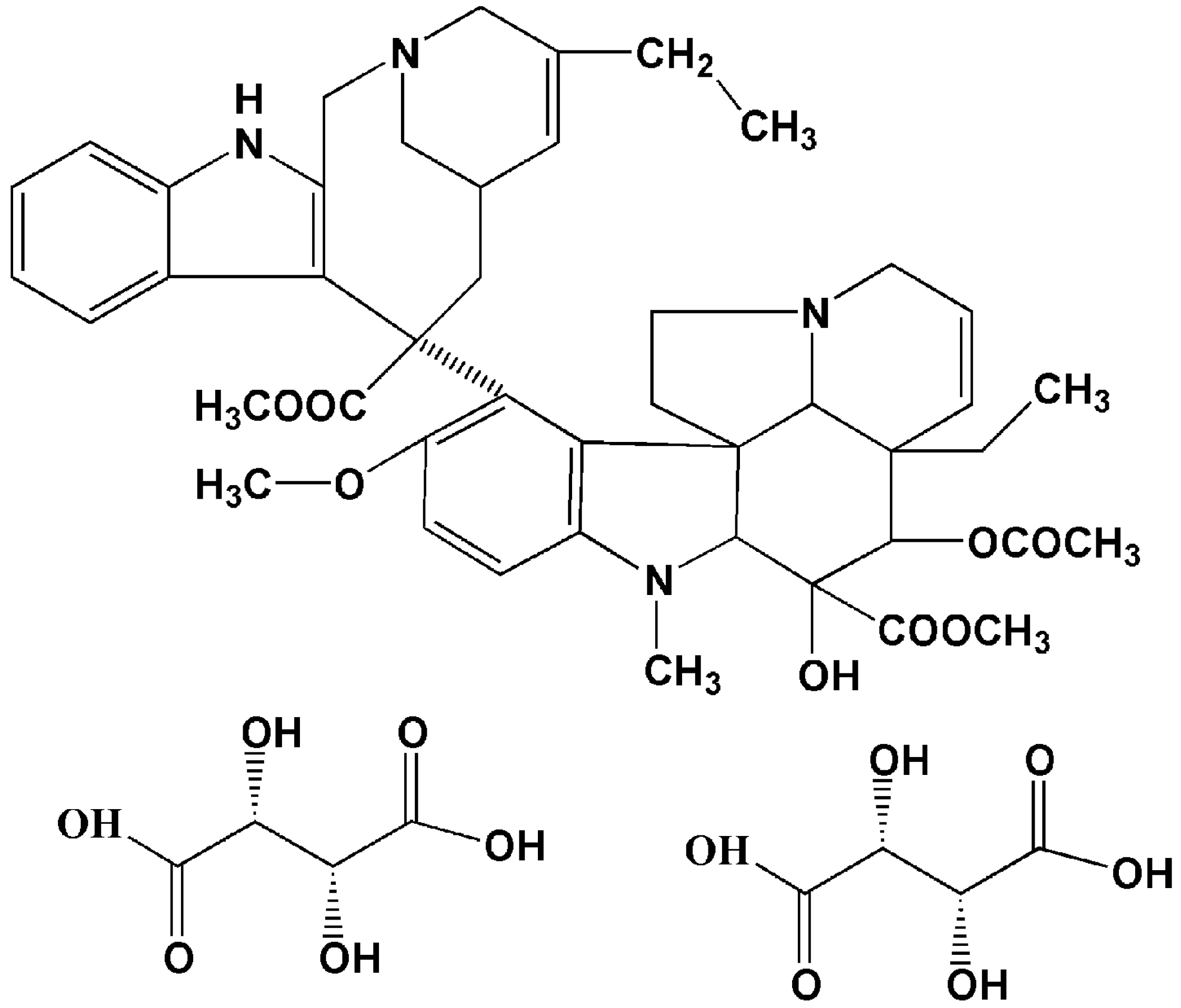
A Novel Active Targeting Preparation, Vinorelbine Tartrate (VLBT) Encapsulated by Folate-Conjugated Bovine Serum Albumin (BSA) Nanoparticles: Preparation, Characterization and in Vitro Release Study
Abstract
:1. Introduction
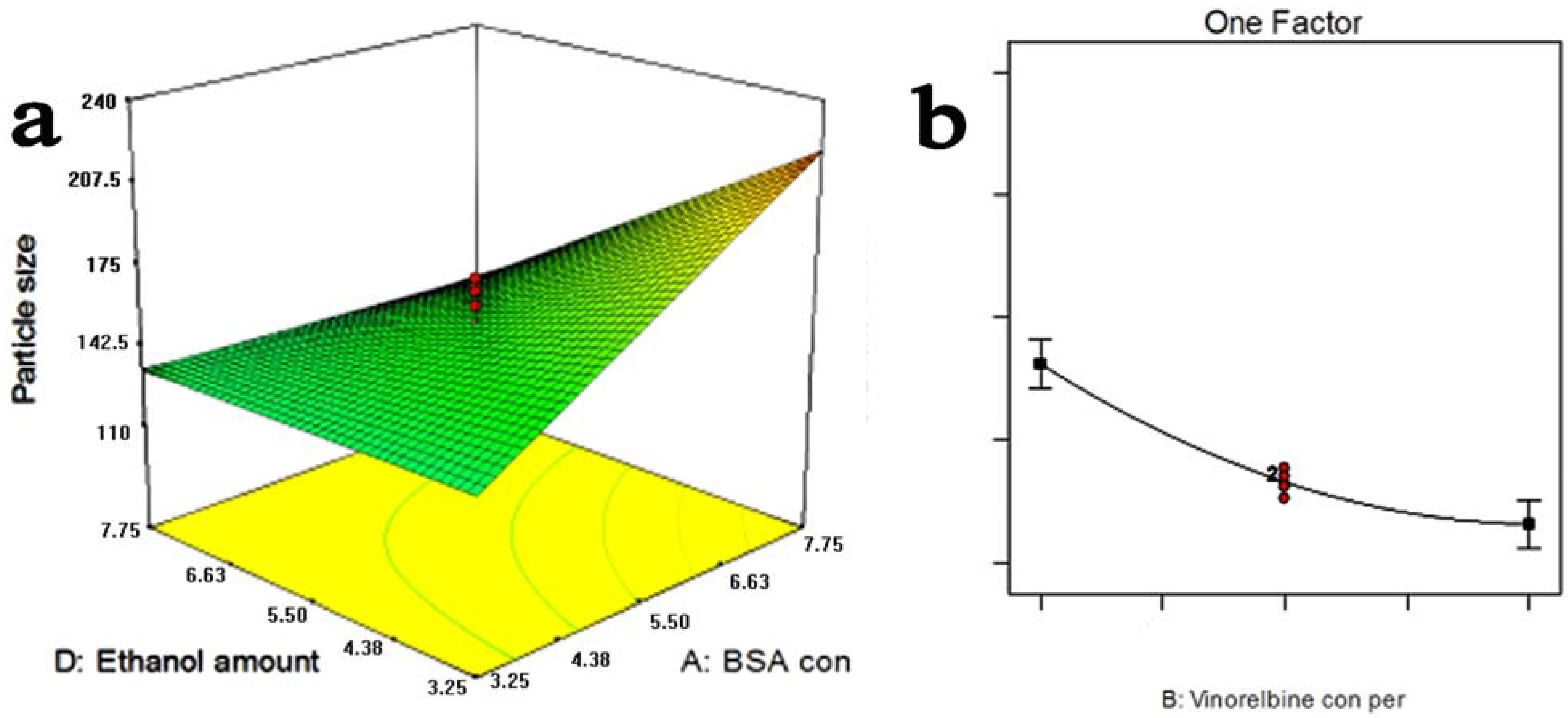
2. Results and Discussion
2.1. Optimization Study


| Run | Variables | Responses | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Y1 (%) | Y2 (nm) | |
| 1 | 5.50 | 5.00 | 3.25 | 5.50 | 13.14 | 151.1 |
| 2 | 7.75 | 3.00 | 4.63 | 7.75 | 22.47 | 114.6 |
| 3 | 7.75 | 7.00 | 4.63 | 3.25 | 11.25 | 219.5 |
| 4 | 3.25 | 3.00 | 1.88 | 3.25 | 21.73 | 167.7 |
| 5 | 3.25 | 7.00 | 1.88 | 7.75 | 8.72 | 79.7 |
| 6 | 5.50 | 5.00 | 3.25 | 5.50 | 12.32 | 159.0 |
| 7 | 3.25 | 3.00 | 4.63 | 7.75 | 25.43 | 119.7 |
| 8 | 1.00 | 5.00 | 3.25 | 5.50 | 12.22 | 111.7 |
| 9 | 5.50 | 9.00 | 3.25 | 5.50 | 9.26 | 142.6 |
| 10 | 5.50 | 5.00 | 3.25 | 1.00 | 31.64 | 230.4 |
| 11 | 7.75 | 3.00 | 1.88 | 3.25 | 19.37 | 246.3 |
| 12 | 5.50 | 5.00 | 3.25 | 5.50 | 11.35 | 164.7 |
| 13 | 7.75 | 7.00 | 1.88 | 3.25 | 10.61 | 176.8 |
| 14 | 3.25 | 7.00 | 4.63 | 3.25 | 8.91 | 158.6 |
| 15 | 3.25 | 7.00 | 4.63 | 7.75 | 6.03 | 142.1 |
| 16 | 3.25 | 3.00 | 1.88 | 7.75 | 17.55 | 147.3 |
| 17 | 5.50 | 1.00 | 3.25 | 5.50 | 46.91 | 171.8 |
| 18 | 3.25 | 3.00 | 4.63 | 3.25 | 21.60 | 54.2 |
| 19 | 3.25 | 7.00 | 1.88 | 3.25 | 9.94 | 141.4 |
| 20 | 5.50 | 5.00 | 0.50 | 5.50 | 11.05 | 210.0 |
| 21 | 5.50 | 5.00 | 3.25 | 5.50 | 13.83 | 170.0 |
| 22 | 5.50 | 5.00 | 3.25 | 5.50 | 13.10 | 149.3 |
| 23 | 10.00 | 5.00 | 3.25 | 5.50 | 14.27 | 192.4 |
| 24 | 7.75 | 7.00 | 4.63 | 7.75 | 9.96 | 115.7 |
| 25 | 5.50 | 5.00 | 3.25 | 10.00 | 10.93 | 162.2 |
| 26 | 5.50 | 5.00 | 3.25 | 5.50 | 12.60 | 167.0 |
| 27 | 7.75 | 7.00 | 1.88 | 7.75 | 8.57 | 185.7 |
| 28 | 5.50 | 5.00 | 6.00 | 5.50 | 12.09 | 103.5 |
| 29 | 7.75 | 3.00 | 4.63 | 3.25 | 9.27 | 220.6 |
| 30 | 7.75 | 3.00 | 1.88 | 7.75 | 22.18 | 55.9 |
2.2. Characterization of FA-BSANPs-VLBT
2.2.1. Folate Content Associated with the VLBT-BSANPs
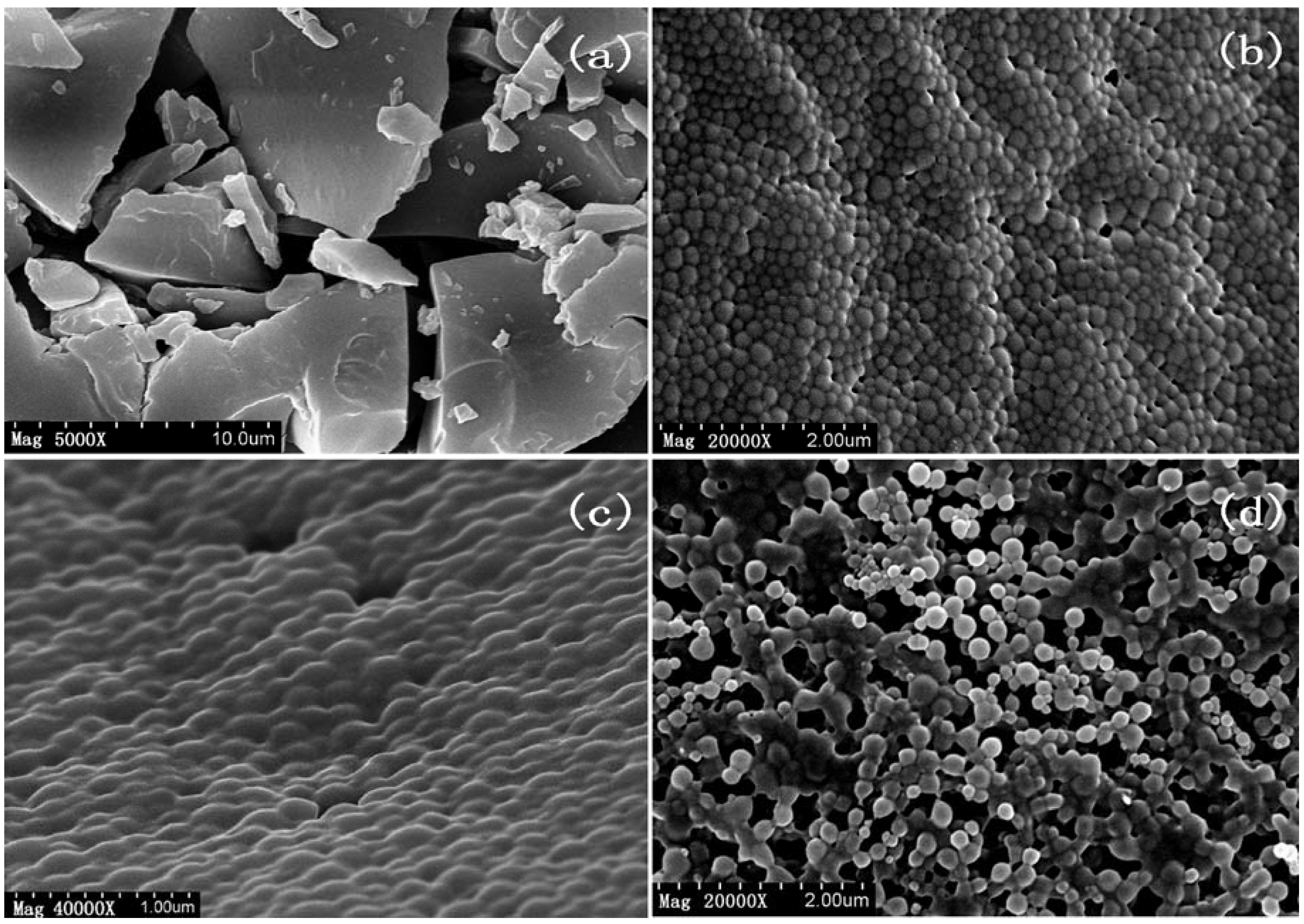
2.2.2. Surface Morphology of Nanoparticles
2.2.3. Surface Chemistry

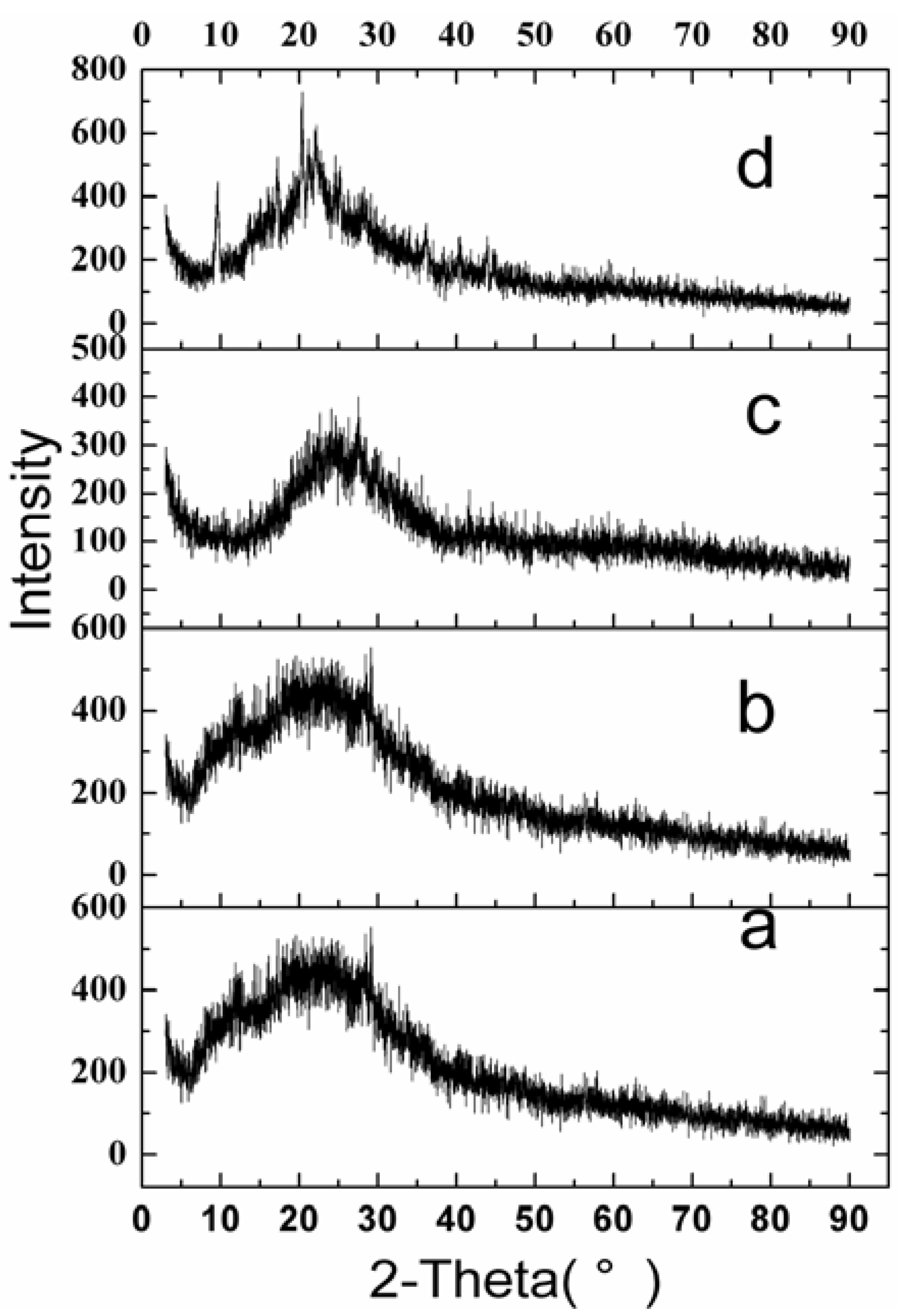
2.2.4. Physical Status of VLBT in Fa-BSANPs-VLBT

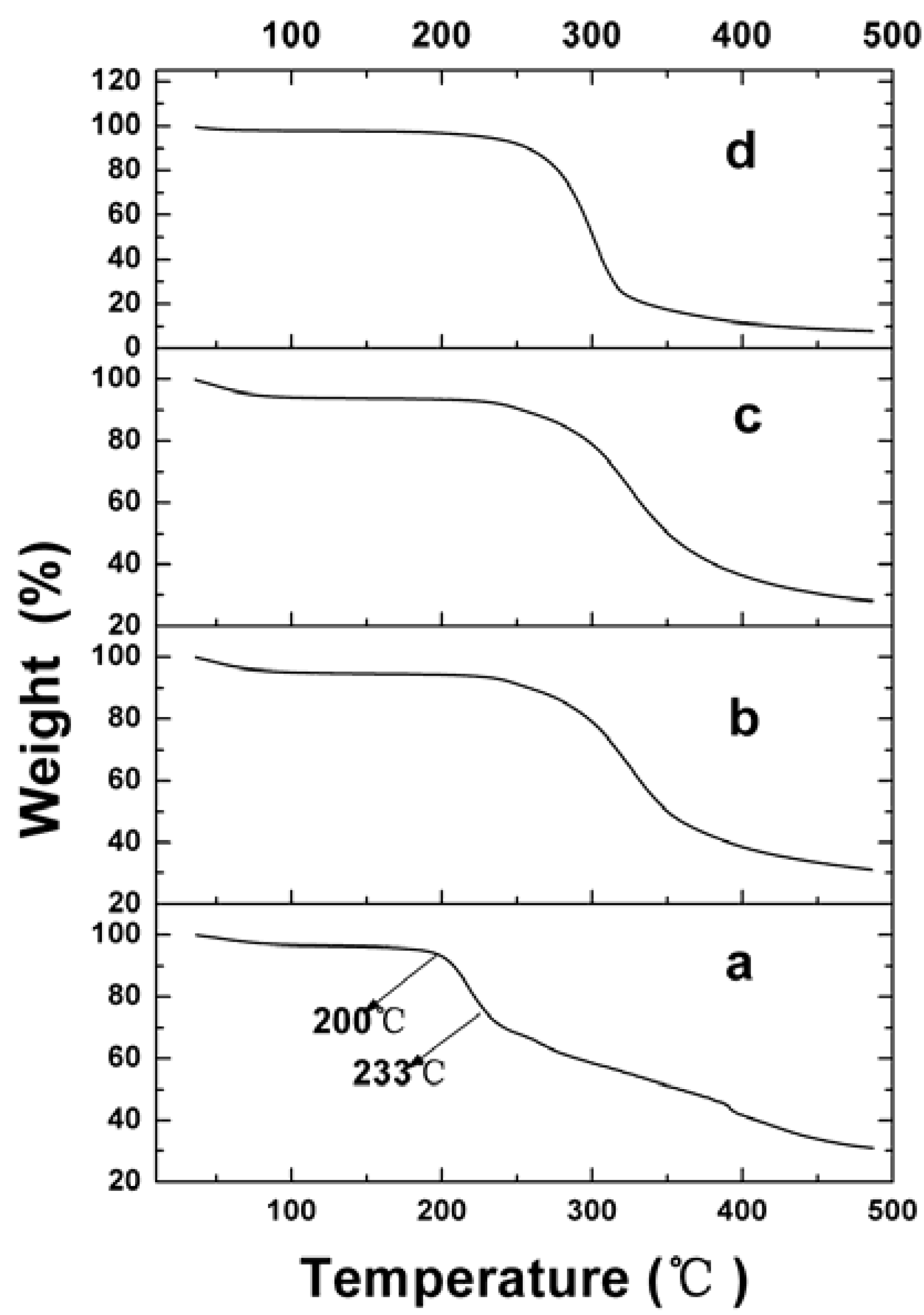
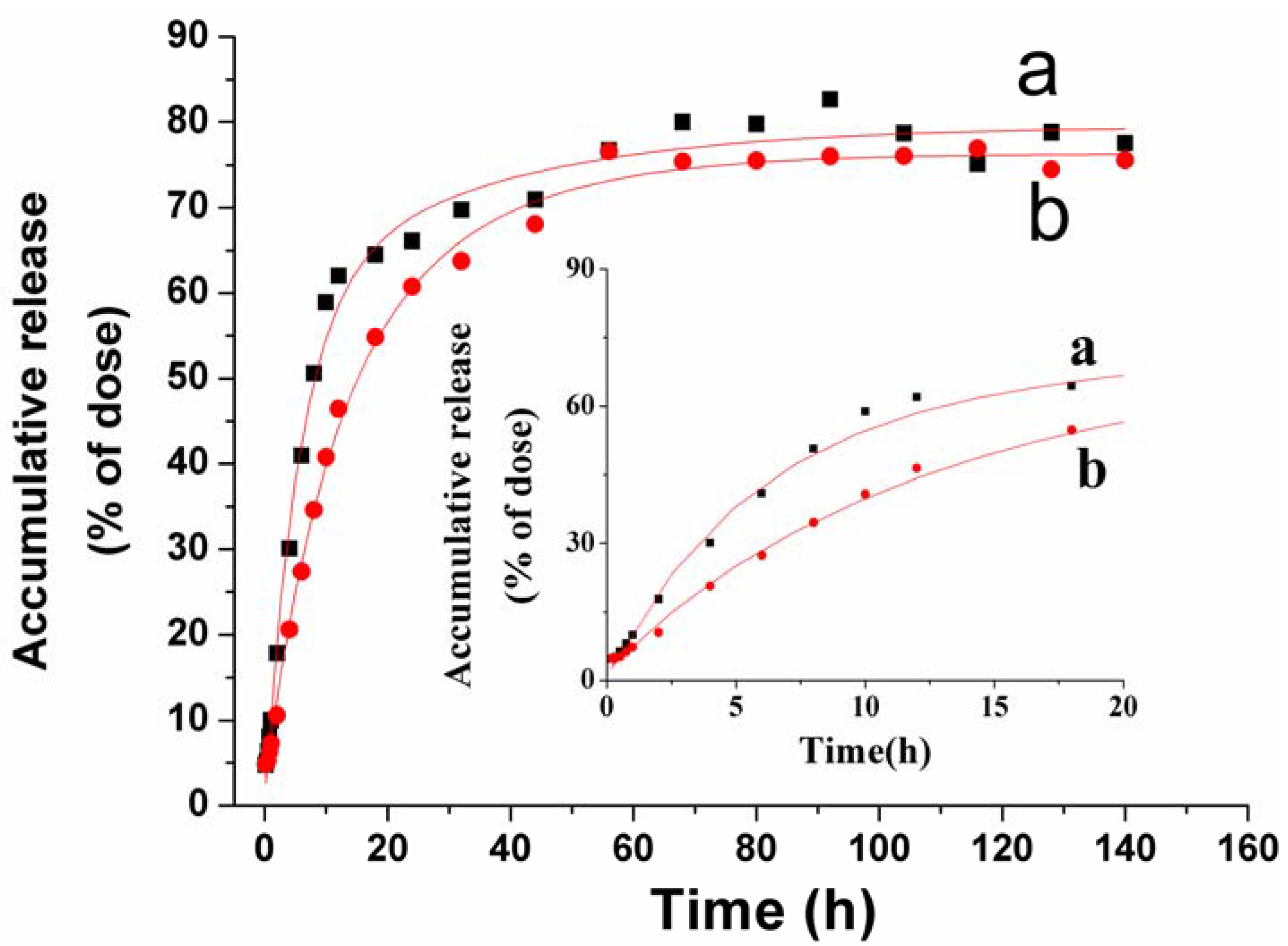
2.3. In Vitro VLBT Release from Fa-BSANPs-VLBT
2.4. Stability of Fa-BSANPs-VLBT
| Time (h) | 0 | 1 | 2 | 4 | 8 |
|---|---|---|---|---|---|
| MPS (nm) | 297.4 | 326.3 | 330.7 | 341.5 | 364.5 |
| ZP (mV) | −17.37 | −15.59 | −17.50 | −17.30 | −16.26 |
3. Experimental Section
3.1. Materials
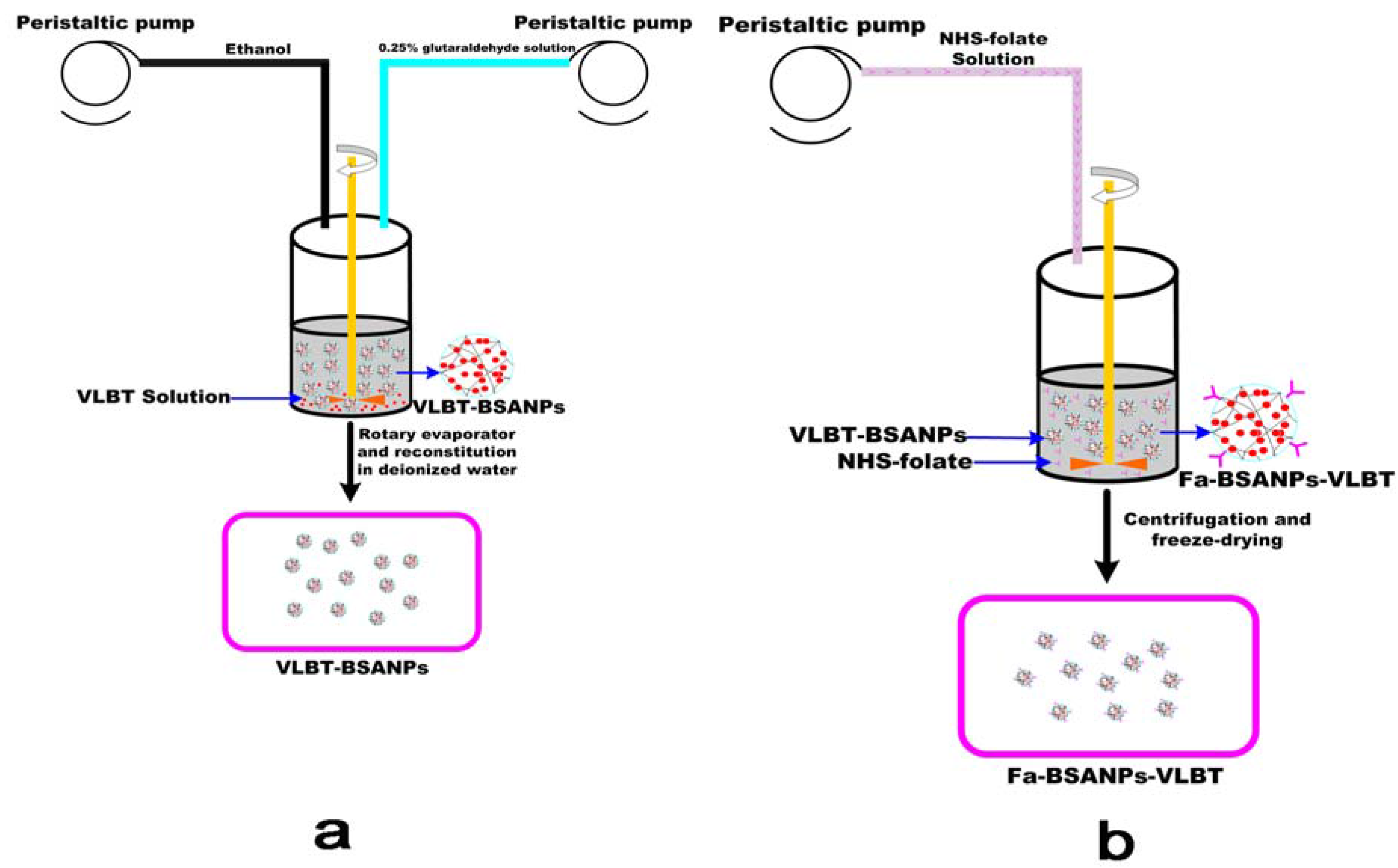
3.2. Preparation of Fa-BSANPs-VLBT
3.2.1. Preparation of the N-Hydroxysuccinimide Ester of Folate (NHS-folate)
3.2.2. Preparation and Optimization of VLBT-Loaded BSANPs by RSM
| Independent variables | Symbol | Coded levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| BSA concentration(mg/mL) | X1 | 3.25 | 5.5 | 7.75 |
| BSA:VLBT (c/c) | X2 | 3 | 5 | 7 |
| ethanol rate(mL/min) | X3 | 1.88 | 3.25 | 4.63 |
| Ethanol:VLBT solution (v/v) | X4 | 3.25 | 5.5 | 7.75 |
3.2.3. Preparation of Fa-BSANPs-VLBT
3.2.4. Lyophilization of Fa-BSANPs-VLBT
3.3. Characterization of the Fa-BSANPs-VLBT
3.3.1. Determination of Folate Content Associated with the VLBT-BSANPs
3.3.2. Drug Loading and Encapsulation Efficiency


3.3.3. MPS and ZP
3.3.4. Surface Morphology of Nanoparticles
3.3.5. Surface Chemistry
3.3.6. Physical Status of VLBT in Fa-BSANPs-VLBT
3.4. In Vitro VLBT Release from Fa-BSANPs-VLBT



3.5. Stability of Fa-BSANPs-VLBT
4. Conclusions
Abbreviations
| ABC | advanced breast cancer |
| BSA | bovine serum albumin |
| BSANPs | bovine serum albumin nanoparticles |
| CCD | central composite design |
| DCC | 1,3-dicyclohexyl-carbodiimide |
| DEE | drug entrapment efficiency |
| DLE | drug loading efficiency |
| DLS | dynamic light scattering |
| DMSO | dimethyl sulfoxide |
| DNR | daunorubicin |
| DSC | differential scanning calorimetry |
| Fa-BSANPs | folate-conjugated bovine serum albumin nanoparticles |
| Fa-BSANPs-VLBT | vinorelbine tartrate loaded folate-conjugated bovine serum albumin nanoparticles |
| F-L-DNR | liposomes loading daunorubicin |
| folate-PEG-CHEMS | folate polyethylene glycol-cholesterol hemisuccinate |
| FTIR | Fourier transform infrared |
| HPLC | high performance liquid chromatography |
| HSA | human serum albumin |
| HSANPs | human serum albumin nanoparticles |
| L-DNR | liposomal daunorubicin |
| MPS | mean particle size |
| NHS-folate | N-hydroxysuccinimide ester of folate |
| NSCLC | non-small-cell lung cancer |
| RSM | response surface methodology |
| SEM | scanning electron microscope |
| TGA | thermogravimetric analyzer |
| VLBT-BSANPs | vinorelbine tartrate loaded bovine serum albumin nanoparticles |
| VLBT | vinorelbine tartrate |
| VLB | vinorelbine |
| XRD | X-ray diffraction |
| ZP | zeta potential |
Acknowledgments
References
- Lee, W.-C.; Hwang, J.-J.; Tseng, Y.-L.; Wang, H.-E.; Chang, Y.-F.; Lu, Y.-C.; Ting, G.; Whang-Peng, J.; Wang, S.-J. Therapeutic efficacy evaluation of 111in-VNB-liposome on human colorectal adenocarcinoma HT-29/luc mouse xenografts. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2006, 569, 497–504. [Google Scholar] [CrossRef]
- Zhigaltsev, I.V.; Maurer, N.; Akhong, Q.-F.; Leone, R.; Leng, E.; Wang, J.; Semple, S.C.; Cullis, P.R. Liposome-encapsulated vincristine, vinblastine and vinorelbine: A comparative study of drug loading and retention. J. Control. Release 2005, 104, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Edinger, M.; Cao, Y.A.; Verneris, M.R.; Bachmann, M.H.; Contag, C.H.; Negrin, R.S. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood 2003, 101, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Toso, C.; Lindley, C. Vinorelbine: A novel vinca alkaloid. Am. J. Health Syst. Pharm. 1995, 52, 1287–1304. [Google Scholar] [PubMed]
- The Elderly Lung Cancer Vinorelbine Italian Study Group. Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J. Natl. Cancer Inst. 1999, 91, 66–72. [Google Scholar]
- Le Chevalier, T.; Brisgand, D.; Douillard, J.Y.; Pujol, J.L.; Alberola, V.; Monnier, A.; Riviere, A.; Lianes, P.; Chomy, P.; Cigolari, S.; et al. Randomized study of vinorelbine and cisplatin versus vindesine and cisplatin versus vinorelbine alone in advanced non-small-cell lung cancer: results of a European multicenter trial including 612 patients. J. Clin. Oncol. 1994, 12, 360–367. [Google Scholar] [PubMed]
- Wozniak, A.J.; Crowley, J.J.; Balcerzak, S.P.; Weiss, G.R.; Spiridonidis, C.H.; Baker, L.H.; Albain, K.S.; Kelly, K.; Taylor, S.A.; Gandara, D.R.; Livingston, R.B. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: a Southwest Oncology Group study. J. Clin. Oncol. 1998, 16, 2459–2465. [Google Scholar] [PubMed]
- Blajman, C.; Balbiani, L.; Block, J.; Coppola, F.; Chacon, R.; Fein, L.; Bonicatto, S.; Alvarez, A.; Schmilovich, A.; Delgado, F.M. A prospective, randomized Phase III trial comparing combination chemotherapy with cyclophosphamide, doxorubicin, and 5-fluorouracil with vinorelbine plus doxorubicin in the treatment of advanced breast carcinoma. Cancer 1999, 85, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Fumoleau, P.; Delgado, F.M.; Delozier, T.; Monnier, A.; Gil Delgado, M.A.; Kerbrat, P.; Garcia-Giralt, E.; Keiling, R.; Namer, M.; Closon, M.T.; et al. Phase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapy. J. Clin. Oncol. 1993, 11, 1245–1252. [Google Scholar] [PubMed]
- Vici, P.; Colucci, G.; Gebbia, V.; Amodio, A.; Giotta, F.; Belli, F.; Conti, F.; Gebbia, N.; Pezzella, G.; Valerio, M.R.; Brandi, M.; Pisconti, S.; Durini, E.; Giannarelli, D.; Lopez, M. First-line treatment with epirubicin and vinorelbine in metastatic breast cancer. J. Clin. Oncol. 2002, 20, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Mano, M. Vinorelbine in the management of breast cancer: New perspectives, revived role in the era of targeted therapy. Cancer Treat. Rev. 2006, 32, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Tang, X.; Li, H.Y.; Liu, X.L. A lipid microsphere vehicle for vinorelbine: Stability, safety and pharmacokinetics. Int. J. Pharm. 2008, 348, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Depierre, A.; Chastang, C.; Quoix, E.; Lebeau, B.; Blanchon, F.; Paillot, N.; Lemarie, E.; Milleron, B.; Moro, D.; Clavier, J.; et al. Vinorelbine versus vinorelbine plus cisplatin in advanced non-small cell lung cancer: a randomized trial. Ann. Oncol. 1994, 5, 37–42. [Google Scholar] [PubMed]
- Le Chevalier, T.; Brisgand, D.; Soria, J.C.; Douillard, J.Y.; Pujol, J.L.; Ruffie, P.; Aberola, V.; Cigolari, S. Long term analysis of survival in the European randomized trial comparing vinorelbine/cisplatin to vindesine/cisplatin and vinorelbine alone in advanced non-small cell lung cancer. Oncologist 2001, 6, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Gridelli, C.; Perrone, F.; Gallo, C.; de Marinis, F.; Ianniello, G.; Cigolari, S.; Cariello, S.; Di Costanzo, F.; D'Aprile, M.; Rossi, A.; Migliorino, R.; Bartolucci, R.; Bianco, A.R.; Pergola, M.; Monfardini, S. Vinorelbine is well tolerated and active in the treatment of elderly patients with advanced non-small cell lung cancer. A two-stage phase II study. Eur. J. Cancer 1997, 33, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Furuse, K.; Kubota, K.; Kawahara, M.; Ogawara, M.; Kinuwaki, E.; Motomiya, M.; Nishiwaki, Y.; Niitani, H.; Sakuma, A. A phase II study of vinorelbine, a new derivative of vinca alkaloid, for previously untreated advanced non-small cell lung cancer. Japan Vinorelbine Lung Cancer Study Group. Lung Cancer 1994, 11, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Mare, M.; Maisano, R.; Caristi, N.; Adamo, V.; Altavilla, G.; Carboni, R.; Munao, S.; La Torre, F. Venous damage prevention by defibrotide in vinorelbine-treated patients. Support. Care Cancer 2003, 11, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Bugat, R.; Variol, P.; Roche, H.; Fumoleau, P.; Robinet, G.; Senac, I. The effects of food on the pharmacokinetic profile of oral vinorelbine. Cancer Chemother. Pharmacol. 2002, 50, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Franssen, E.; Fitch, M.I.; Warner, E. Patient preferences for oral versus intravenous palliative chemotherapy. J. Clin. Oncol. 1997, 15, 110–115. [Google Scholar] [PubMed]
- Bonneterre, J.; Chevalier, B.; Focan, C.; Mauriac, L.; Piccart, M. Phase I and pharmacokinetic study of weekly oral therapy with vinorelbine in patients with advanced breast cancer (ABC). Ann. Oncol. 2001, 12, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.C.; Leone, R.; Wang, J.; Leng, E.C.; Klimuk, S.K.; Eisenhardt, M.L.; Yuan, Z.N.; Edwards, K.; Maurer, N.; Hope, M.J.; Cullis, P.R.; Ahkong, Q.F. Optimization and characterization of a sphingomyelin/cholesterol liposome formulation of vinorelbine with promising antitumor activity. J. Pharm. Sci. 2005, 94, 1024–1038. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Lin, C.C.; Lin, Z.Z.; Tseng, Y.L.; Hong, R.L. A phase I and pharmacokinetic study of liposomal vinorelbine in patients with advanced solid tumor. Invest. New Drugs 2012, 30, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Cui, J.X.; Wang, C.X.; Zhang, L.; Li, Y.H.; Xiu, X.; Li, Y.F.; Wei, N. Development of pegylated liposomal vinorelbine formulation using “post-insertion” technology. Int. J. Pharm. 2010, 391, 230–236. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Wan, F.; de Cui, F.; Sun, Y.; Du, Y.Z.; Hu, F.Q. Preparation and characteristic of vinorelbine bitartrate-loaded solid lipid nanoparticles. Int. J. Pharm. 2007, 343, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; You, J.; Sun, Y.; Zhang, X.G.; Cui, F.D.; Du, Y.Z.; Yuan, H.; Hu, F.Q. Studies on PEG-modified SLNs loading vinorelbine bitartrate (I): preparation and evaluation in vitro. Int. J. Pharm. 2008, 359, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.G.; Zhang, Y.; Zhao, X.H.; Zhang, Q.; Liu, Y.; Jiang, R. Optimization of the preparation process of vinblastine sulfate (VBLS)-loaded folate-conjugated bovine serum albumin (BSA) nanoparticles for tumor-targeted drug delivery using response surface methodology (RSM). Int. J. Nanomed. 2009, 4, 321–333. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–84. [Google Scholar] [CrossRef] [PubMed]
- Momekova, D.; Rangelov, S.; Yanev, S.; Nikolova, E.; Konstantinov, S.; Romberg, B.; Storm, G.; Lambov, N. Long-circulating, pH-sensitive liposomes sterically stabilized by copolymers bearing short blocks of lipid-mimetic units. Eur. J. Pharm. Sci. 2007, 32, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Michaelis, M.; Rothweiler, F.; Knobloch, T.; Sithisarn, P.; Cinatl, J.; Kreuter, J. Interaction of folate-conjugated human serum albumin (HSA) nanoparticles with tumour cells. Int. J. Pharm. 2011, 406, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Rollett, A.; Reiter, T.; Nogueira, P.; Cardinale, M.; Loureiro, A.; Gomes, A.; Cavaco-Paulo, A.; Moreira, A.; Carmo, A.M.; Guebitz, G.M. Folic acid-functionalized human serum albumin nanocapsules for targeted drug delivery to chronically activated macrophages. Int. J. Pharm. 2012, 427, 460–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.J.; Low, P.S. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J. Biol. Chem. 1994, 269, 3198–3204. [Google Scholar] [PubMed]
- Huang, S.J.; Sun, S.L.; Feng, T.H.; Sung, K.H.; Lui, W.L.; Wang, L.F. Folate-mediated chondroitin sulfate-Pluronic 127 nanogels as a drug carrier. Eur. J. Pharm. Sci. 2009, 38, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Lee, S.B.; Lee, Y.M. Preparation and characterization of methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) amphiphilic block copolymeric nanospheres for tumor-specific folate-mediated targeting of anticancer drugs. Biomaterials 2005, 26, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, T.; Takagi, A.; Maeda, A.; Kagatani, S.; Konno, Y.; Hashida, M. In vivo fate of folate-BSA in non-tumor- and tumor-bearing mice. J. Pharm. Sci. 1998, 87, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Kapoor, D.N.; Kapil, R.; Chhabra, N.; Dhawan, S. Design and development of paclitaxel-loaded bovine serum albumin nanoparticles for brain targeting. Acta Pharm. 2011, 61, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhao, X.; Zu, Y.; Li, J.; Zhang, Y.; Jiang, R.; Zhang, Z. Preparation, characterization, and in vitro targeted delivery of folate-decorated paclitaxel-loaded bovine serum albumin nanoparticles. Int. J. Nanomed. 2010, 5, 669–677. [Google Scholar]
- Tang, Q.S.; Chen, D.Z.; Xue, W.Q.; Xiang, J.Y.; Gong, Y.C.; Zhang, L.; Guo, C.Q. Preparation and biodistribution of 188Re-labeled folate conjugated human serum albumin magnetic cisplatin nanoparticles (188Re-folate-CDDP/HSA MNPs) in vivo. Int. J. Nanomed. 2011, 6, 3077–3085. [Google Scholar]
- Zhang, L.; Hou, S.; Mao, S.; Wei, D.; Song, X.; Lu, Y. Uptake of folate-conjugated albumin nanoparticles to the SKOV3 cells. Int. J. Pharm. 2004, 287, 155–162. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Y.; Zhao, X.; Zu, Y.; Han, X.; Ge, Y.; Wang, W.; Yu, X. A Novel Active Targeting Preparation, Vinorelbine Tartrate (VLBT) Encapsulated by Folate-Conjugated Bovine Serum Albumin (BSA) Nanoparticles: Preparation, Characterization and in Vitro Release Study. Materials 2012, 5, 2403-2422. https://doi.org/10.3390/ma5112403
Li Y, Zhao X, Zu Y, Han X, Ge Y, Wang W, Yu X. A Novel Active Targeting Preparation, Vinorelbine Tartrate (VLBT) Encapsulated by Folate-Conjugated Bovine Serum Albumin (BSA) Nanoparticles: Preparation, Characterization and in Vitro Release Study. Materials. 2012; 5(11):2403-2422. https://doi.org/10.3390/ma5112403
Chicago/Turabian StyleLi, Yong, Xiuhua Zhao, Yuangang Zu, Xue Han, Yunlong Ge, Weiguo Wang, and Xinyang Yu. 2012. "A Novel Active Targeting Preparation, Vinorelbine Tartrate (VLBT) Encapsulated by Folate-Conjugated Bovine Serum Albumin (BSA) Nanoparticles: Preparation, Characterization and in Vitro Release Study" Materials 5, no. 11: 2403-2422. https://doi.org/10.3390/ma5112403






