, 1148–1154. [Google Scholar] [CrossRef]
- Gupta, M.C.; Umare, S.S. Studies on poly(o-methoxyaniline). Macromolecules 1992, 25, 138–142. [Google Scholar] [CrossRef]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Dual functionality of pTSA as electrolyte and dopant in the electrochemical synthesis of polyaniline, and its effect on electrical properties. Polym. Int. 2007, 56, 919–927. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Chiang, J.C.; Halpern, M.; Huang, W.S.; Mu, S.L.; Somasiri, L.D.; Wu, W.Q.; Yaniger, S.I. Polyaniline”: Interconversion of Metallic and Insulating Forms. Mol. Cryst. Liq. Cryst. 1985, 121, 173–180. [Google Scholar] [CrossRef]
- Bekri-Abbes, I.; Srasra, E. Characterization and AC conductivity of polyaniline–montmorillonite nanocomposites synthesized by mechanical/chemical reaction. React. Func. Polym. 2010, 70, 11–18. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Dubal, D.P.; Jamadade, V.S.; Salunkhe, R.R.; Lokhande, C.D. Fuzzy nanofibrous network of polyaniline electrode for supercapacitor application. Synth. Met. 2010, 160, 519–522. [Google Scholar] [CrossRef]
- Toda, F. Organic Solid-State Reactions; Springer: London, UK, 2002. [Google Scholar]
- Huang, J.X.; Moore, J.A.; Acquaye, J.H.; Kaner, R.B. Mcchanochemical route to the conducting polymer polyaniline. Macromolecules 2005, 38, 317–321. [Google Scholar] [CrossRef]
- Abdiryim, T.; Jamal, R.; Nurulla, I. Doping effect of organic sulphonic acids on the solid-state synthesized polyaniline. J. Appl. Polym. Sci. 2007, 105, 576–584. [Google Scholar] [CrossRef]
- Bhadra, S.; Kim, N.H.; Rhee, K.Y.; Lee, J.H. Preparation of nanosize polyaniline by solid-state polymerization and determination of crystal structure. Polym. Int. 2009, 58, 1173–1180. [Google Scholar] [CrossRef]
- Bekri-Abbes, I.; Srasra, E. Investigation of structure and conductivity properties of polyaniline synthesized by solid-solid reaction. J. Polym. Res. 2011, 18, 659–665. [Google Scholar] [CrossRef]
- Du, X.S.; Zhou, C.F.; Wang, G.T.; Mai, Y.W. Novel solid-state and template-free synthesis of branched polyaniline Nanofibers. Chem. Mater. 2008, 20, 3806–3808. [Google Scholar] [CrossRef]
- Gong, J.; Cui, X.J.; Xie, Z.W.; Wang, S.G.; Qu, L.Y. The solid-state synthesis of polyaniline/H4SiW12O40 materials. Synth. Met. 2002, 129, 187–192. [Google Scholar]
- Abdiryim, T.; Zhang, X.G.; Jamal, R. Comparative studies of solid-state synthesized polyaniline doped with inorganic acids. Mater. Chem. Phys. 2005, 90, 367–372. [Google Scholar] [CrossRef]
- Jamal, R.; Abdiryim, T.; Ding, Y.; Nurulla, I. Comparative studies of solid-state synthesized poly (o-methoxyaniline) doped with organic sulfonic acids. J. Polym. Res. 2008, 15, 75–82. [Google Scholar] [CrossRef]
- Wei, Y.; Hsueh, K.F.; Jang, G.W. A study of leucoemeraldine and effect of redox reactions on molecular weight of chemically prepared polyaniline. Macromolecules 1994, 27, 518–525. [Google Scholar] [CrossRef]
- He, Y. A novel emulsion route to sub-micrometer polyaniline/nano-ZnO composite fibers. Appl. Surf. Sci. 2005, 249, 1–6. [Google Scholar] [CrossRef]
- Liu, H.; Hu, X.B.; Wang, J.Y.; Boughton, R.I. Structure, conductivity, and thermopower of crystalline polyaniline synthesized by the ultrasonic irradiation polymerization method. Macromolecules 2002, 35, 9414–9419. [Google Scholar] [CrossRef]
- Nagaraja, M.; Pattar, J.; Shashank, N.; Manjanna, J.; Kamada, Y.; Rajanna, K.; Mahesh, H.M. Electrical, structural and magnetic properties of polyaniline/pTSA-TiO2 nanocomposites. Synth. Met. 2009, 159, 718–722. [Google Scholar] [CrossRef]
- Wang, J.S.; Wang, J.X.; Yang, Z.; Wang, Z.; Zhang, F.B.; Wang, S.C. A novel strategy for the synthesis of polyaniline nanostructures with controlled morphology. React. Funct. Polym. 2008, 68, 1435–1440. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D. Extrinsic and intrinsic structural change during heat treatment of polyaniline. Polym. Degrad. Stabil. 2008, 93, 1094–1099. [Google Scholar] [CrossRef]
- Bhadra, S.; Chattopadhyay, S.; Singha, N.K.; Khastgir, D. Improvement of conductivity of electrochemically synthesized polyaniline. J. Appl. Polym. Sci. 2008, 108, 57–64. [Google Scholar] [CrossRef]
- Gospodinova, N.; Trlemezyan, L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Prog. Polym. Sci. 1998, 23, 1443–1484. [Google Scholar] [CrossRef]
- Dai, L.M.; Wang, Q.; Wan, M.X. Direct observation of conformational transitions for polyaniline chains intercalated in clay particles upon secondary doping. J. Mater. Sci. Lett. 2000, 19, 1645–1647. [Google Scholar] [CrossRef]
- Chiou, N.-R.; Lee, L.J.; Epstein, A. Self-assembled polyaniline nanofibers/nanotubes. Chem. Mater. 2007, 19, 3589–3591. [Google Scholar] [CrossRef]
- Chabukswar, V.; Bhavsar, S. Synthesis and characterization of organically soluble and electrically conducting acids doped polyaniline. Chem. Chem. Technol. 2010, 4, 277–280. [Google Scholar]
- Oh, M.; Park, S.-J.; Jung, Y.; Kim, S. Electrochemical properties of polyaniline composite electrodes prepared by in-situ polymerization in titanium dioxide dispersed aqueous solution. Synth. Met. 2012, 162, 695–701. [Google Scholar] [CrossRef]
- Pant, H.C.; Patra, M.K.; Negi, S.C.; Bhatia, A.; Vadera, S.R.; Kumar, N. Studies on conductivity and dielectric properties of polyaniline–zinc sulphide composites. Bull. Mater. Sci. 2006, 29, 379–384. [Google Scholar] [CrossRef]
- Reddy, K.R.; Sin, B.C.; Ryu, K.S.; Noh, J.; Lee, Y. In situ self-organization of carbon black-polyaniline composites from nanospheres to nanorods: Synthesis, morphology, structure and electrical conductivity. Synth. Met. 2009, 159, 1934–1939. [Google Scholar] [CrossRef]
- Chaudhari, H.K.; Kelkar, D.S. Investigation of structure and electrical conductivity in doped polyaniline. Polym. Int. 1997, 42, 380–384. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.L.; Gao, F.; Li, W.S.; Wu, K.Z.; Wang, X.D. Activated carbon coated with polyaniline as an electrode material in supercapacitors. New Carbon Mater. 2008, 23, 275–280. [Google Scholar] [CrossRef]
- Pouget, J.P.; Hsu, C.H.; MacDiarmid, A.G.; Epstein, A.J. Structural investigation of metallic PAN-CSA and some of its derivatives. Synth. Met. 1995, 69, 119–120. [Google Scholar] [CrossRef]
- Mi, H.Y.; Zhang, X.G.; An, S.Y.; Ye, X.G.; Yang, S.D. Microwave-assisted synthesis and electrochemical capacitance of polyaniline/multi-wall carbon nanotubes composite. Electrochem. Commun. 2007, 9, 2859–2862. [Google Scholar] [CrossRef]
- Su, H.; Wang, T.; Zhang, S.; Song, J.; Mao, C.; Niu, H.; Jin, B.; Wu, J.; Tian, Y. Facile synthesis of polyaniline/TiO2/graphene oxide composite for high performance supercapacitors. Solid State Sci. 2012, 14, 677–681. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Li, H.L. Preparation of polyaniline nanowire arrayed electrodes for electrochemical supercapacitors. Microporous Mesoporous Mater. 2008, 110, 590–594. [Google Scholar] [CrossRef]
- An, K.H.; Jeon, K.K.; Heo, J.K.; Lim, S.C.; Bae, D.J.; Lee, Y.H. High-capacitance supercapacitor using a nanocomposite electrode of single-walled carbon nanotube and polypyrrole. J. Electrochem. Soc. 2002, 149, 1058–1062. [Google Scholar] [CrossRef]
- Bian, C.Q.; Yu, A.S.; Wu, H.Q. Fibriform polyaniline/nano-TiO2 composite as an electrode material for aqueous redox supercapacitors. Electrochem. Commun. 2009, 11, 266–269. [Google Scholar] [CrossRef]
- Chen, W.; Fan, Z.-L.; Gu, L.; Bao, X.-H.; Wang, C.-L. Enhanced capacitance of manganese oxide via confinement inside carbon nanotubes. Chem. Commun. 2010, 46, 3905–3907. [Google Scholar] [CrossRef]
- Mi, H.Y.; Zhang, X.G.; Ye, X.G.; Yang, S.D. Preparation and enhanced capacitance of core-shell polypyrrole/polyaniline composite electrode for supercapacitors. J. Power Sources 2008, 176, 403–409. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shao, W.; Jamal, R.; Xu, F.; Ubul, A.; Abdiryim, T. The Effect of a Small Amount of Water on the Structure and Electrochemical Properties of Solid-State Synthesized Polyaniline. Materials 2012, 5, 1811-1825. https://doi.org/10.3390/ma5101811
Shao W, Jamal R, Xu F, Ubul A, Abdiryim T. The Effect of a Small Amount of Water on the Structure and Electrochemical Properties of Solid-State Synthesized Polyaniline. Materials. 2012; 5(10):1811-1825. https://doi.org/10.3390/ma5101811
Chicago/Turabian StyleShao, Weiwei, Ruxangul Jamal, Feng Xu, Aminam Ubul, and Tursun Abdiryim. 2012. "The Effect of a Small Amount of Water on the Structure and Electrochemical Properties of Solid-State Synthesized Polyaniline" Materials 5, no. 10: 1811-1825. https://doi.org/10.3390/ma5101811




