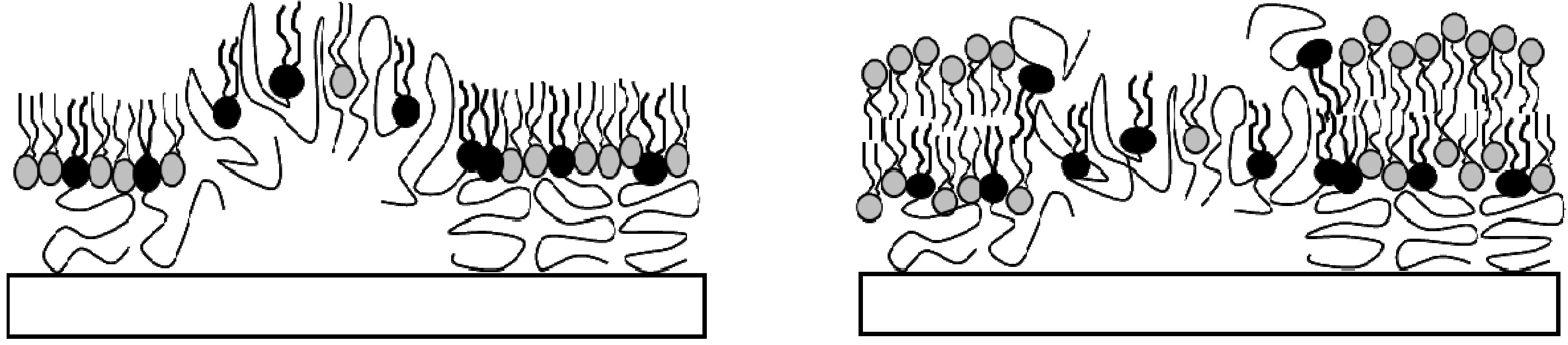
2.1. Physisorbed Polymer-Tethered Phospholipid Bilayer with Gradual Change in Lipopolymer Concentration (TYPE 1)
Previously, we demonstrated that physisorbed polymer-tethered phospholipid bilayers with different concentrations of lipopolymers in their inner monolayer display distinct, lipopolymer concentration-specific buckling structures [
23,
24]. The formation of these structures was confirmed by atomic force microscopy (AFM) and was explained in terms of a stress relaxation phenomenon caused by stress-inducing lipopolymers in the membrane system. In the case of lipopolymers with amphiphilic polymer moieties, such as poly(ethylene glycol) (PEG) and poly(2-ethyl-2-oxazoline), buckling structures were easily resolvable by epifluorescence microscopy (EPI). At low lipopolymer concentrations, buckling structures were found to exist as circular or straight-sided blisters. With increasing lipopolymer concentration, blisters were reported to become more elaborate and branched and to eventually develop into a compartmentalizing buckling pattern.
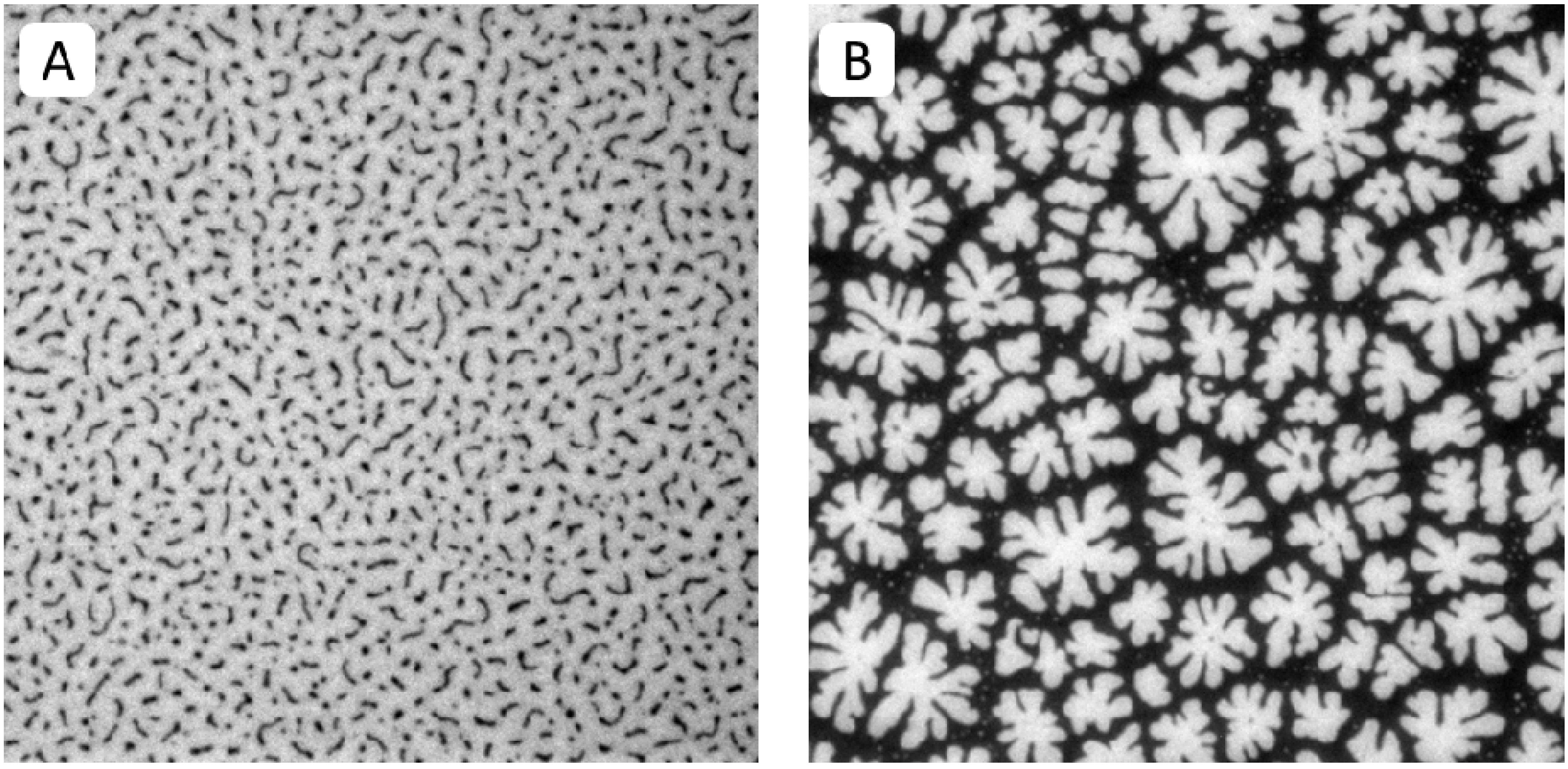
Figure 2A,B illustrate typical EPI micrographs of buckling structures in polymer-tethered lipid bilayers containing different amounts of DSPE-PEG5000. At 5 mol %, lateral stress is comparably low and membrane buckling regions exist as straight-sided blisters (
Figure 2A). In contrast, at 40 mol %, lateral stress is high resulting in the formation of membrane-compartmentalizing buckling regions (
Figure 2B).
Figure 2.
EPI micrographs of physisorbed polymer-tethered lipid bilayers of 5 mol % (A) and 40 mol % DSPE-PEG5000 (B). All pictures were taken with 40× objective and 1.6× Optovar magnification. The size of the micrographs is 100 µm × 100 µm.
Figure 2.
EPI micrographs of physisorbed polymer-tethered lipid bilayers of 5 mol % (A) and 40 mol % DSPE-PEG5000 (B). All pictures were taken with 40× objective and 1.6× Optovar magnification. The size of the micrographs is 100 µm × 100 µm.
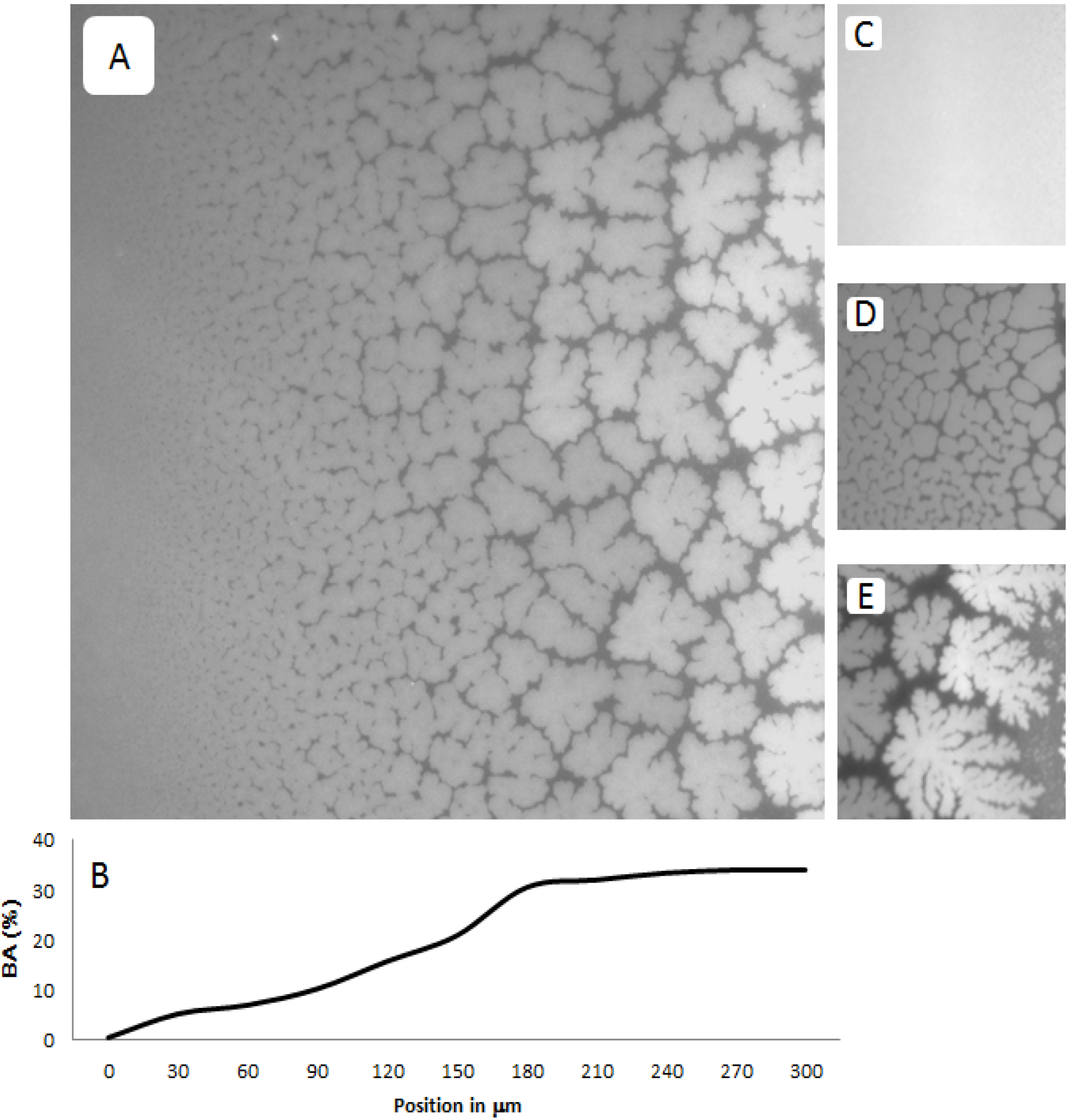
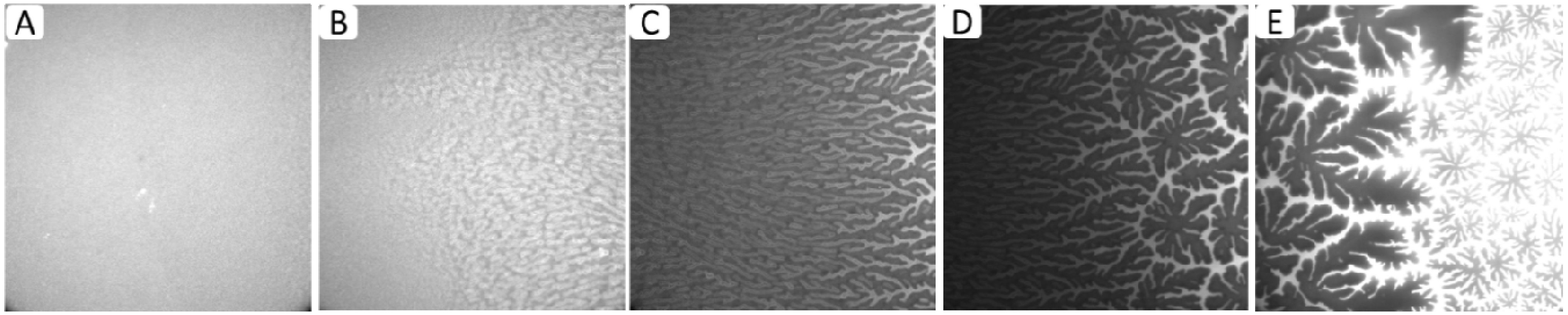
Figure 3A–E shows representative EPI micrographs obtained from a TYPE 1 polymer-tethered lipid bilayer system. As outlined in the
Experimental Section, the lipopolymer gradient in TYPE 1 membranes was allowed to build up at the air-water interface over an incubation time period of 40 min, prior to film transfer to the solid substrate.
Figure 3A presents a lower magnification micrograph captured using a 20× objective, which clearly illustrates the gradual transition from a region without optically resolvable buckling structures to one with a well-developed, bilayer-compartmentalizing buckling pattern. As the corresponding bearing area,
BA, data (
BA quantifies percentage of buckled membrane region) in
Figure 3B illustrate, the length scale of the gradient between buckling-free regions (low lipopolymer concentrations) and those with well-developed compartmentalizing buckling structures (high lipopolymer concentrations) for this incubation time period is about 200 µm.
Figure 3C–E depict higher magnification micrographs using a 40× objective of different regions of a TYPE 1 bilayer sample, which are distinct in terms of buckling formation.
Figure 3C exemplifies the region without optically resolvable buckling structures, which suggests a polymer-tethered lipid bilayer with less than 5 mol % DSPE-PEG5000. The bilayer area in
Figure 3D is characterized by straight-sided, partially branched blisters, indicative of a local DSPE-PEG5000 molar concentration of 5–10 mol % [
24]. This micrograph also illustrates the tendency of sufficiently long buckling ridges to compartmentalize the lipid bilayer.
Figure 3E shows a region of well-developed, bilayer-compartmentalizing buckling structures indicating a local DSPE-PEG5000 molar concentration of 15–20 mol %.
Figure 3.
Representative EPI micrographs of a TYPE 1 physisorbed polymer-tethered lipid bilayer. The gradual change of buckling structures in
Figure 2A indicates the existence of a lateral lipopolymer gradient in the membrane system (20× magnification).
Figure 3B illustrates the corresponding change in bearing area,
BA (percentage of buckled membrane region).
Figure 3C–E show magnified micrographs (40× magnification) of bilayer regions characterized by differences in buckling formation: no buckling (
C), partially branched blisters (
D), and well developed, bilayer-compartmentalizing buckles (
E). The image size of A is 320 µm × 320 µm, whereas that of C, D and E is 160 µm × 160 µm.
Figure 3.
Representative EPI micrographs of a TYPE 1 physisorbed polymer-tethered lipid bilayer. The gradual change of buckling structures in
Figure 2A indicates the existence of a lateral lipopolymer gradient in the membrane system (20× magnification).
Figure 3B illustrates the corresponding change in bearing area,
BA (percentage of buckled membrane region).
Figure 3C–E show magnified micrographs (40× magnification) of bilayer regions characterized by differences in buckling formation: no buckling (
C), partially branched blisters (
D), and well developed, bilayer-compartmentalizing buckles (
E). The image size of A is 320 µm × 320 µm, whereas that of C, D and E is 160 µm × 160 µm.
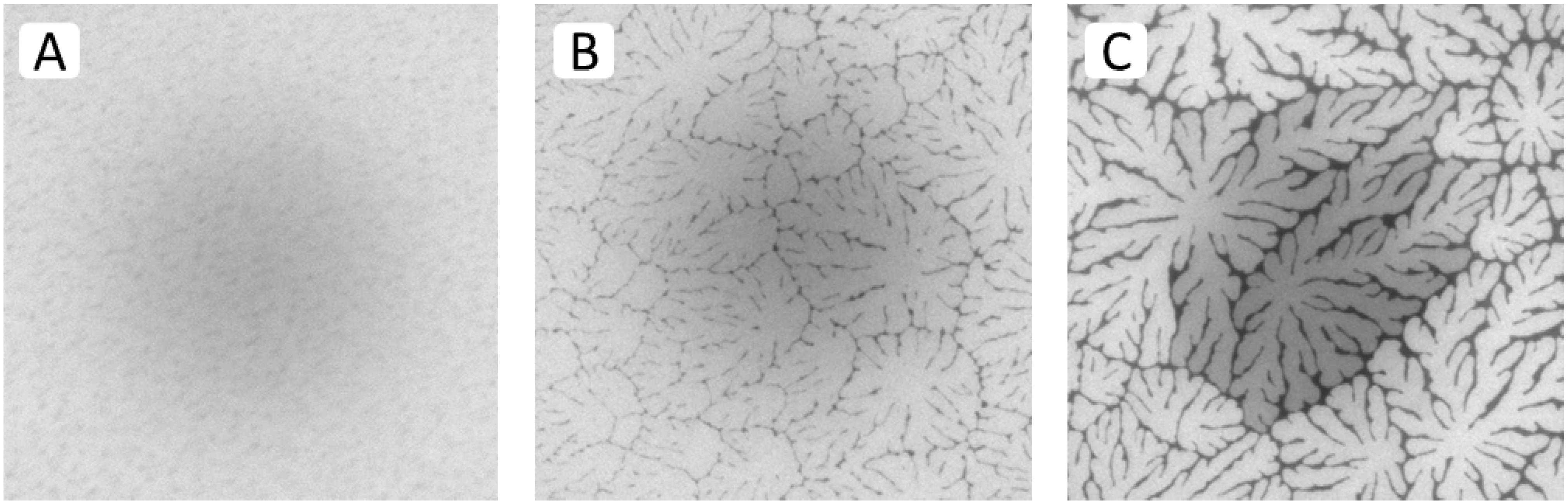
Results from spot bleaching experiments in
Figure 4A–C illustrate the influence of buckling structures on lipid lateral fluidity in different regions of a TYPE 1 bilayer. In the region without optically resolvable buckling structures (
Figure 4A), the circular bleaching spot exhibits a gradual transition of the bleaching intensity indicating good fluidity within the bilayer (images taken 1.5 min after spot photobleaching). In the region of straight-sided, partially branched blisters (
Figure 4B), qualitatively similar fluorescence recovery can be observed, which displays lateral fluidity outside buckling areas.
Figure 4C best demonstrates that buckling areas act as efficient lipid diffusion barriers, as reported previously for physisorbed polymer-tethered lipid bilayers containing poly(2-ethyl-oxazoline) or PEG lipopolymers [
23,
24].
Figure 4.
Fluorescence recovery after photobleaching of dye-labeled lipids in different regions of a TYPE 1 bilayer sample (images taken 1.5 min after spot photobleaching) exhibiting a buckling-free region (
A); a region with branched buckling structures (
B); and a region with bilayer-compartmentalizing buckles (
C). The micrographs illustrate the fluidity of lipids in the bright (buckle-free) regions of the bilayer and confirm the ability of buckling structures to act as efficient lipid diffusion barriers, as reported previously [
23]. (Image size: 160 µm × 160 µm).
Figure 4.
Fluorescence recovery after photobleaching of dye-labeled lipids in different regions of a TYPE 1 bilayer sample (images taken 1.5 min after spot photobleaching) exhibiting a buckling-free region (
A); a region with branched buckling structures (
B); and a region with bilayer-compartmentalizing buckles (
C). The micrographs illustrate the fluidity of lipids in the bright (buckle-free) regions of the bilayer and confirm the ability of buckling structures to act as efficient lipid diffusion barriers, as reported previously [
23]. (Image size: 160 µm × 160 µm).
Combined AFM and spot photobleaching experiments revealed that in such cases no lipid bilayer can form on top of buckling regions [
23]. Consequently, in TYPE 1 bilayers, these regions of “buckling-induced dewetting” cause the formation of diffusion obstacles at low to medium lipopolymer concentrations and the compartmentalization of the lipid bilayer system at high lipopolymer concentrations. It should be pointed out that the spot photobleaching method does not provide reliable quantitative information about lipid diffusion, as the size of the bleaching spot exceeds the average distance between buckling structures. Previously, such quantitative information was obtained using single molecule tracking experiments of dye-labeled and quantum dot-conjugated lipids in polymer-tethered membranes [
15,
23]. Notably, the available single molecule tracking data reveal a complex length scale-dependent lipid diffusion behavior in physisorbed polymer-tethered lipid bilayers, which exhibits remarkable parallels to those observed in plasma membranes. At sub-optical resolution length scale (~100 nm), wide-field single molecule fluorescence microscopy experiments show that lipid diffusion is well described by a model of obstacle-induced obstructed diffusion [
15]. Here the degree of obstruction is determined by the density of lipopolymers in the membrane system. Interestingly, the observed obstruction of lipid diffusion at this length scale seems to be, in part, associated with a lipopolymer-induced roughening of the bilayer, which alters membrane tension [
20]. At micron-size length scale, the formation of diffusion barriers in buckled regions reveals a second type of obstructed lipid diffusion. In this case, the degree of obstruction is determined by the length and connectivity of buckles. The complex lipid diffusion behavior in physisorbed polymer-tethered membranes was recently demonstrated through long-term tracking of photostable quantum dot-conjugated lipids [
23]. These experiments not only showed a lipopolymer density-dependent obstruction of lipid diffusion over the entire detected length scale range, but also exhibited the feature of hop diffusion at a particular length scale (qualitatively similar to plasma membranes) [
20]. It should be noted that the described lipid diffusion properties are distinct from those reported on chemisorbed polymer-tethered lipid bilayers [
14].
Physisorbed polymer-tethered lipid bilayers not only show fascinating diffusion behavior, but are also characterized by interesting mechanical properties. Previously, mean-field calculations have shown that the mechanical properties of polymer-tethered membranes depend on lipopolymer density [
21,
22]. Interestingly, the bending elasticity,
Kc, of a typical red blood cell membrane of about 50
kBT corresponds to that of a polymer-tethered lipid bilayer of 5 mol % DSPE-PEG5000 and
Kc = 400
kBT of a typical membrane of Dictyostelium discoideum (wild type) is comparable to
Kc values in polymer-tethered membranes of 20 mol % DSPE-PEG5000 [
31,
32] In contrast, a fluid lipid bilayer without lipopolymers is notably softer than typical cell membranes. Importantly, there is an empirical correlation between the extent of buckling formation and membrane elastic properties. A more quantitative relationship between buckle formation and membrane elasticity can be developed by linking experimentally determined buckling parameters, such as the buckling width,
2b, or the maximum height of buckles,
wmax, to mean-field calculations of polymer-lipopolymer mixtures and buckling theory of an Euler column [
24]. In this case, the Euler column approximation can be applied because the buckling width is notably larger than the overall membrane thickness,
h, and because the Young’s modulus of the glass substrate is much higher than that of the polymer-tethered membrane [
33]. In the case of compartment-forming buckling structures, information about the density of lipopolymers and the corresponding membrane elasticity can be also obtained by determining the compartment density,
Ncorr [
24]. The buckling parameter information needed for quantitative correlation can be best acquired from the analysis of EPI and AFM micrographs of polymer-tethered lipid monolayers.
Figure 5A-E illustrates representative EPI micrographs of different regions within a TYPE 1 polymer-tethered monolayer sample. The micrographs depict the gradual transition from regions of low lipopolymer concentration (≤5 mol % DSPE-PEG5000) (
Figure 5A) to those of elevated lipopolymer concentration (~30 mol % DSPE-PEG5000) (
Figure 5E). Monolayer micrographs show typical phase inversion (relative to corresponding bilayer system) observed on polymer-tethered membranes with PEG lipopolymers (
i.e., bright phase represents buckling regions in monolayer , while dark phase represents buckling regions in bilayer) [
24].
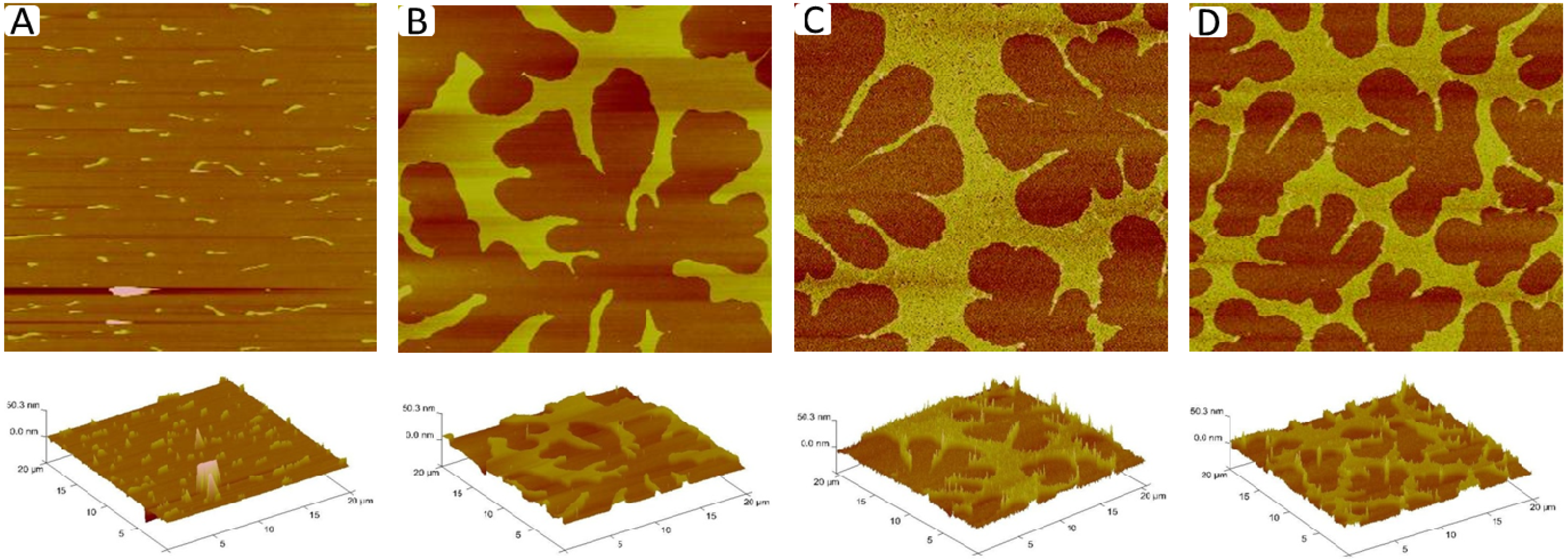
Figure 6A–D displays corresponding AFM micrographs of different regions of a typical TYPE 1 monolayer (length scale 20 µm × 20 µm). While
Figure 6A shows a monolayer region, which is characterized by straight-sided blisters,
Figure 6B–D depict a compartmentalizing buckling pattern of decreasing compartment size. Using previously applied protocols [
23], analysis of buckling width,
2b (
Figure 6A), and compartment density,
Ncorr (
Figure 6B–D), suggests lipopolymer molar concentrations of 4 mol % (
Figure 6A), 16 mol % (
Figure 6B), 31 mol % (
Figure 6C), and 36 mol % (
Figure 6D), associated with a change in the plane strain modulus,
, of the membrane from 1.9 to 7.3 MPa.
Figure 5.
EPI fluorescence micrographs of different regions of a TYPE 1 physisorbed polymer-tethered monolayer illustrating the gradient in terms of buckling structures representative of changes in lipopolymer density: no optically resolvable buckles (A); straight-sided blisters (B); increasingly branched blisters (C); branched blisters and compartmentalizing buckles (D); and compartmentalizing buckles (E). Image size: 160 µm × 160 µm.
Figure 5.
EPI fluorescence micrographs of different regions of a TYPE 1 physisorbed polymer-tethered monolayer illustrating the gradient in terms of buckling structures representative of changes in lipopolymer density: no optically resolvable buckles (A); straight-sided blisters (B); increasingly branched blisters (C); branched blisters and compartmentalizing buckles (D); and compartmentalizing buckles (E). Image size: 160 µm × 160 µm.
Figure 6.
AFM micrographs of different regions of a TYPE 1 physisorbed polymer-tethered monolayer exhibiting distinct degrees of membrane buckling: straight-sided blisters (
A); and compartmentalizing buckles of decreasing compartment size (
B–
D). The decreasing compartment size indicates increasing lipopolymer molar concentrations. Image size: 20 µm × 20 µm. The buckle amplitude is about 7.5 ± 1.5 Å [
24].
Figure 6.
AFM micrographs of different regions of a TYPE 1 physisorbed polymer-tethered monolayer exhibiting distinct degrees of membrane buckling: straight-sided blisters (
A); and compartmentalizing buckles of decreasing compartment size (
B–
D). The decreasing compartment size indicates increasing lipopolymer molar concentrations. Image size: 20 µm × 20 µm. The buckle amplitude is about 7.5 ± 1.5 Å [
24].
The data presented for TYPE 1 membranes in
Figure 3,
Figure 4,
Figure 5 and
Figure 6 bring to light a fascinating model membrane system with gradually changing properties of membrane organization, dynamics, and elasticity. Notably, the length scale of the gradient in TYPE 1 membranes depends on the incubation time after addition of the POPC solution to the mixed POPC-DSPE-PEG5000 monolayer at the air-water interface. Longer incubation times lead to increasing length scales of gradients between buckling-free regions (low lipopolymer concentrations) and those with compartmentalizing buckling structures (high lipopolymer concentrations), due to diffusional relaxation processes of lipids and lipopolymers at the air-water interface. The significance of the TYPE 1 architecture is that gradients become static and do not change over time after LB transfer. This static behavior is caused by the physisorption of lipopolymers onto the glass substrate preventing the gradual relaxation of the lipopolymer gradient. Resulting differences in lipopolymer density in TYPE 1 systems demonstrate the ability to maintain regions of different lateral stress within one membrane sample. These regions manifest themselves in terms of clearly distinguishable buckling structures. Such buckling structures represent a buckle delamination of the membrane seen as stress relaxation phenomena. Furthermore, the lateral lipopolymer gradient leads to remarkable length scale-dependent lipid fluidity in TYPE 1 bilayer systems ranging from regions of low obstruction of lipid diffusion to those characterized by significant lipopolymer-induced obstructed and hop diffusion processes. Here it is important to recognize that the physisorption of lipopolymers on the glass substrate does cause the obstruction of lipid diffusion, but typically not to the degree of complete membrane immobilization. A simple fluid lipid bilayer system with a comparable static gradient does not appear to be feasible as the lateral mobility of lipids will decrease any previously formed gradient over time. This is beautifully illustrated by the analysis of transient gradients of charged lipids in micropatterned solid-supported lipid bilayers [
28]. In this case, the gradient of charged, dye-labeled lipids was created by applying an electric field and the time evolution of the gradient was analyzed after turning off the applied electric field, thus providing information about lipid diffusivity. However, in the case of engineered solid substrates with specific gradient properties (e.g., surface charge or curvature), lipid bilayer structures with membrane constituent gradients seem possible. An alternative gradient strategy could be the usage of polymerizable lipids to build a lipid bilayer system with a lateral gradient in lipid crosslinking density.
2.2. Physisorbed Polymer-Tethered Phospholipid Bilayer with Sharp Boundary between Regions of Low and High Lipopolymer Concentrations (TYPE 2)
The immobilization of physisorbed lipopolymers on the glass surface not only offers the possibility to fabricate membrane systems with lateral lipopolymer density gradients, but also those with a sharp boundary between regions of low and high lipopolymer molar concentrations. As described in the
Experimental Section, TYPE 2 membranes were built by regulating the phospholipid-lipopolymer mixing ratio at the air-water interface and by conducting partial LB transfers at altered lipopolymer concentrations.
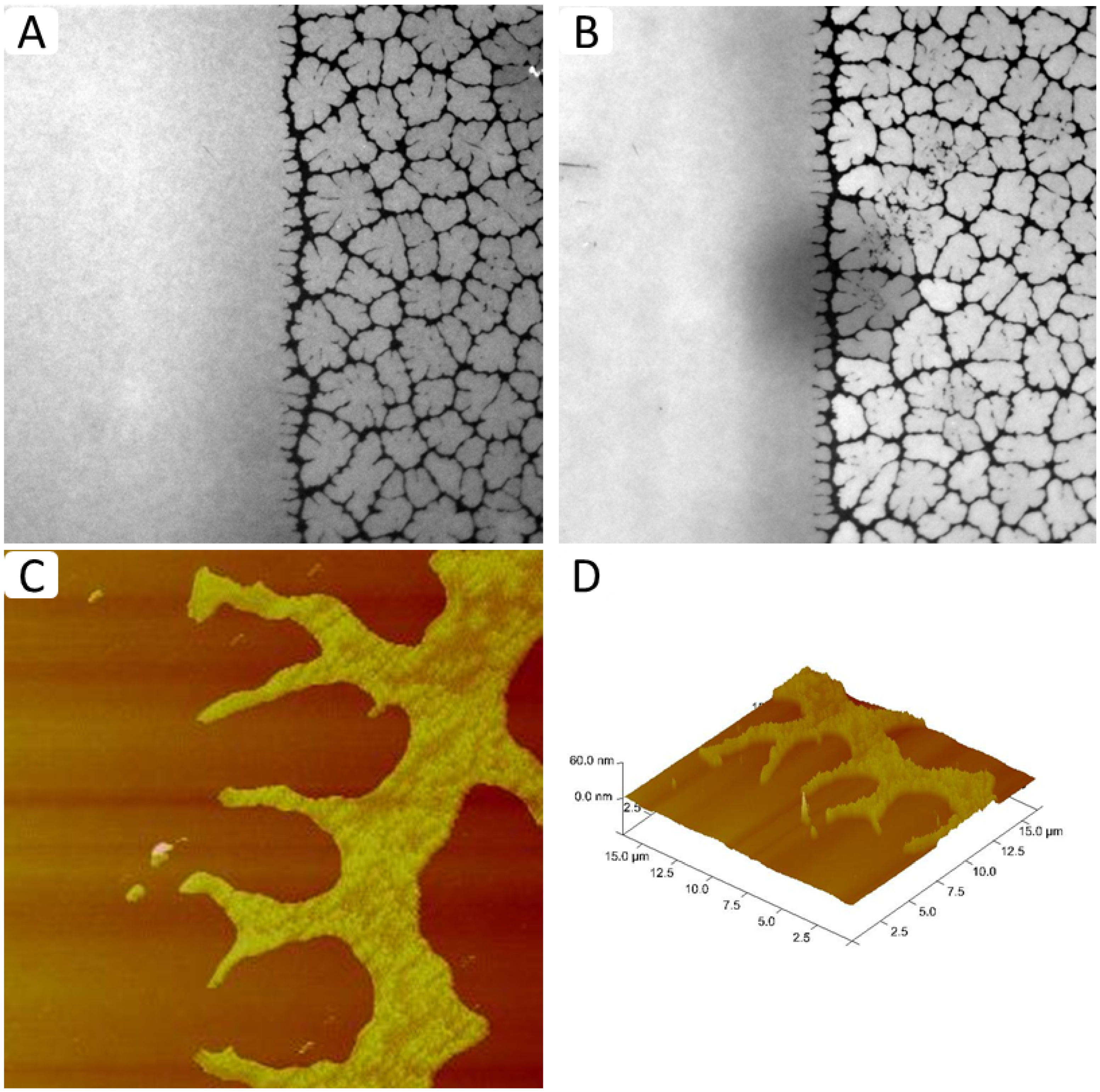
Figure 7A–D shows representative EPI and AFM micrographs of such a physisorbed polymer-tethered lipid membrane. The EPI micrograph in
Figure 7A illustrates two sharply separated membrane regions, a homogeneous region and a region characterized by compartmentalizing buckling structures. As outlined in the Materials and Methods section, the homogeneous and non-homogeneous buckled regions contain approximately 5 and 30 mol % DSPE-PEG5000, respectively. The shape of the bleaching spot in
Figure 7B demonstrates the good bilayer fluidity in the homogeneous region of the membrane with the low lipopolymer density. In contrast, the partially recovered bleaching spot in the non-homogeneous region shows that the “dark phase” acts as a lipid diffusion barrier. This behavior suggests that the non-homogeneous region is not caused by phospholipid-lipopolymer phase separation, but instead is a typical fingerprint of membrane buckling and buckling-induced “dewetting” [
24]. Indeed, the presence of buckling structures is confirmed by AFM micrographs in
Figure 7C,D that show representative AFM data from the boundary region of a typical TYPE 2 polymer-tethered monolayer. Again it should be emphasized that the sharp boundary between regions of low and high lipopolymer densities, which exhibit distinctly different dynamic end elastic properties, remains unchanged over an extended period of time. Of course, the concept of TYPE 2 membranes should not remain limited to those with one sharp boundary. Modifications to the membrane fabrication process can be envisioned, which lead to well-defined patterned polymer-tethered bilayer systems. Previously, several successful strategies have been pursued to build patterned solid-supported lipid bilayers. For example, Groves
et al. used patterned grids of photoresist, aluminum oxide, or gold on oxidized silicon substrates to form patterned solid-supported lipid bilayers [
28]. Other patterning strategies include the photochemical patterning [
29] and patterning via the controlled crosslinking of polymerizable lipids [
30]. An interesting example of patterning in polymer-supported membranes represents the controlled formation of stripe phases in polymer-tethered lipid bilayers comprised of lipids and lipopolymers, in which stripe formation was controlled through changing LB transfer conditions [
34].
Figure 7.
EPI (
A,
B) and AFM micrographs (
C,
D) of TYPE 2 physisorbed polymer-tethered lipid bilayer and monolayer, respectively. Micrographs confirm the existence of a sharp boundary between regions of low and high lipopolymer densities with distinct properties of membrane dynamics and elasticity. (EPI micrograph image size: 160 µm × 160 µm; AFM micrograph image size: 20 µm × 20 µm. The buckle amplitude is about 7.5 ± 1.5 Å [
24].
Figure 7.
EPI (
A,
B) and AFM micrographs (
C,
D) of TYPE 2 physisorbed polymer-tethered lipid bilayer and monolayer, respectively. Micrographs confirm the existence of a sharp boundary between regions of low and high lipopolymer densities with distinct properties of membrane dynamics and elasticity. (EPI micrograph image size: 160 µm × 160 µm; AFM micrograph image size: 20 µm × 20 µm. The buckle amplitude is about 7.5 ± 1.5 Å [
24].