Properties of PEMA-NH4CF3SO3 Added to BMATSFI Ionic Liquid
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
3. Results and Discussion
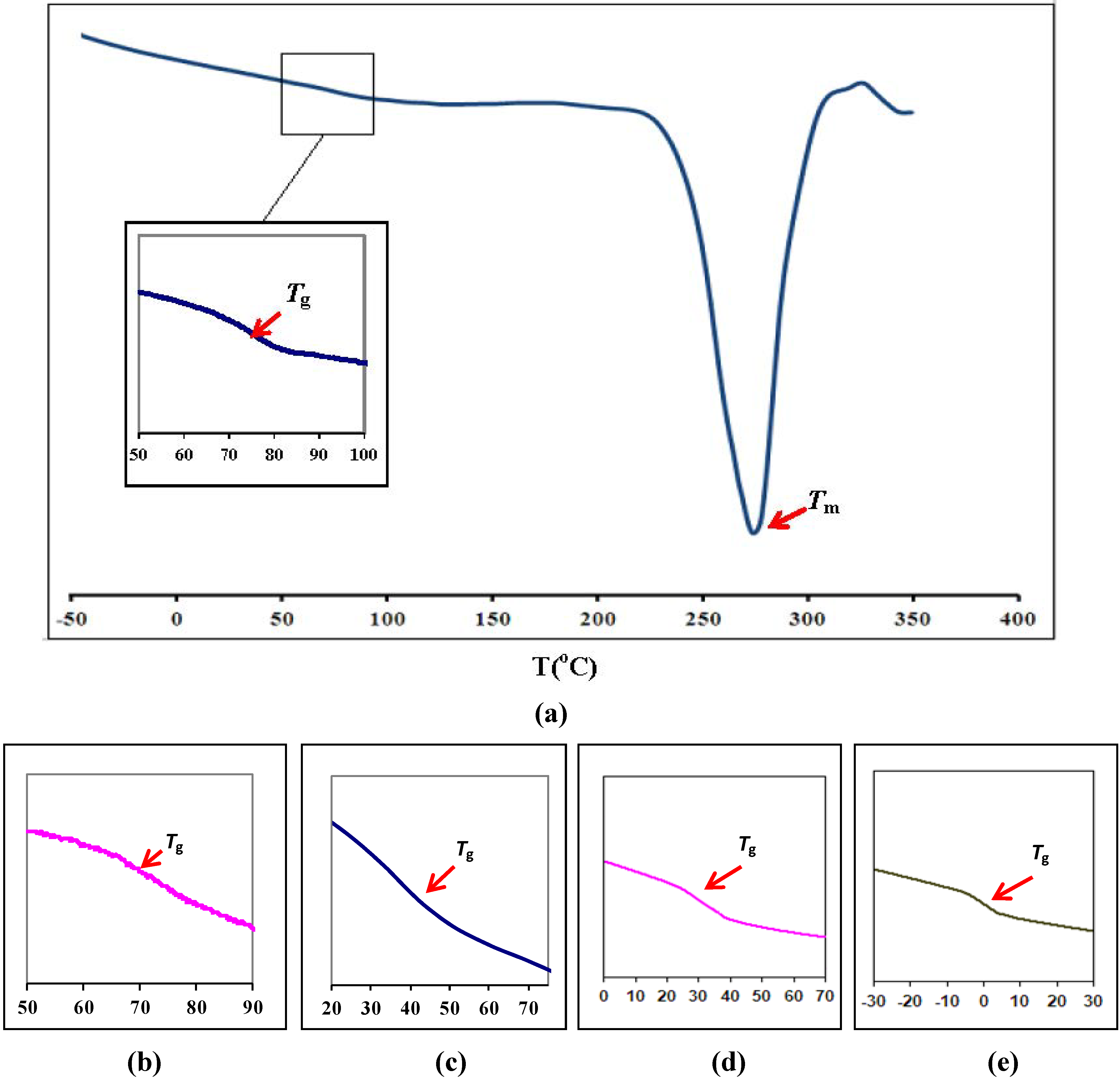
3.1. Differential Scanning Calorimetry
| Polymer film | Glass transition temperature, Tg (°C) |
|---|---|
| Pure PEMA | 72 |
| (PEMA-NH4SO3CF3) | 68 |
| (PEMA-NH4SO3CF3)-BMATFSI 15 wt % | 43 |
| (PEMA-NH4SO3CF3)-BMATFSI 25 wt % | 29 |
| (PEMA-NH4SO3CF3)-BMATFSI 35 wt % | 2 |
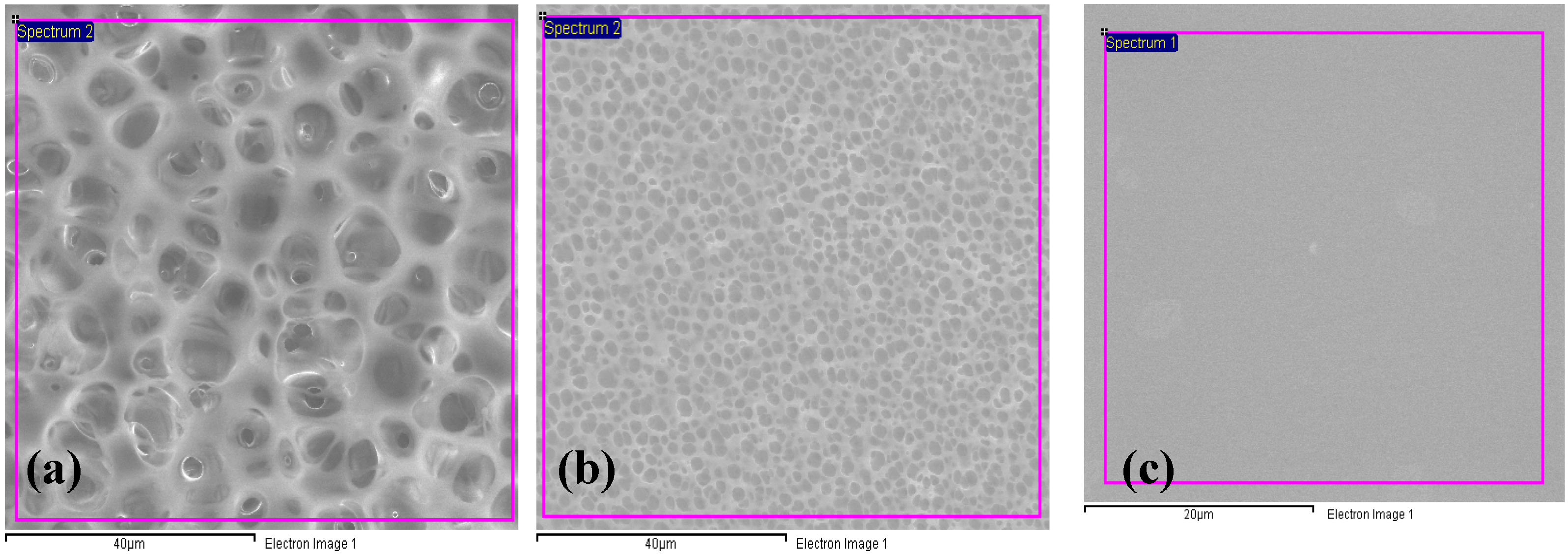
3.2. Scanning Electron Microscopy (SEM)
3.3. Fourier Transform Infrared

3.4. Conductivity Study
3.4.1. Composition Dependence of Conductivity
| Polymer film | Conductivity, σ (S cm−1) |
|---|---|
| PEMA | 8.60 × 10−11 |
| (PEMA-NH4SO3CF3) | 1.02 × 10−5 |
| (PEMA-NH4SO3CF3)-BMATFSI 15 wt % | 4.05 × 10−5 |
| (PEMA-NH4SO3CF3)-BMATFSI 25 wt % | 7.47 × 10−5 |
| (PEMA-NH4SO3CF3)-BMATFSI 35 wt % | 8.35 × 10−4 |
3.4.2. Temperature Dependence of Conductivity
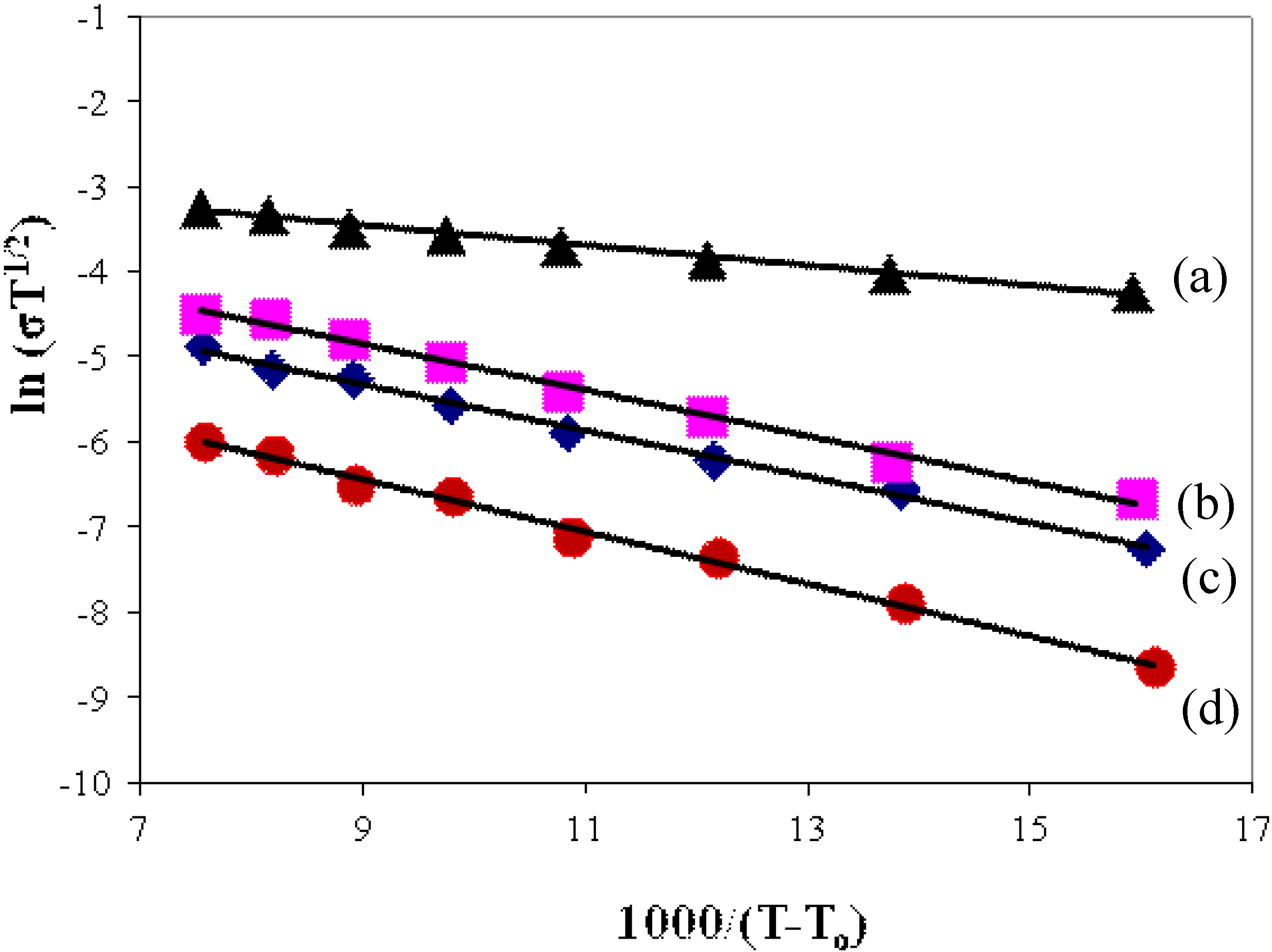
3.5. Activation Energy for Proton Ion Transport
| Polymer film | Tg (K) | EA (kJ) |
|---|---|---|
| (PEMA-NH4SO3CF3) | 340.93 | 2.5 |
| (PEMA-NH4SO3CF3)-BMATFSI 15 wt % | 315.65 | 2.3 |
| (PEMA-NH4SO3CF3)-BMATFSI 25 wt % | 302.31 | 2.2 |
| (PEMA-NH4SO3CF3)-BMATFSI 35 wt % | 275.25 | 1.8 |
3.6. Electrochemical Stability Determination
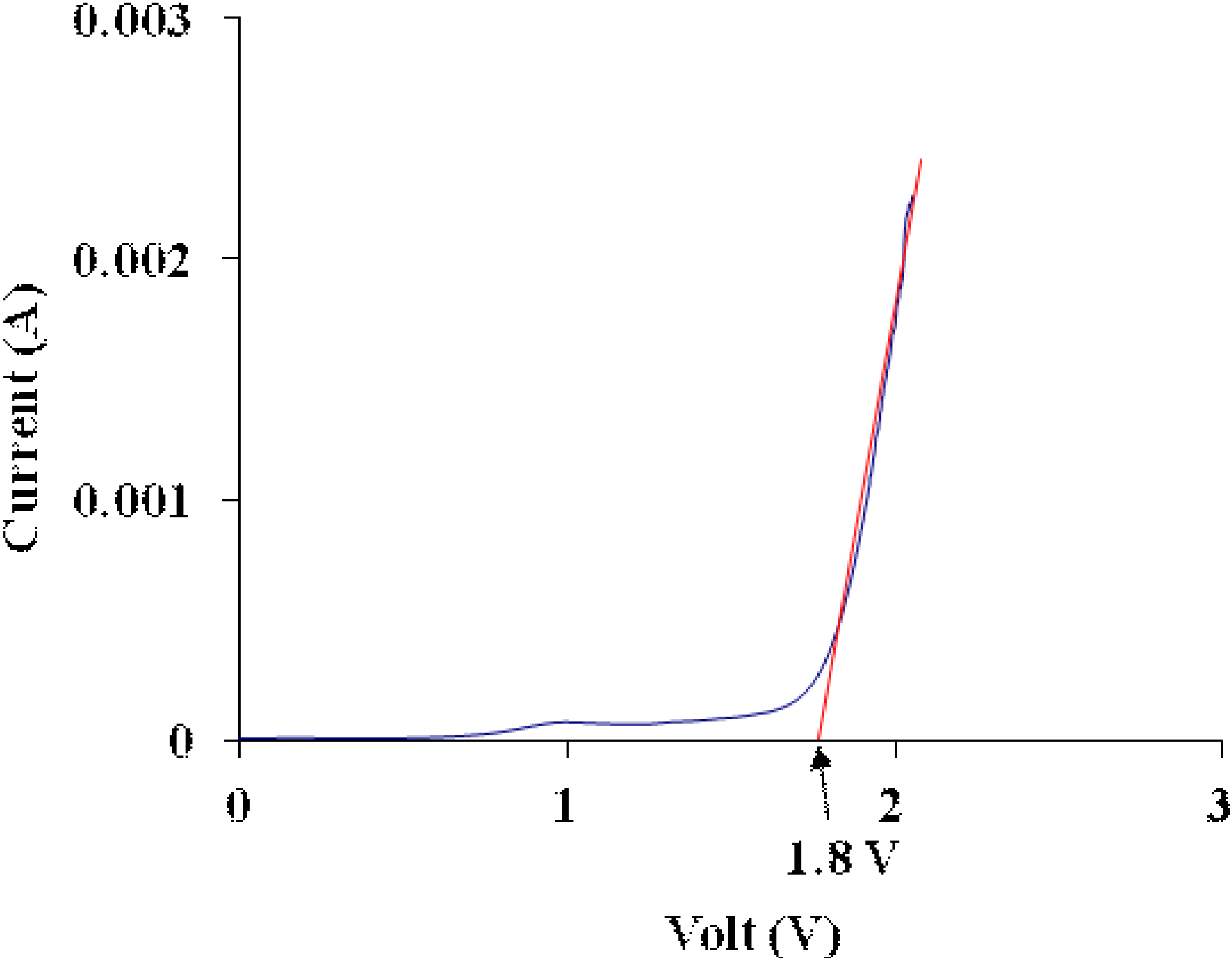
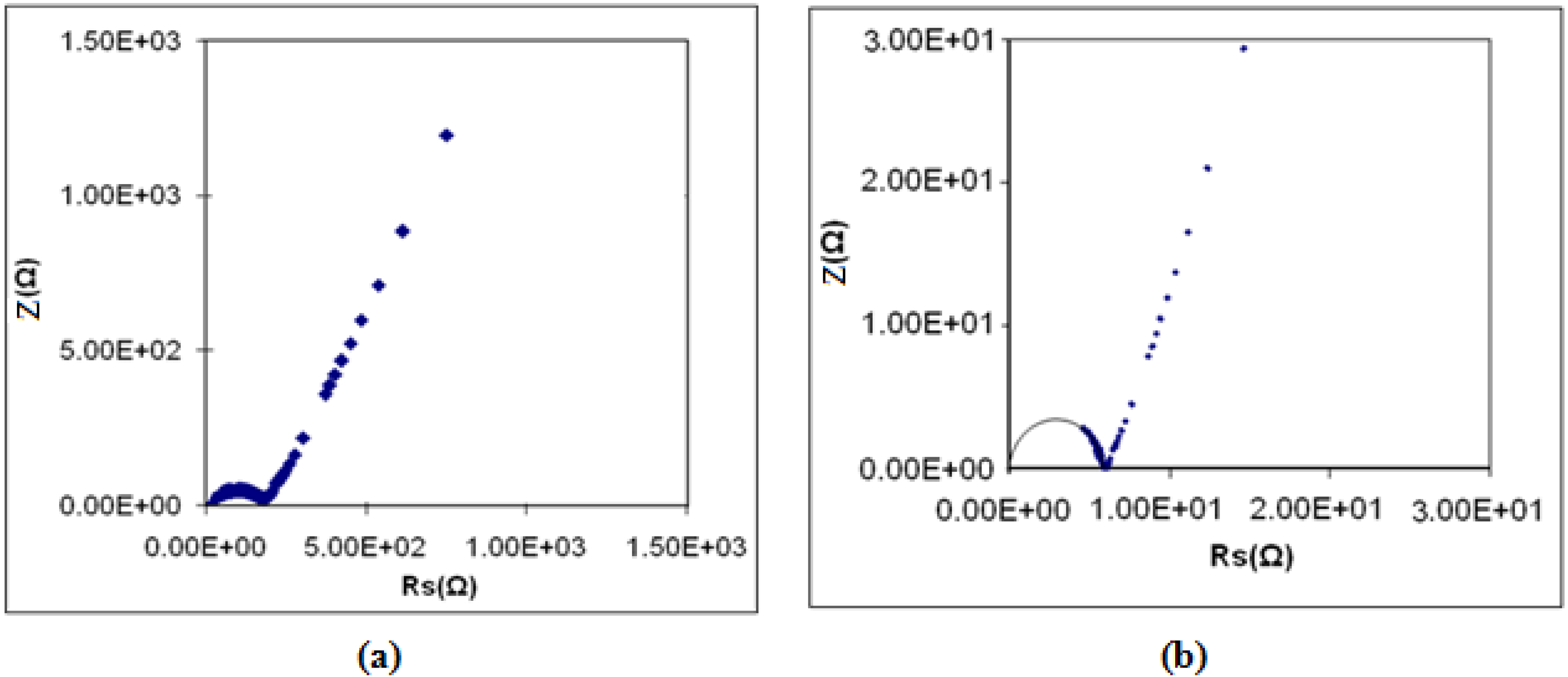
3.7. Ionic Transference Number Measurements
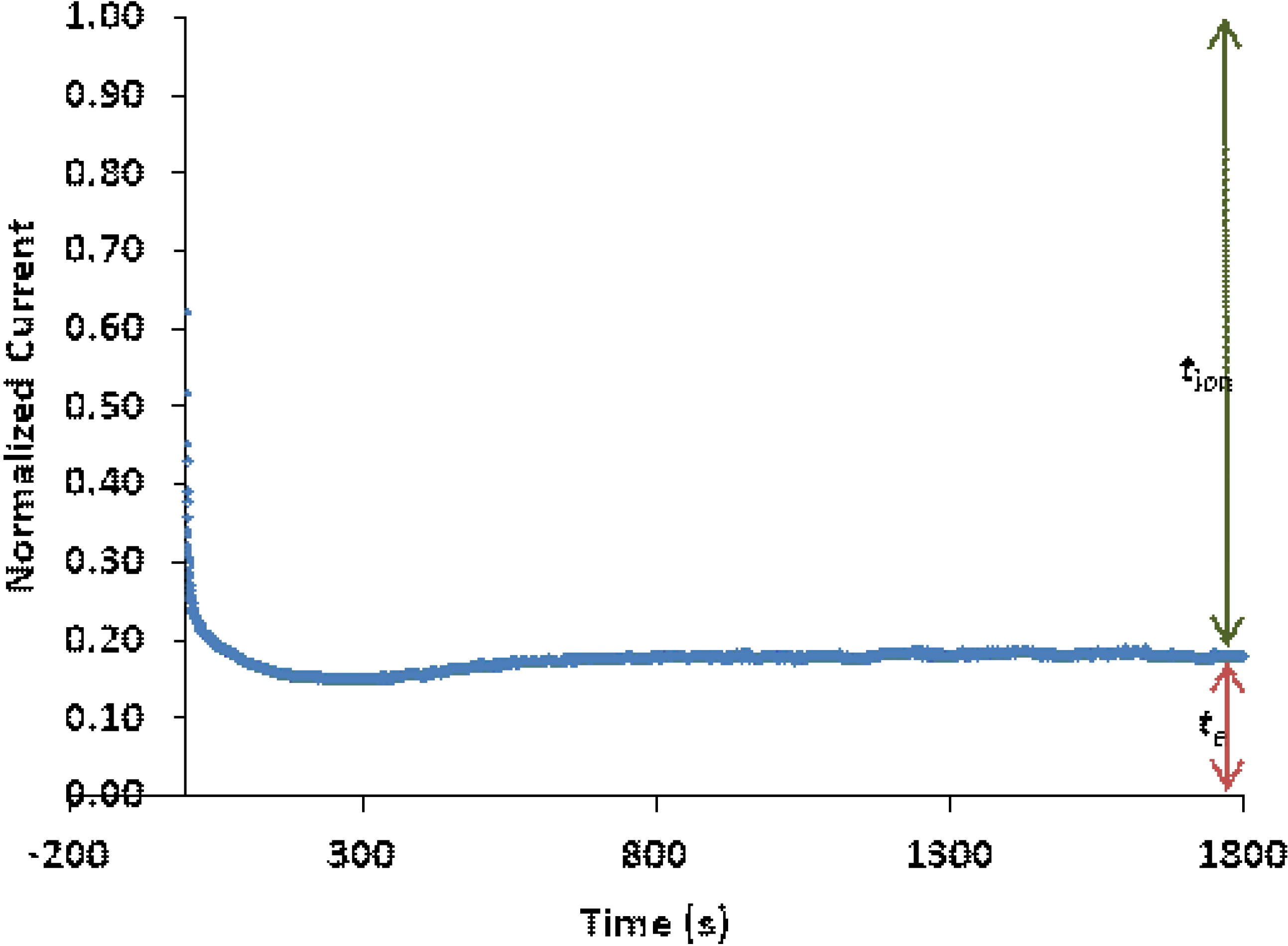
| Polymer film | tion |
|---|---|
| (PEMA-NH4SO3CF3) | 0.999 |
| (PEMA-NH4SO3CF3)-BMATFSI 25 wt % | 0.930 |
| (PEMA-NH4SO3CF3)-BMATFSI 35 wt % | 0.820 |
4. Conclusions
References
- Stainer, M.; Hardy, L.C.; Whitmore, D.H.; Shriver, D. Stoichiometry of formation and conductivity response of amorphous and crystalline complexes formed between poly(ethylene oxide) and ammonium salts: PEOx·NH4SCN and PEOx·NH4SO3CF3. J. Electrochem. Soc. 1984, 131, 784–790. [Google Scholar] [CrossRef]
- Ansari, S.M.; Browdwin, M.; Stainer, M.; Druger, S.D.; Ratner, M.A.; Shriver, D.F. Conductivity and dielectric constant of the polymeric solid electrolyte, (PEO)8NH4SO3CF3, in the 100 Hz to 1010 Hz range. Solid State Ionics 1985, 17, 101–106. [Google Scholar] [CrossRef]
- Hashmi, S.A.; Kumar, A.; Maurya, K.K.; Chandra, S. Proton-conducting polymer electrolyte I: The polyethylene oxide + NH4ClO4 system. J. Phys. D Appl. Phys. 1990, 23, 1307–1314. [Google Scholar] [CrossRef]
- Khatijah, S.; Subban, R.H.Y.; Mohamed, N.S. Ionic conductivity of PVC-NH4I-EC proton conducting polymer electrolytes. Adv. Mater. Res. 2012, 545, 312–316. [Google Scholar] [CrossRef]
- Reiter, J.; Velicka, J.; Mika, M. Proton-conducting polymer electrolytes based on methacrylates. Electrochim. Acta 2008, 53, 7769–7774. [Google Scholar]
- Han, H.S.; Kang, H.R.; Kim, S.W.; Kim, H.T. Phase-separated polymer electrolyte based on poly(vinyl chloride)/poly(ethyl methacrylate) blend. J. Power Source 2002, 112, 461–468. [Google Scholar] [CrossRef]
- Fahmy, T.; Ahmed, M.T. Thermal induced structural change investigations in PVC/PEMA polymer blend. Polym. Test. 2001, 20, 477–484. [Google Scholar] [CrossRef]
- Rajendran, S.; Prabhu, M.R.; Rani, M.U. Ionic conduction in poly(vinyl chloride)/poly(ethyl methacrylate)-based polymer blend electrolytes complexed with different lithium salts. J. Power Sources 2008, 180, 880–883. [Google Scholar] [CrossRef]
- Zakaria, N.A.; Isa, M.I.N.; Mohamed, N.S.; Subban, R.H.Y. Characterization of polyvinyl chloride/polyethyl methacrylate polymer blend for use as polymer host in polymer electrolytes. J. Appl. Polym. Sci. 2012, 126, E419–E424. [Google Scholar] [CrossRef]
- Rudziah, S.; Ibrahim, S.; Mohamed, N.S. PVDF-HFP/PEMA-NH4CF3SO3-TiO2 polymer electrolyte and its application in proton batteries. Adv. Mater. Res. Appl. Eng. Mater. 2011, 287–290, 285–288. [Google Scholar]
- Rudhziah, S.; Muda, N.; Ibrahim, S.; Rahman, A.A.; Mohamed, N.S. Proton conducting polymer electrolytes based on PVDF-HFP and PVDF-HFP/PEMA blend. Sains Malays. 2011, 40, 707–712. [Google Scholar]
- Anuar, N.K.; Zainal, N.; Mohamed, N.S.; Subban, R.H.Y. Studies of poly(ethyl methacrylate) complexed with ammonium trifluoromethanesulfonate. Adv. Mater. Res. 2012, 501, 19–23. [Google Scholar] [CrossRef]
- Noda, A.; Watanabe, M. Highly conductive polymer electrolytes prepared by in situ polymerization of vinyl monomers in room temperature molten salts. Electrochim. Acta 2000, 45, 1265–1270. [Google Scholar] [CrossRef]
- Fuller, J.; Breda, A.C.; Carlin, R.T. Ionic liquid–polymer gel electrolytes from hydrophilic and hydrophobic ionic liquids. J. Electroanal. Chem. 1998, 459, 29–34. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, S.Y.; Choi, S.; Lee, H. Ionic liquid–polymer gel electrolytes based on morpholinium salt and PVdF(HFP) copolymer. J. Power Sources 2006, 155, 385–390. [Google Scholar] [CrossRef]
- Bansal, D.; Cassel, F.; Croce, F.; Hendrickson, M.; Plichta, E.; Salomon, M. Conductivities and Transport Properties of Gelled Electrolytes with and without an Ionic Liquid for Li and Li-Ion Batteries. J. Phys. Chem. B 2005, 109, 4492–4496. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Henderson, W.A.; Tizzani, C.; Passerini, S.; Jeong, S.S.; Kim, K.W. Characterization of Solvent-Free Polymer Electrolytes Consisting of Ternary PEO-LiTFSI-PYR14 TFSI. J. Electrochem. Soc. 2006, 153, A1649–A1654. [Google Scholar] [CrossRef]
- Singh, B.; Sekhon, S.S. Polymer electrolytes based on room temperature ionic liquid: 2,3-dimethyl-1-octylimidazolium triflate. J. Phys. Chem. B 2005, 109, 16539–16543. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhu, C.; Huang, B.; Lu, M.; Yang, Y. Synthesis and electrochemical characterization of PEO-based polymer electrolytes with room temperature ionic liquids. Electrochim. Acta 2007, 52, 5789–5794. [Google Scholar] [CrossRef]
- Singh, P.K.; Jadhav, N.A.; Mishra, S.K.; Singh, U.P.; Bhattacharya, B. Application of ionic liquid doped solid polymer electrolyte. Ionics 2010, 16, 645–648. [Google Scholar] [CrossRef]
- Singh, P.K.; Bhattacharya, B. Present scenario of solid state photoelectrochemical solar cell and dye sensitized solar cell using PEO-based polymer electrolytes. In Dye-Sensitized Solar Cells and Solar Cell Performance; Travino, M.R., Ed.; Nova Science Publishers: Hauppauge, New York, NY, USA, 2011; Chapter 10; pp. 235–266. [Google Scholar]
- Axenov, K.V.; Laschat, S. Thermotropic ionic liquid crystals. Materials 2011, 4, 206–259. [Google Scholar] [CrossRef]
- Wagner, J.B.; Wagner, C. Electrical Conductivity Measurements on Cuprous Halides. J. Chem. Phys. 1957, 26, 1597–1601. [Google Scholar] [CrossRef]
- Cai, H.; Hu, R.; Egami, T.; Farrington, C.G. The effect of salt concentration on the local atomic structure and conductivity of PEO-based NiBr2 electrolytes. Solid State Ionics 1992, 52, 333–338. [Google Scholar] [CrossRef]
- Ghiya, V.P.; Dave, V.; Gross, R.A.; McCarthy, S.P. Biodegradability of cellulose acetate plasticized with citrate esters. J. Macromol. Sci. A 1996, 33, 627–638. [Google Scholar] [CrossRef]
- Singh, P.K.; Nagarale, R.K.; Pandey, S.P.; Rhee, H.W.; Bhattacharya, B. Present status of solid state photoelectrochemical solar cells and dye sensitized solar cells using PEO-based polymer electrolytes. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 023002:1–13. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, X.; Yang, B.; Tang, X. A novel ionic-conduction mechanism based on polyurethane electrolyte. J. Polymer Sci. B 2001, 39, 1246–1254. [Google Scholar] [CrossRef]
- Selvasekarapandian, S.; Baskaran, R.; Hema, M. Complex AC impedance, transference number and vibrational spectroscopy studies of proton conducting PVAc–NH4SCN polymerelectrolytes. Phys. B Condens. Matter 2005, 357, 412–419. [Google Scholar] [CrossRef]
- Wieczoreck, W.; Stevens, J.R. Impedance spectroscopy and phase structure of polyether-poly(methyl methacrylate)-LiCF3SO3 blend-based electrolytes. J. Phys. Chem. B 1997, 101, 1529–1534. [Google Scholar] [CrossRef]
- Rhoo, H.J.; Kim, H.T.; Park, J.M.; Hwang, T.S. Ionic conduction in plasticized PVC/PMMA blend polymer electrolytes. Electrochim. Acta 1997, 42, 1571–1579. [Google Scholar] [CrossRef]
- Aravindan, V.; Lakshmi, C.; Vickraman, P. Investigations on Na+ ion conducting polyvinylidenefluoride-co-hexafluoropropylene/poly ethylmetharcrylate blend polymer electrolytes. Curr. Appl. Phys. 2009, 9, 1106–1111. [Google Scholar] [CrossRef]
- Pratap, R.; Singh, B.; Chandra, S. Polymeric rechargeable solid-state proton battery. J. Power Sources 2006, 161, 702–706. [Google Scholar] [CrossRef]
- Ng, L.S.; Mohamad, A.A. Effect of temperature on the performance of proton batteries based on chitosan-NH4NO3-EC membrane. J. Membr. Sci. 2008, 325, 653–657. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Anuar, N.K.; Subban, R.H.Y.; Mohamed, N.S. Properties of PEMA-NH4CF3SO3 Added to BMATSFI Ionic Liquid. Materials 2012, 5, 2609-2620. https://doi.org/10.3390/ma5122609
Anuar NK, Subban RHY, Mohamed NS. Properties of PEMA-NH4CF3SO3 Added to BMATSFI Ionic Liquid. Materials. 2012; 5(12):2609-2620. https://doi.org/10.3390/ma5122609
Chicago/Turabian StyleAnuar, Norwati Khairul, Ri Hanum Yahaya Subban, and Nor Sabirin Mohamed. 2012. "Properties of PEMA-NH4CF3SO3 Added to BMATSFI Ionic Liquid" Materials 5, no. 12: 2609-2620. https://doi.org/10.3390/ma5122609




