Cholesterol Organization in Phosphatidylcholine Liposomes: A Surface Plasmon Resonance Study
Abstract
:1. Introduction
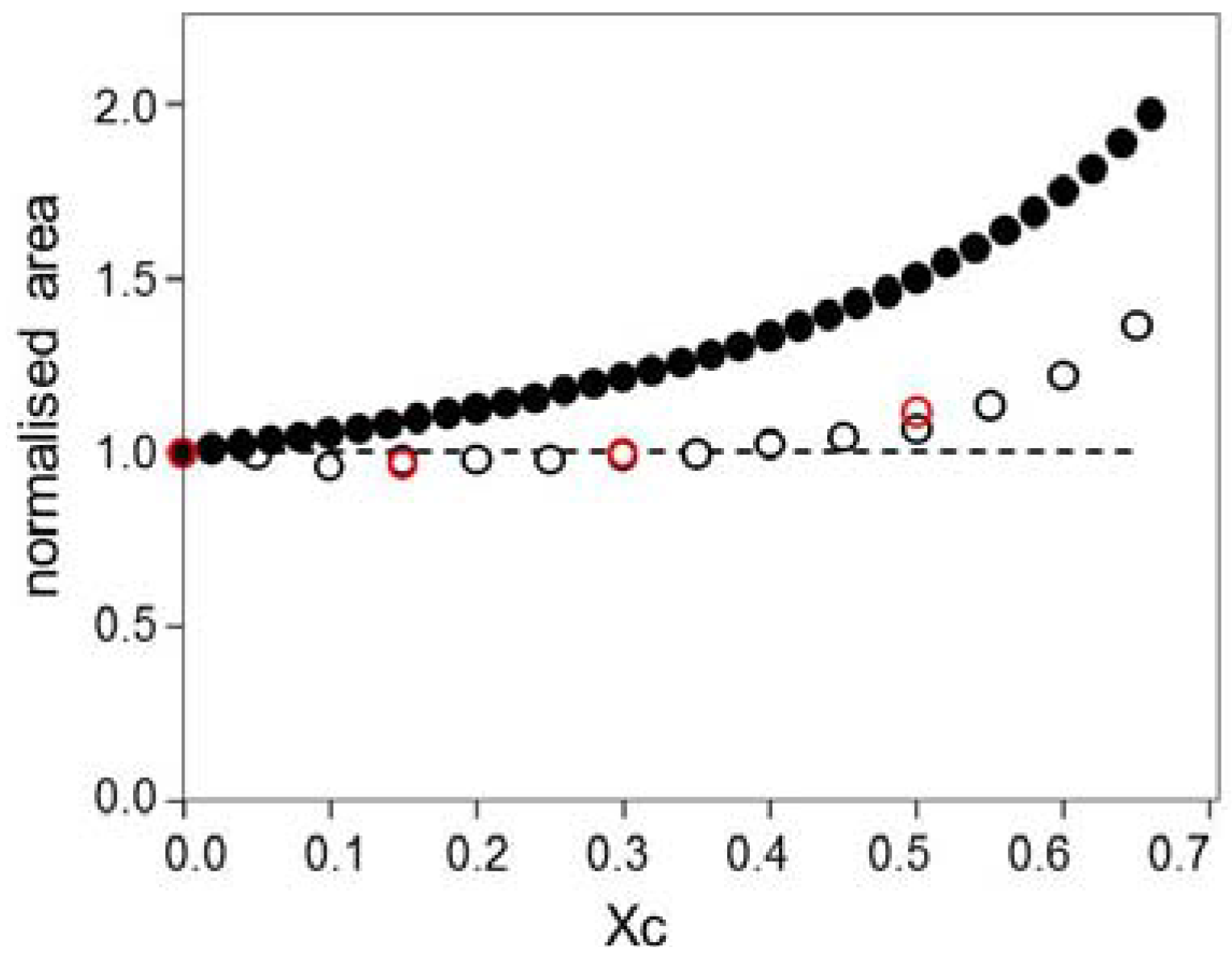

2. Results
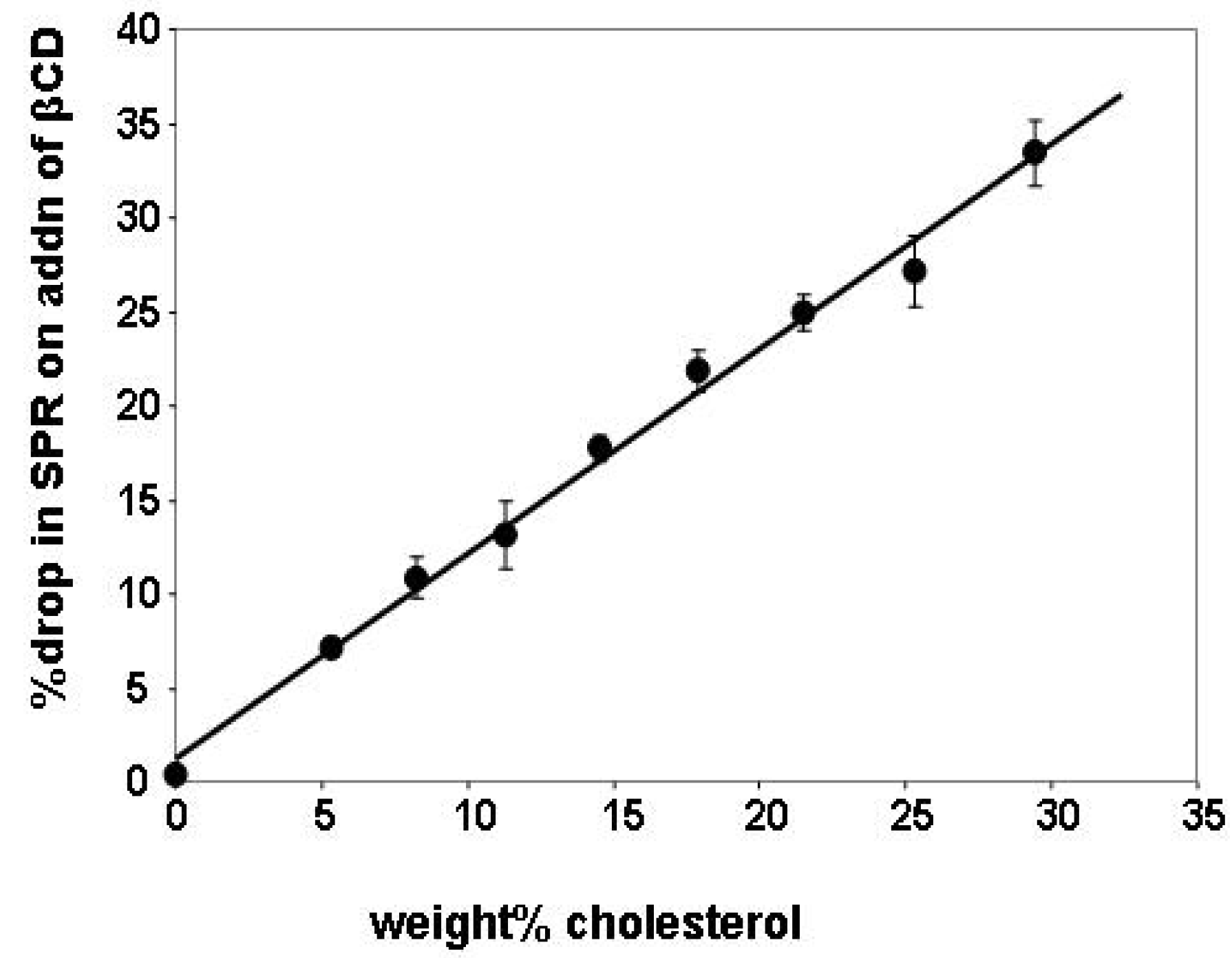
2.1. Cholesterol Removal and the SPR Response
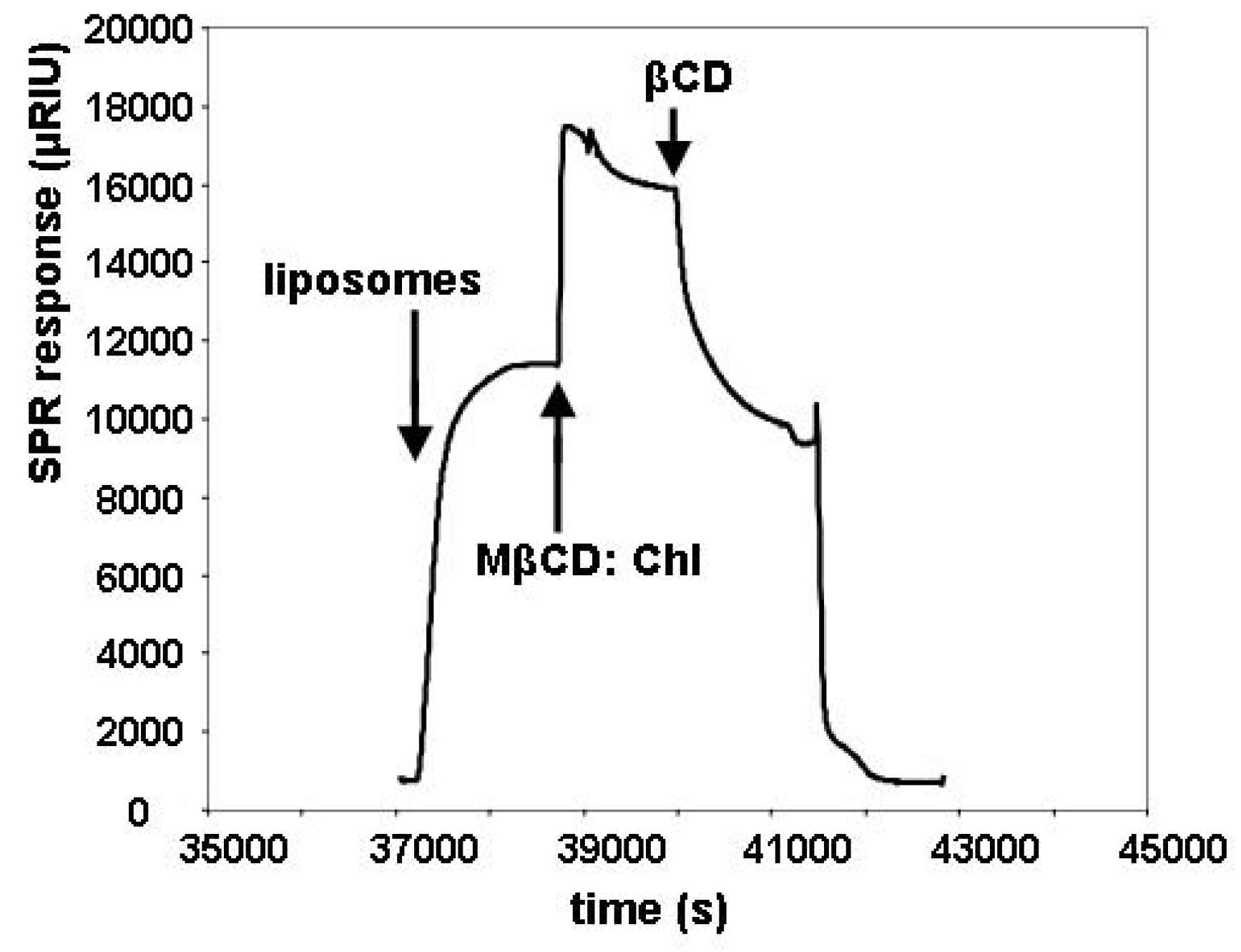
2.2. Insertion of Chl with MβCD:Chl
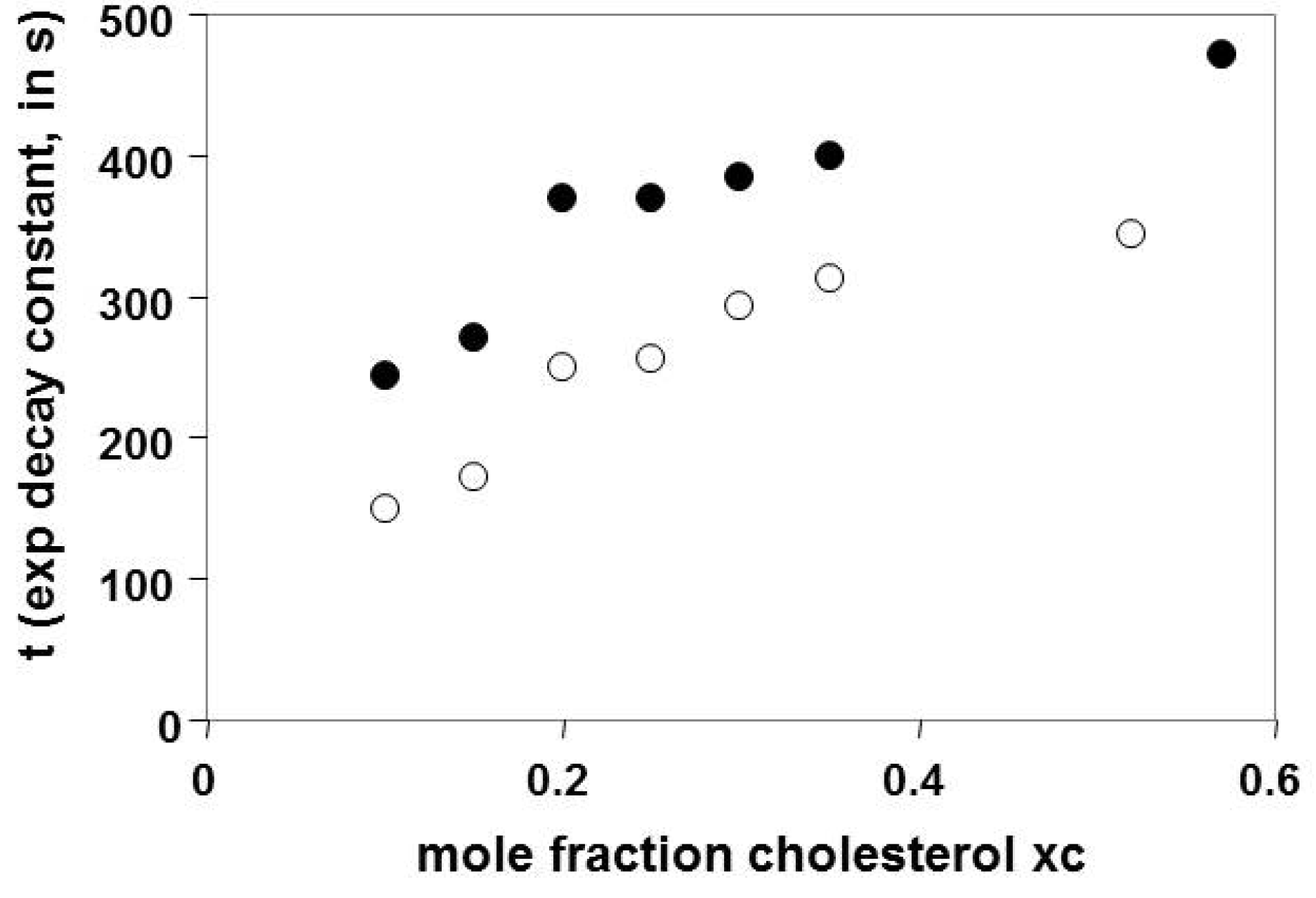
2.3. Kinetics of Cholesterol Removal from Liposomes with χC ≤ 0.35
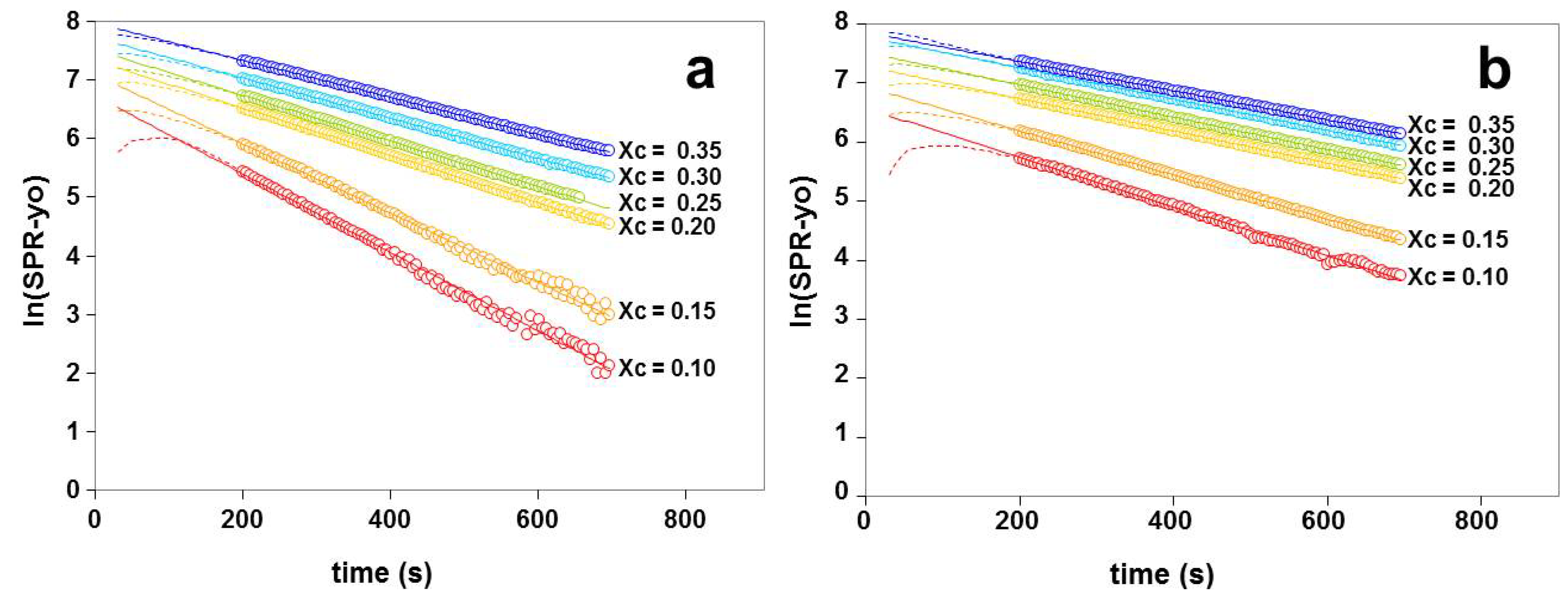
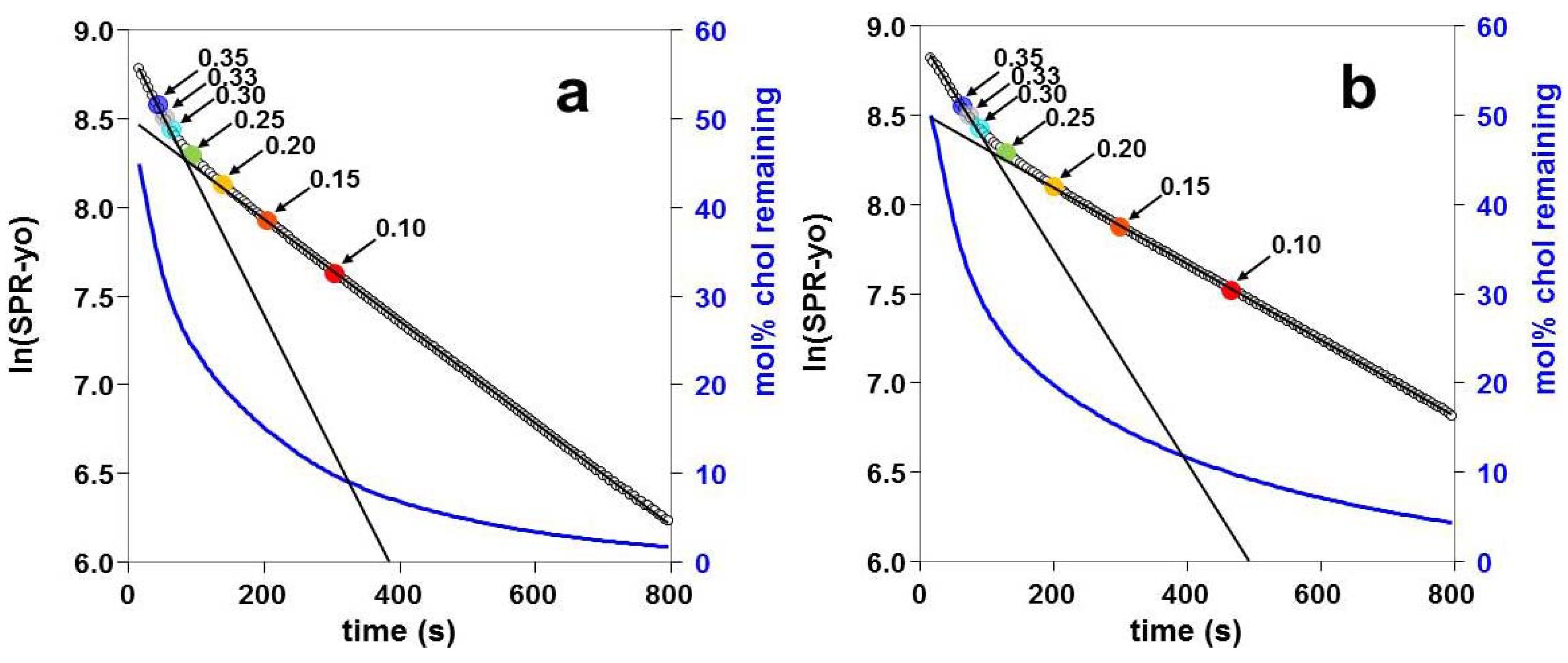
2.4. Kinetics of Cholesterol Removal from Liposomes Saturated with Cholesterol
3. Discussion
3.1. Effect of Sample History: Rate of Cholesterol Removal a Function OF Initial χC
3.2. Time Constants in the Slow Pool Increase with Increasing χC
3.3. Two Kinetic Pools of Cholesterol in PC/Cholesterol Mixtures
3.4. Discontinuity in the Time Constants Describing the Rate of Cholesterol Removal, Near χC = 0.2–0.25
3.5. Some Possible Structural Arrangements for PC/Cholesterol Bilayers
3.6. Modified Lattice Model
4. Experimental Section
4.1. Materials
4.2. Modification of the SPR Slides with PMMA and Silicate
4.3. Surface Plasmon Resonance
4.4. Liposome Preparation
4.5. Formation of Vesicle Layers on Silicate
4.6. Addition of β-Cyclodextrin and MβCD:Chl
4.7. Analyzing Kinetics of Cholesterol Removal
5. Conclusions
References
- Smaby, J.M.; Brockman, H.L.; Brown, R.E. Cholesterol’s interfacial interactions with sphingomyelins and phosphatidylcholines: Hydrocarbon chain structure determines the magnitude of condensation. Biochemistry 1994, 33, 9135–9142. [Google Scholar] [PubMed]
- Ali, M.R.; Cheng, K.H.; Huang, J. Assess the nature of cholesterol-lipid interactions through the chemical potential of cholesterol in phosphatidylcholine bilayers. Proc. Nat. Acad. Sci. USA 2007, 104, 5372–5377. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.L-.G.; Zhu, W.; Venegas, B. On the lateral structure of model membranes containing cholesterol. Biochim. Biophys. Acta 2009, 1788, 2–11. [Google Scholar] [CrossRef]
- Somerharju, P.; Virtanen, J.A.; Cheng, K.H. Lateral organisation of membrane lipids: The superlattice view. Biochim. Biophys. Acta. 1999, 1440, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, J.M.; Westerman, P.W.; Carey, M.C. Fluorocholesterols, in contrast to hydroxycholesterols, exhibit interfacial properties similar to cholesterol. J. Lipid Res. 2000, 41, 991–1003. [Google Scholar] [PubMed]
- Huang, S.; Buboltz, J.T.; Feigenson, G.W. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim. Biophys. Acta 1999, 1417, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.L-.G. Evidence for regular distribution of sterols in liquid crystalline phosphatidylcholine bilayers. Proc. Nat. Acad. Sci. USA 1994, 91, 10069–10073. [Google Scholar] [CrossRef]
- Roger, J.; Lee, A.G.; Wilton, D.C. The organisation of cholesterol and ergosterol in lipid bilayers based on studies using non-perturbing fluorescent sterol probes. Biochim. Biophys. Acta 1979, 552, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.H.; Cannon, B.; Metze, J.; Lewis, A.; Huang, J.Y.; Vaughn, M.W.; Zhu, Q.; Somerharju, P.; Virtanen, J. Lipid headgroup superlattice modulates the activity of surface-acting cholesterol oxidase in ternary phospholipid/cholesterol bilayers. Biochemistry 2006, 45, 10855–10864. [Google Scholar] [CrossRef]
- Wang, M.M.; Olsher, M.; Sugár, I.; Chong, P.L.-G. Cholesterol superlattice modulates the activity of cholesterol oxidase in lipid membranes. Biochemistry 2004, 43, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chong, P.L-.G. Evidence for regulatory role of cholesterol superlattices in the hydrolytic activity of secretory phospholipase A2 in lipid membranes. Biochemistry 1999, 38, 3867–3873. [Google Scholar] [CrossRef]
- Olsher, M.; Chong, P.L-.G. Sterol supperlattice affects antioxidant potentency and can be used to assess adverse effects of antioxidants. Anal. Biochem. 2008, 382, 1–8. [Google Scholar] [CrossRef]
- Zidovetzki, R.; Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim. Biophys. Acta 2007, 1768, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Christian, A.E.; Haynes, M.P.; Phillips, M.C.; Rothblat, G.H. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 1997, 38, 2264–2272. [Google Scholar] [PubMed]
- Irie, T.; Otagiri, M.; Sunada, M.; Uekama, K.; Ohtani, Y.; Yamada, Y.; Sugiyama, Y. Cyclodextrin-induced hemolysis and shape changes of human erythrocytes in vitro. J. Pharm. Dyn. 1982, 5, 741–744. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sensor. Actuator. B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Podlesnik Beseničar, M.; Bavdek, A.; Kladnik, A.; Maček, P.; Anderluh, G. Kinetics of cholesterol extraction from lipid membranes by methyl-β-cyclodextrin—A surface plasmon resonance approach. Biochim. Biophys. Acta 2008, 1778, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; van der Meer, B.W.; Chen, S.Y. Evidence for a regular distribution of cholesterol in phospholipid bilayers from diphenylhexatriene fluorescence. Biophys. J. 1995, 68, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J.A.; Pekerarc, S.; Le, H.B.; Patterson, J.; Montez, B.; Oldfield, E. Molecular order and dynamics of phosphatidylcholine bilayer membranes in the presence of cholesterol, ergosterol and lanosterol: A comparative study using 2H-, 13C- and 31P-NMR spectroscopy. Biochim. Biophys. Acta 1995, 1238, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Ohvo-Rekilä, H.; Ramstedt, B.; Leppimäki, P.; Slotte, J.P. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 2002, 41, 66–97. [Google Scholar] [CrossRef] [PubMed]
- Olżyńskaa, A.; Zańa, A.; Jurkiewicza, P.; Sýkoraa, J.; Gröbnerb, G.; Langnerc, M.; Hofa, M. Molecular interpretation of fluorescence solvent relaxation of Patman and 2H NMR experiments in phosphatidylcholine bilayers. Chem. Phys. Lipids 2007, 147, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.G.; Rodrigueza, W.V.; Kilsdonk, E.P.C.; Stoudt, G.W.; Johnson, W.J.; Rothblat, G.H. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J. Biol. Chem. 1996, 271, 16026–16034. [Google Scholar] [CrossRef] [PubMed]
- Venegas, B.; Zhu, W.; Haloupek, N.B.; Lee, J.; Zellhart, E.; Sugár, I.P.; Kianni, M.F.; Chong, P.L.-G. Cholesterol superlattice modulates CA4P release from liposomes and CA4P cytotoxicity on mammary cancer cells. Biohys. J. 2012, 102, 2086–2094. [Google Scholar]
- Melzak, K.A.; Tsortos, A.; Gizeli, E. Use of acoustic sensors to probe the mechanical properties of liposomes. Methods Enzymol. 2009, 465, 21–41. [Google Scholar] [PubMed]
- Leventis, R.; Silvius, J.R. Use of cyclodextrins to monitor transbilayer movement and differential lipid affinities of cholesterol. Biophys. J. 2001, 81, 2257–2267. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, R.F.M.; Fedorov, A.; Prieto, M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J. 2003, 85, 2406–2416. [Google Scholar] [CrossRef] [PubMed]
- Halling, K.K.; Ramstedt, B.; Nyström, J.H.; Slotte, J.P.; Nyholm, K.M. Cholesterol interactions with fluid-phase phospholipids: effect on the lateral organization of the bilayer. Biophys. J. 2008, 95, 3861–3871. [Google Scholar] [CrossRef] [PubMed]
- Snyder, B.; Freire, E. Compositional domain structure in phosphatidylcholine-cholesterol and sphingomyelin-cholesterol bilayers. Proc. Natl. Acad. Sci. USA 1980, 77, 4055–4059. [Google Scholar] [CrossRef] [PubMed]
- Houslay, M.D.; Stanley, K.K. Dynamics of Biological Membranes: Influence on Synthesis Structure and Function; Wiley: Chichester, UK, 1982. [Google Scholar]
- Radhakrishnan, A.; Anderson, T.G.; McConnell, H.M. Condensed complexes, rafts, and the chemical activity of cholesterol in membranes. Proc. Natl. Acad. Sci. USA 2000, 97, 12422–12427. [Google Scholar] [CrossRef] [PubMed]
- Venegas, B.; Sugár, I.P.; Chong, P.L.-G. Critical factors for detection of biphasic changes in membrane properties at specific sterol mole fractions for maximal superlattice formation. J. Phys. Chem. B 2007, 111, 5180–5192. [Google Scholar] [CrossRef] [PubMed]
- Sugár, I.P.; Chong, P.L.-G. A statistical mechanical model of cholesterol/phospholipid mixtures: linking condensed complexes, superlattices, and the phase diagram. J. Am. Chem. Soc. 2012, 134, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Miles, K.; Cheng, K.H.; Huang, J. Lateral distribution of cholesterol in dioleoylphosphatidylcholine lipid bilayers: Cholesterol-phospholipid interactions at high cholesterol limit. Biophys. J. 2004, 86, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Sugár, I.; Chong, P.L.-G. Role of the sterol superlattice in the partitioning of the antifungal drug nystation into lipid membranes. Biochemistry 1998, 37, 11797–11805. [Google Scholar] [CrossRef] [PubMed]
- Melzak, K.A.; Gizeli, E. A silicate gel for promoting deposition of lipid bilayers. J. Colloid Interface Sci. 2002, 246, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Melzak, K.A.; Bender, F.; Tsortos, A.; Gizeli, E. Probing mechanical properties of liposomes using acoustic sensors. Langmuir 2008, 24, 9172–9180. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Melzak, K.A.; Melzak, S.A.; Gizeli, E.; Toca-Herrera, J.L. Cholesterol Organization in Phosphatidylcholine Liposomes: A Surface Plasmon Resonance Study. Materials 2012, 5, 2306-2325. https://doi.org/10.3390/ma5112306
Melzak KA, Melzak SA, Gizeli E, Toca-Herrera JL. Cholesterol Organization in Phosphatidylcholine Liposomes: A Surface Plasmon Resonance Study. Materials. 2012; 5(11):2306-2325. https://doi.org/10.3390/ma5112306
Chicago/Turabian StyleMelzak, Kathryn A., Shirley A. Melzak, Electra Gizeli, and José L. Toca-Herrera. 2012. "Cholesterol Organization in Phosphatidylcholine Liposomes: A Surface Plasmon Resonance Study" Materials 5, no. 11: 2306-2325. https://doi.org/10.3390/ma5112306





