Commercial Coffee Wastes as Materials for Adsorption of Heavy Metals from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Types of Coffee Wastes
2.3. Adsorption Experiments
2.3.1. Effect of pH
2.3.2. Effect of Contact Time
2.3.3. Effect of Initial Metal Concentration—Isotherms
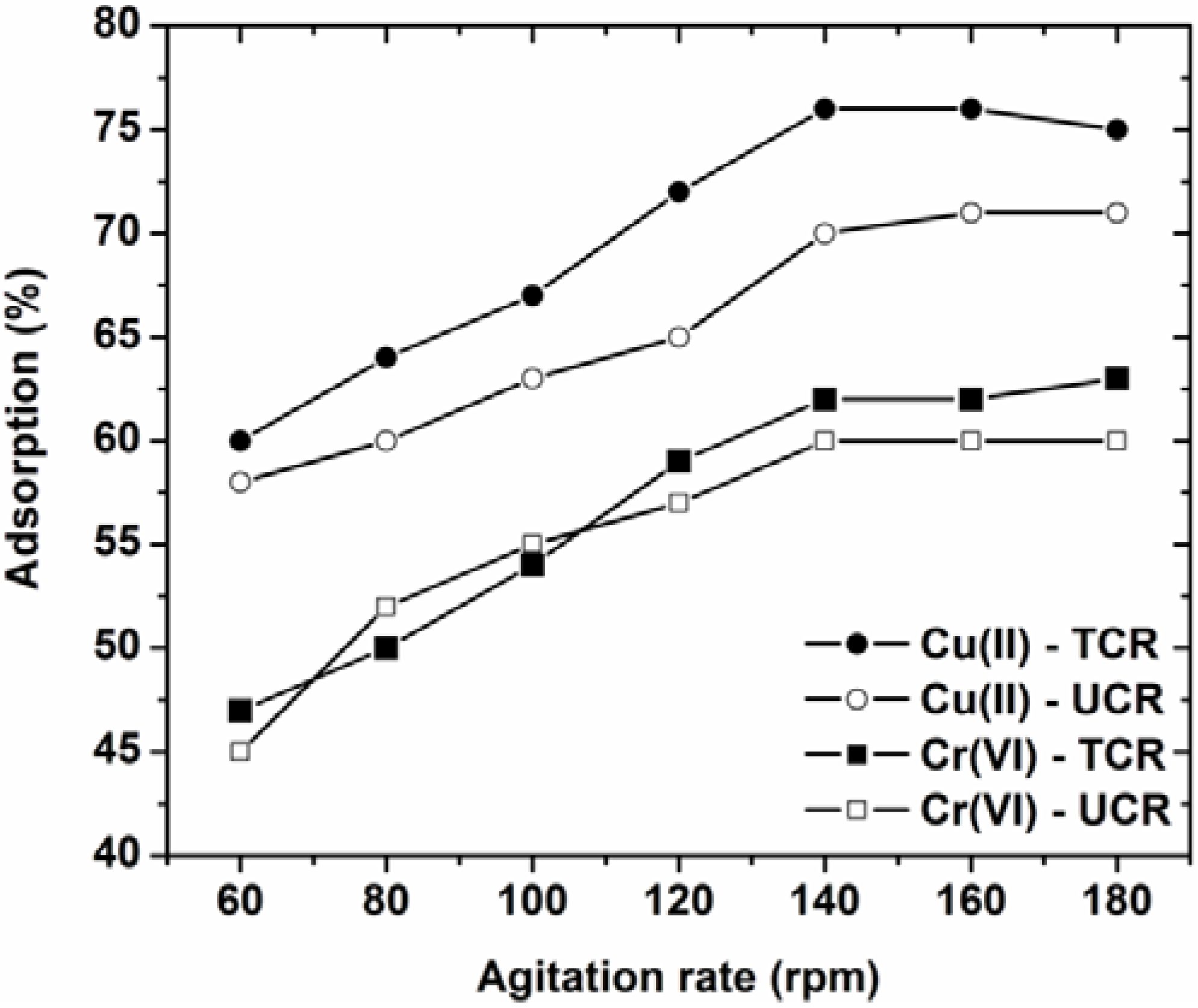
2.3.4. Effect of Agitation Rate
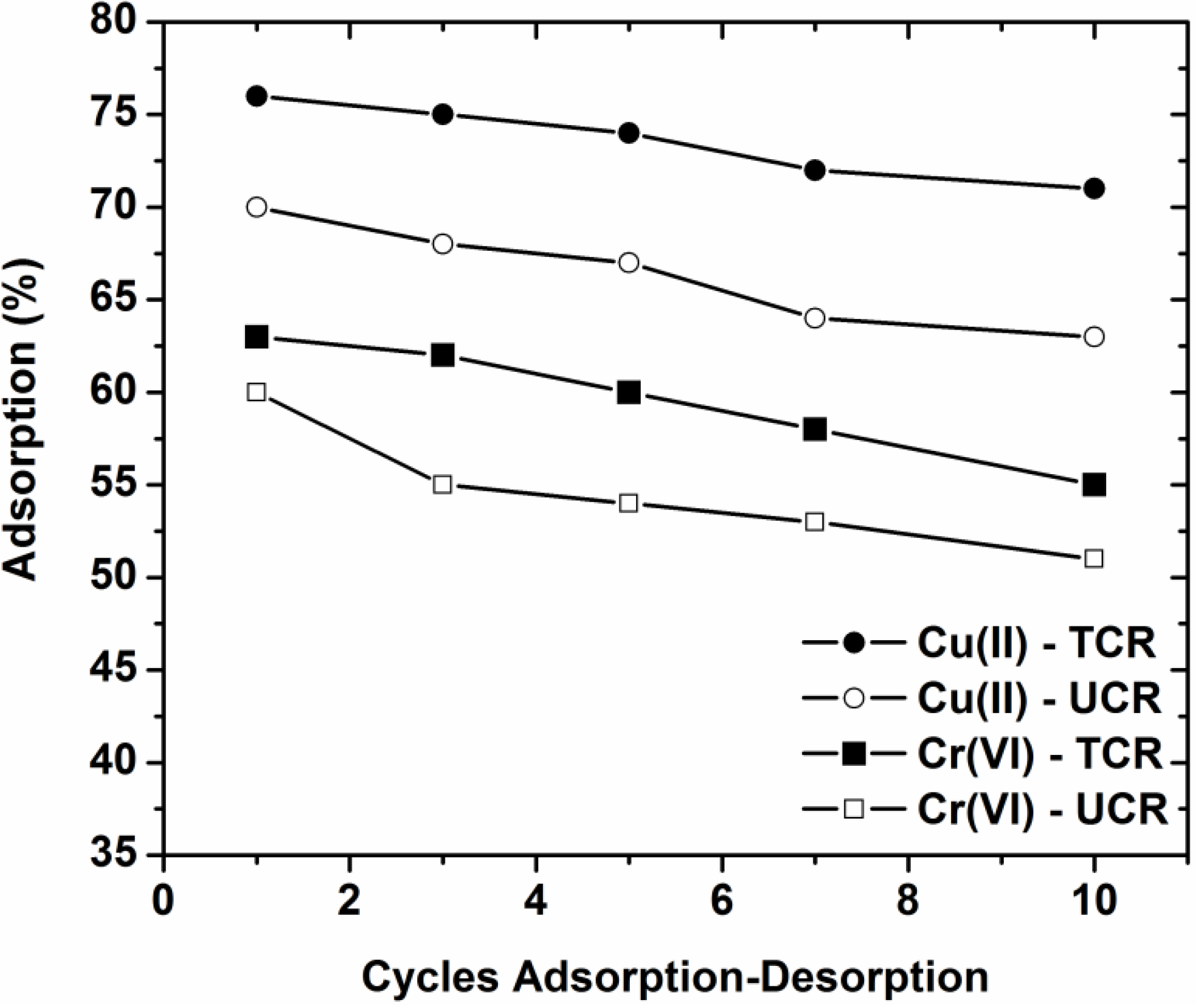
2.4. Desorption and Reuse Experiments
2.5. Analysis
3. Results and Discussion
3.1. Effect of pH on Adsorption—Characterization
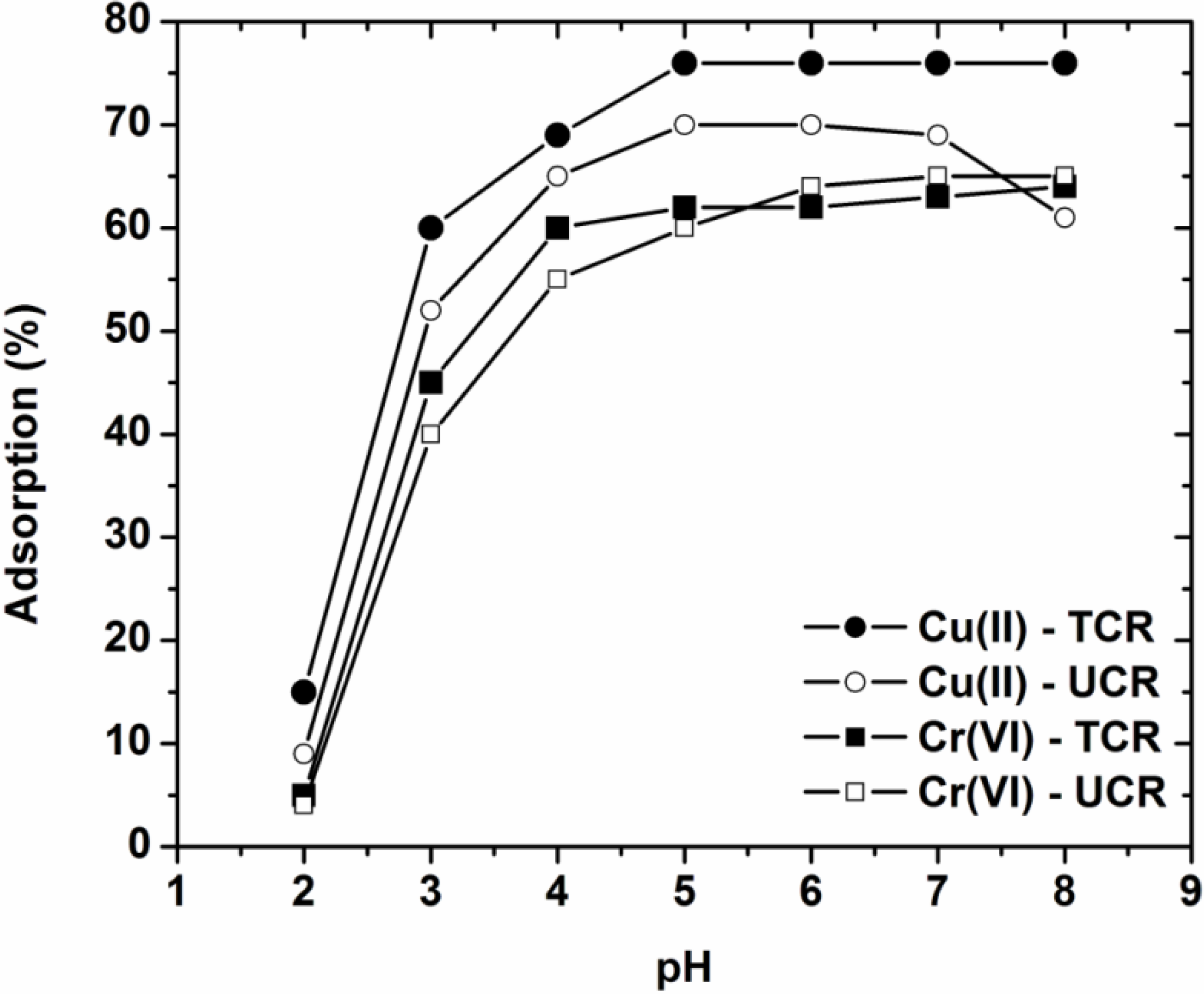
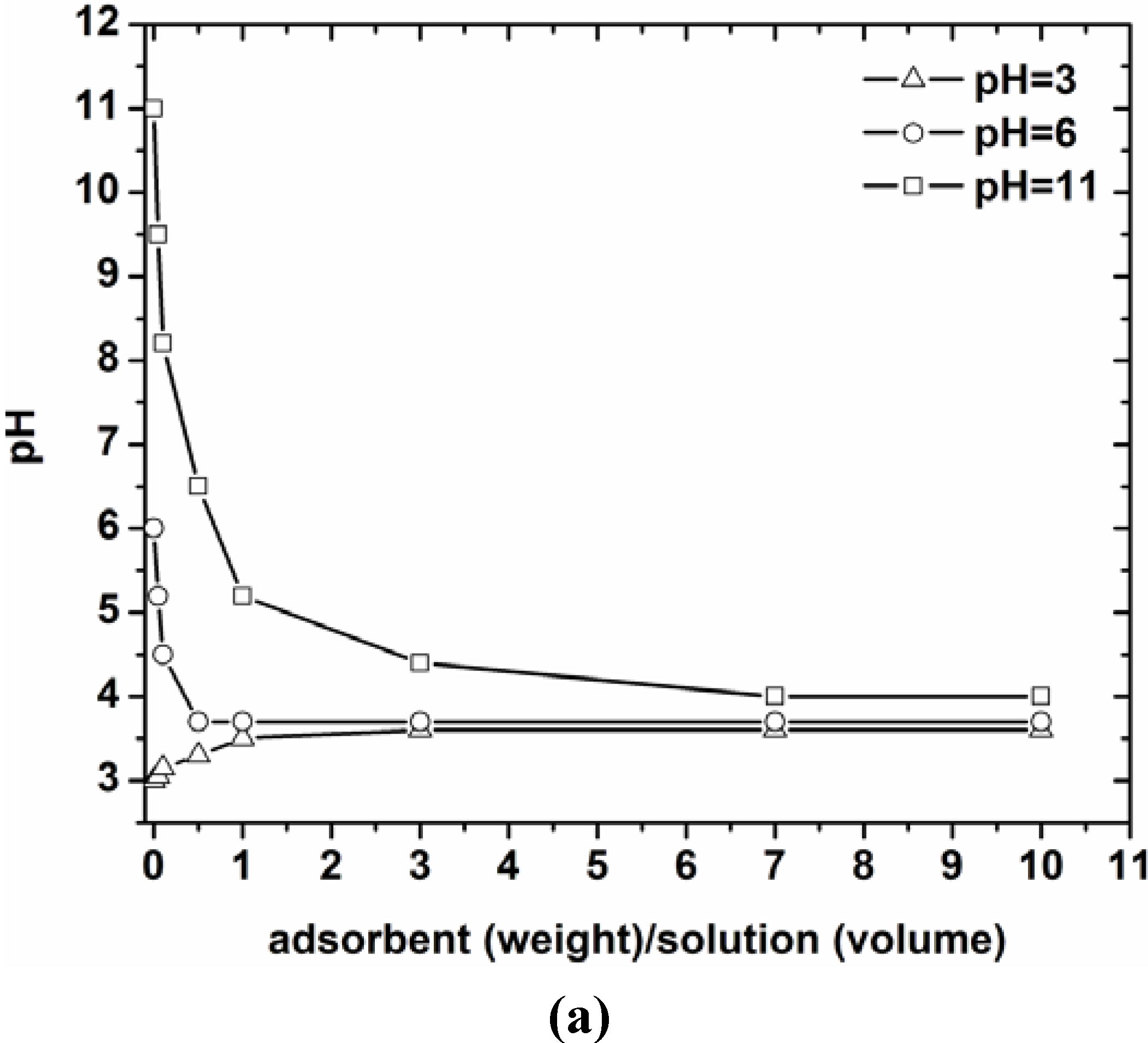
3.2. Effect of Contact Time
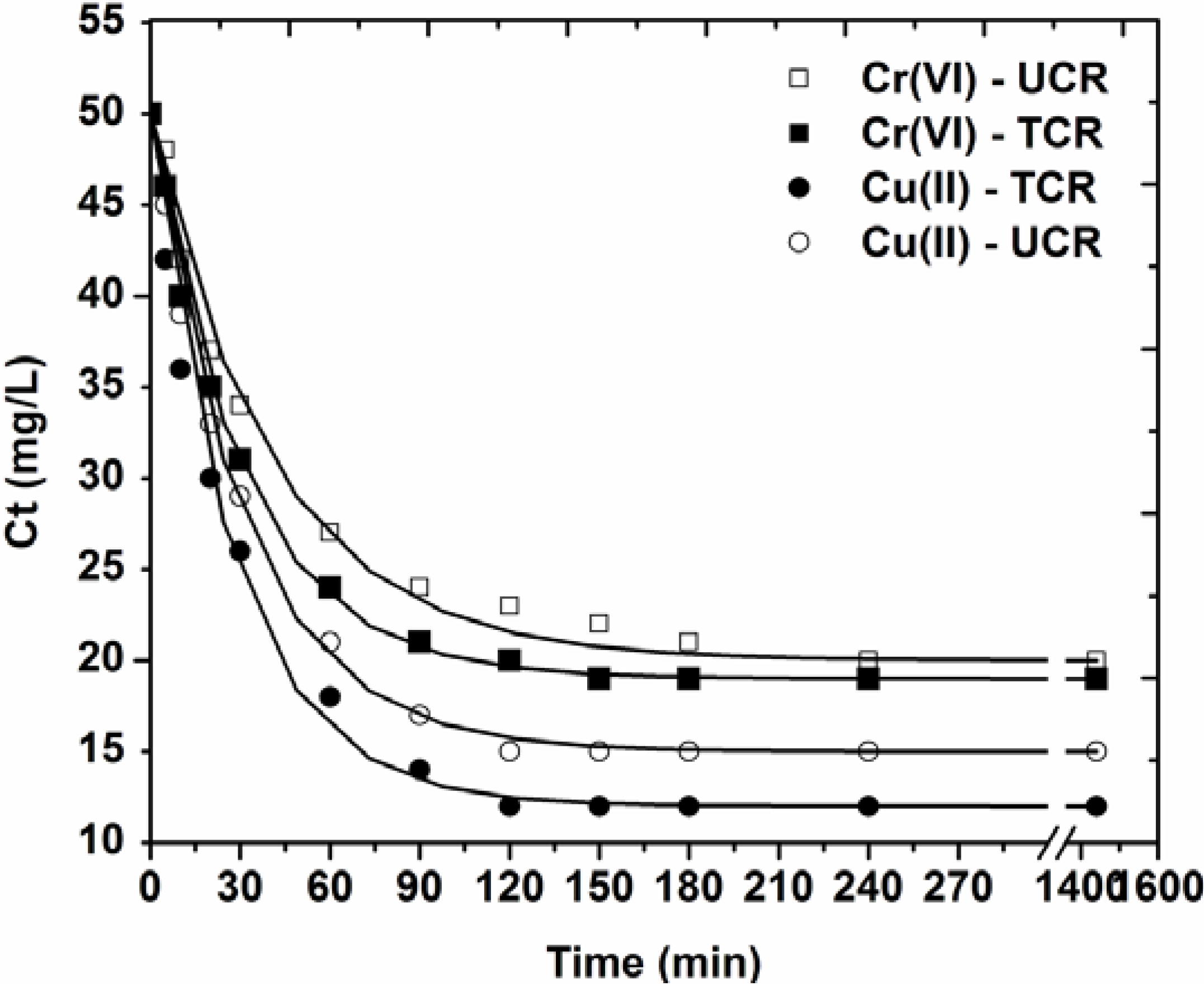
| Adsorbate-Adsorbent | Pseudo-first order | Pseudo-second order | Pseudo-third order | |||
|---|---|---|---|---|---|---|
| k1 | R2 | k2 | R2 | k3 | R2 | |
| min−1 | – | min−1 | – | min−1 | – | |
| Cu(II)-UCR | 0.032 | 0.998 | 0.061 | 0.963 | 0.128 | 0.872 |
| Cu(II)-TCR | 0.037 | 0.995 | 0.070 | 0.972 | 0.153 | 0.887 |
| Cr(VI)-UCR | 0.025 | 0.994 | 0.046 | 0.973 | 0.093 | 0.889 |
| Cr(VI)-TCR | 0.032 | 0.998 | 0.061 | 0.966 | 0.127 | 0.876 |
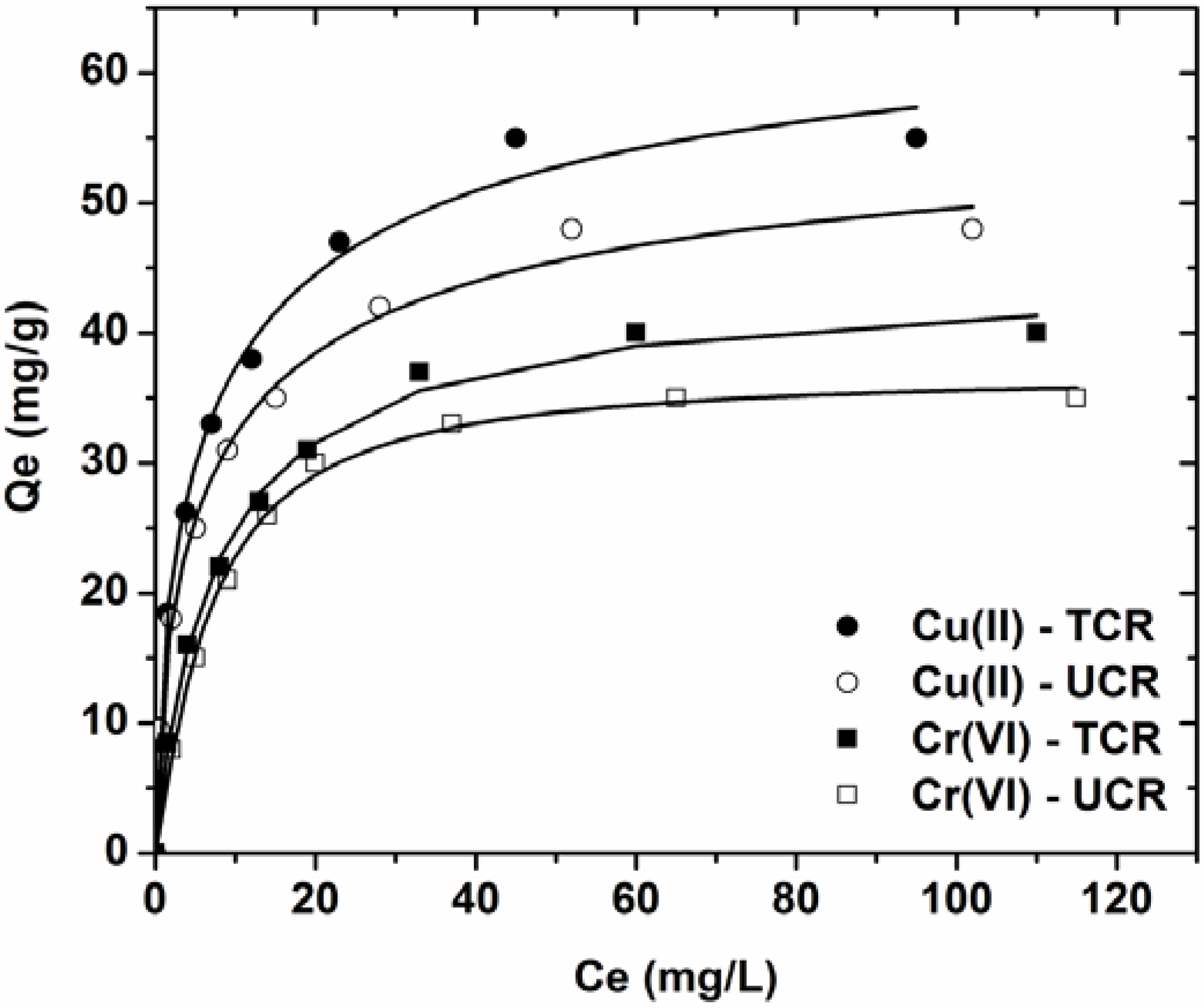
3.3. Effect of Initial Metal Concentration
| Ion-Adsorbent | Langmuir equation | Freundlich equation | L-F equation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qmax | KL | R2 | KF | n | R2 | Qmax | KLF | b | R2 | |
| mg/g | L/mg | – | mg1−1/n L1/n g−1 | – | – | mg/g | (L/mg)1/b | – | – | |
| Cu(II)-UCR | 49.34 | 0.222 | 0.978 | 16.842 | 4.02 | 0.964 | 59.18 | 0.132 | 0.637 | 0.996 |
| Cu(II)-TCR | 56.90 | 0.229 | 0.973 | 19.245 | 3.94 | 0.924 | 69.97 | 0.125 | 0.612 | 0.993 |
| Cr(VI)-UCR | 38.68 | 0.139 | 0.995 | 11.739 | 3.90 | 0.915 | 37.03 | 0.150 | 1.180 | 0.998 |
| Cr(VI)-TCR | 43.75 | 0.136 | 0.996 | 12.505 | 3.67 | 0.940 | 44.82 | 0.129 | 0.932 | 0.996 |
3.4. Effect of Agitation Rate
3.5. Desorption and Reuse

3.6. Economic Perspectives
4. Conclusions
- •
- The pH selected as optimum for further adsorption experiments was pH = 5, where the adsorbents presented the maximum removal just before the pH-zone of 5–8 where precipitation and hydrolysis phenomena dominate. The surface charge of the adsorbents was confirmed through the determination of PZC for each material.
- •
- Equilibrium data were fitted to the Lanmguir, Freundlich and Langmuir-Freundlich (L-F) model. The best correlation was for L-F model (R2 ~ 0.998).
- •
- Kinetic data were fitted to the pseudo-first, -second and -third order model. The best correlation was for pseudo-first order equation (R2 ~ 0.996).
- •
- The optimum agitation rate for the current adsorption phenomenon was determined to be 140 rpm.
- •
- The optimum pH found after desorption experiments was pH = 2 both for Cu(II) and Cr(VI).
- •
- After 10 cycles of adsorption-desorption, the reduction in adsorption percentages from the 1st to 10th cycle was approximately 7% for both coffee residues and ions.
Acknowledgments
References
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K. Copper and chromium(VI) removal by chitosan derivatives—Equilibrium and kinetic studies. Chem. Eng. J. 2009, 152, 440–448. [Google Scholar] [CrossRef]
- Boonamnuayvitaya, V.; Chaiya, C.; Tanthapanichakoon, W.; Jarudilokkul, S. Removal of heavy metals by adsorbent prepared from pyrolyzed coffee residues and clay. Sep. Purif. Technol. 2004, 35, 11–22. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; Ng, J.C.Y.; McKay, G. Kinetics of pollutant sorption by biosorbents: Review. Separ. Purif. Method. 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Amer. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Tien, C. Adsorption Calculations and Modeling; Butterworth-Heinemann: Boston, MA, USA, 1994. [Google Scholar]
- Ajmal, M.; Rao, R.A.K.; Ahmad, R.; Ahmad, J. Adsorption studies on Citrus reticulata (fruit peel of orange): Removal and recovery of Ni(II) from electroplating wastewater. J. Hazard. Mater. 2000, 79, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lei, L.; Li, Y.; Ni, Y. Chromium adsorption on high-performance activated carbons from aqueous solution. Sep. Purif. Technol. 2003, 31, 13–18. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P.; Singh, V.K. Removal of hexavalent chromium from aqueous solution using low-cost activated carbons derived from agricultural waste materials and activated carbon fabric cloth. Ind. Eng. Chem. Res. 2005, 44, 1027–1042. [Google Scholar] [CrossRef]
- Nuhoglu, Y.; Oguz, E. Removal of copper(II) from aqueous solutions by biosorption on the cone biomass of Thuja orientalis. Process Biochem. 2003, 38, 1627–1631. [Google Scholar] [CrossRef]
- Azouaou, N.; Sadaoui, Z.; Djaafri, A.; Mokaddem, H. Adsorption of cadmium from aqueous solution onto untreated coffee grounds: Equilibrium, kinetics and thermodynamics. J. Hazard. Mater. 2010, 184, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.S.; Franca, A.S. Low-cost adsorbents from agri-food wastes. Food Sci. Technol. New Res. 2008, 171–209. [Google Scholar]
- Oliveira, L.S.; Franca, A.S.; Alves, T.M.; Rocha, S.D.F. Evaluation of untreated coffee husks as potential biosorbents for treatment of dye contaminated waters. J. Hazard. Mater. 2008, 155, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Valdés, H.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Zaror, C.A. Effect of ozone treatment on surface properties of activated carbon. Langmuir 2002, 18, 2111–2116. [Google Scholar] [CrossRef]
- Boehm, H.P. Chemical identification of surface groups. Advan. Catal. 1966, 16, 179–274. [Google Scholar]
- Wang, L.; Wang, A. Adsorption properties of congo red from aqueous solution onto N,O-carboxymethyl-chitosan. Bioresource Technol. 2008, 99, 1403–1408. [Google Scholar] [CrossRef]
- Baek, M.H.; Ijagbemi, C.O.; O, S.J.; Kim, D.S. Removal of Malachite Green from aqueous solution using degreased coffee bean. J. Hazard. Mater. 2010, 176, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Kostoglou, M.; Vassiliou, A.A.; Lazaridis, N.K. Treatment of real effluents from dyeing reactor: Experimental and modeling approach by adsorption onto chitosan. Chem. Eng. J. 2011, 168, 577–585. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kyzas, G.Z. Commercial Coffee Wastes as Materials for Adsorption of Heavy Metals from Aqueous Solutions. Materials 2012, 5, 1826-1840. https://doi.org/10.3390/ma5101826
Kyzas GZ. Commercial Coffee Wastes as Materials for Adsorption of Heavy Metals from Aqueous Solutions. Materials. 2012; 5(10):1826-1840. https://doi.org/10.3390/ma5101826
Chicago/Turabian StyleKyzas, George Z. 2012. "Commercial Coffee Wastes as Materials for Adsorption of Heavy Metals from Aqueous Solutions" Materials 5, no. 10: 1826-1840. https://doi.org/10.3390/ma5101826





