Substrate Effects on the Formation Process, Structure and Physicochemical Properties of Supported Lipid Bilayers
Abstract
:1. Introduction
2. Substrate Effects on SLB Formation Process
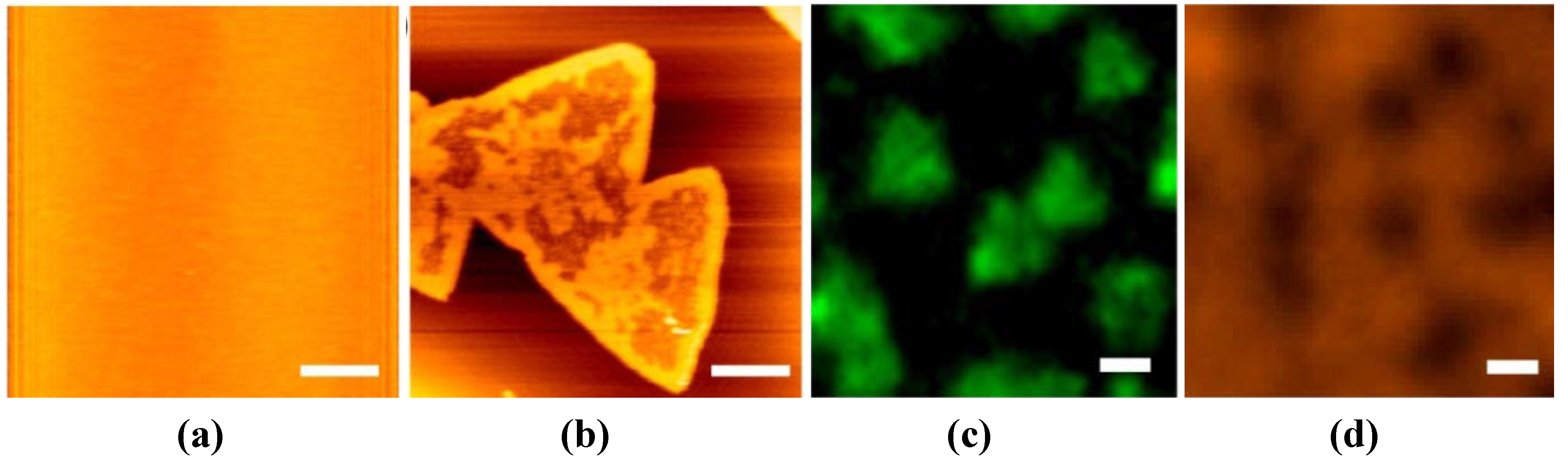
2.1. Vesicle Fusion Method for SLB Formation
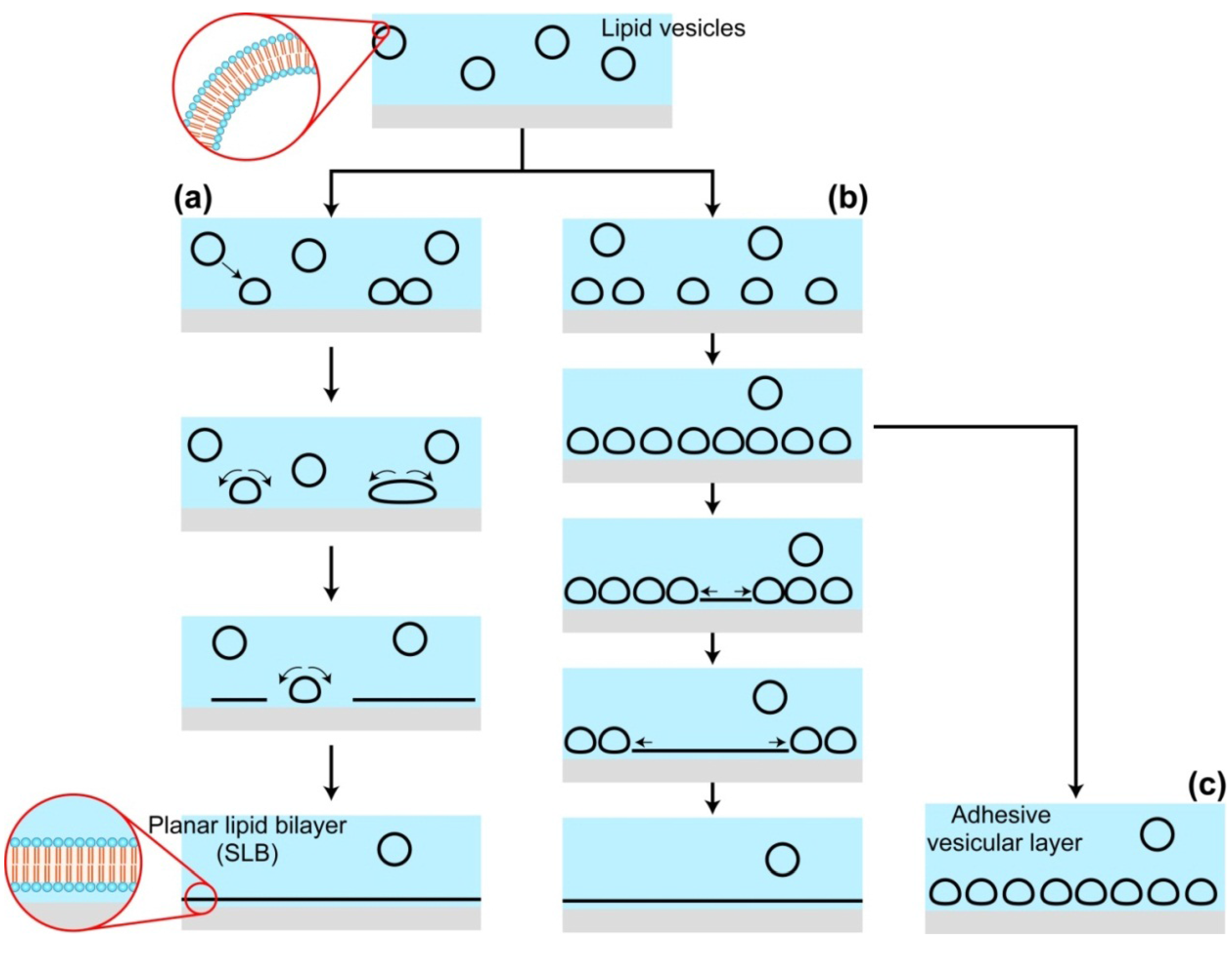
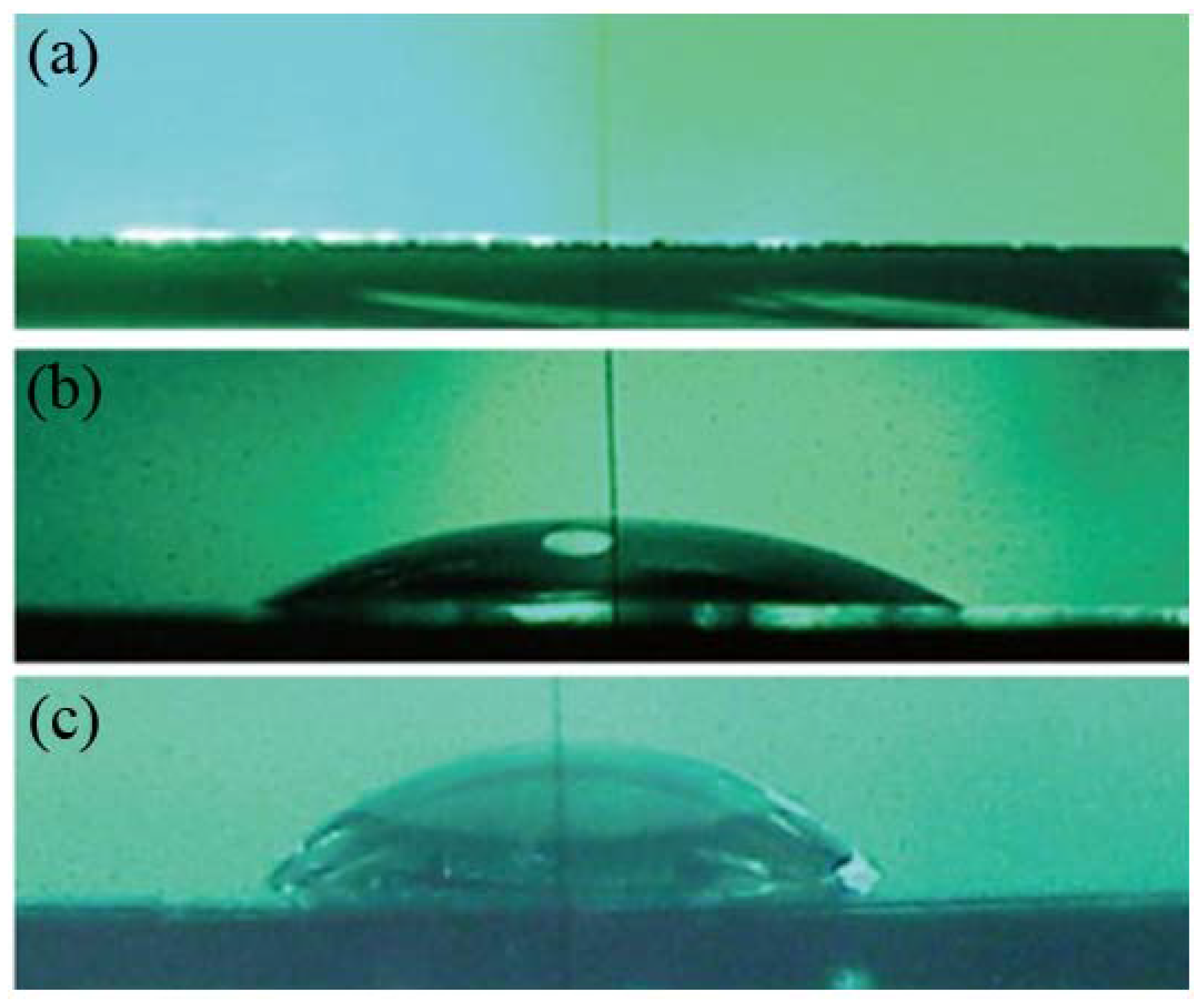
2.2. Effects of Substrates during the SLB Formation by the Vesicle Fusion Method
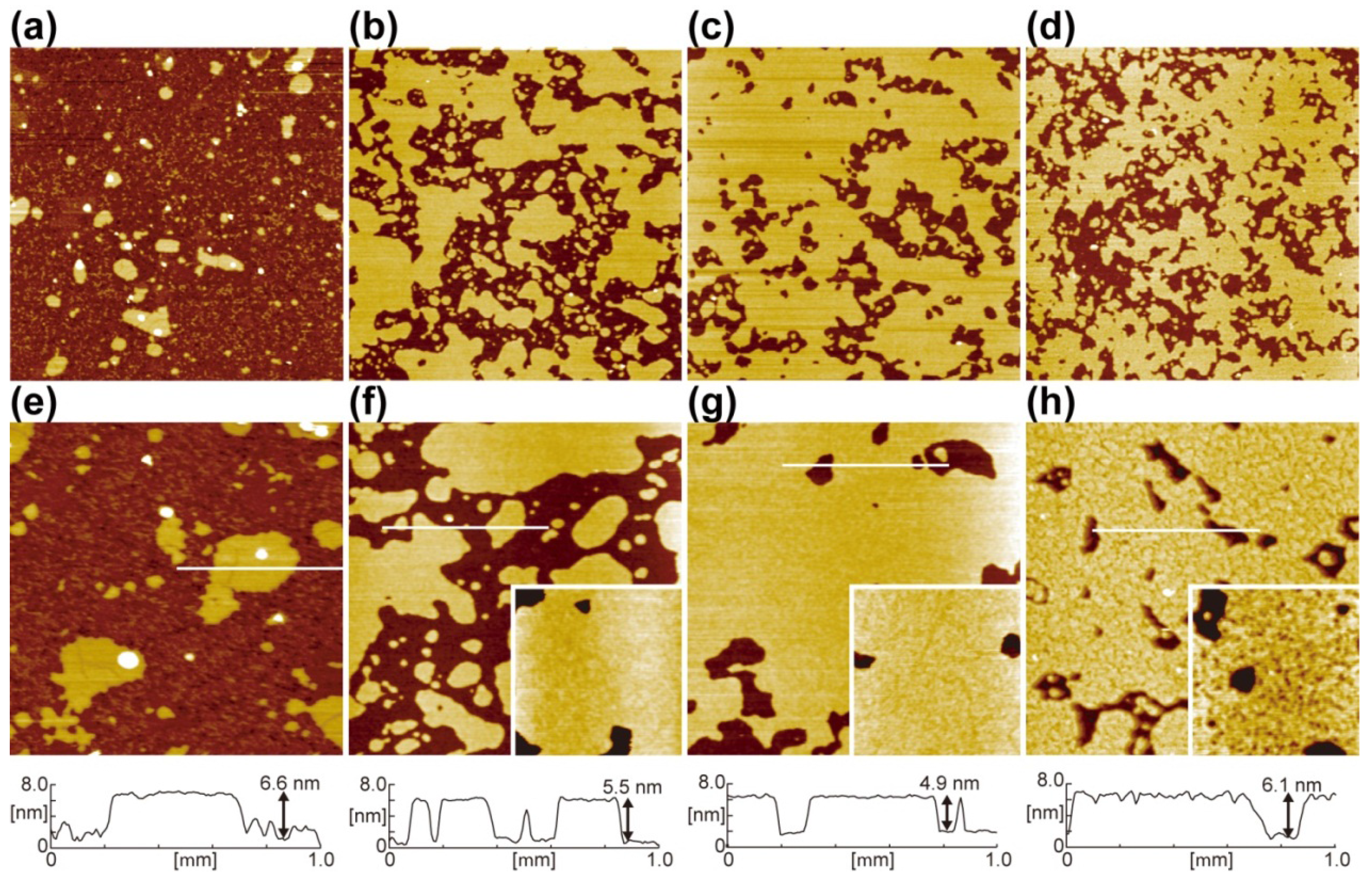
| Annealing condition | Water contact angle | SLB coverage |
|---|---|---|
| Without annealing | <5° | 0.12 |
| 480 °C for 10 min | 11° | 0.63 |
| 700 °C for 10 min | 24° | 0.78 |
| 700 °C for 60 min | 67° | 0.67 |
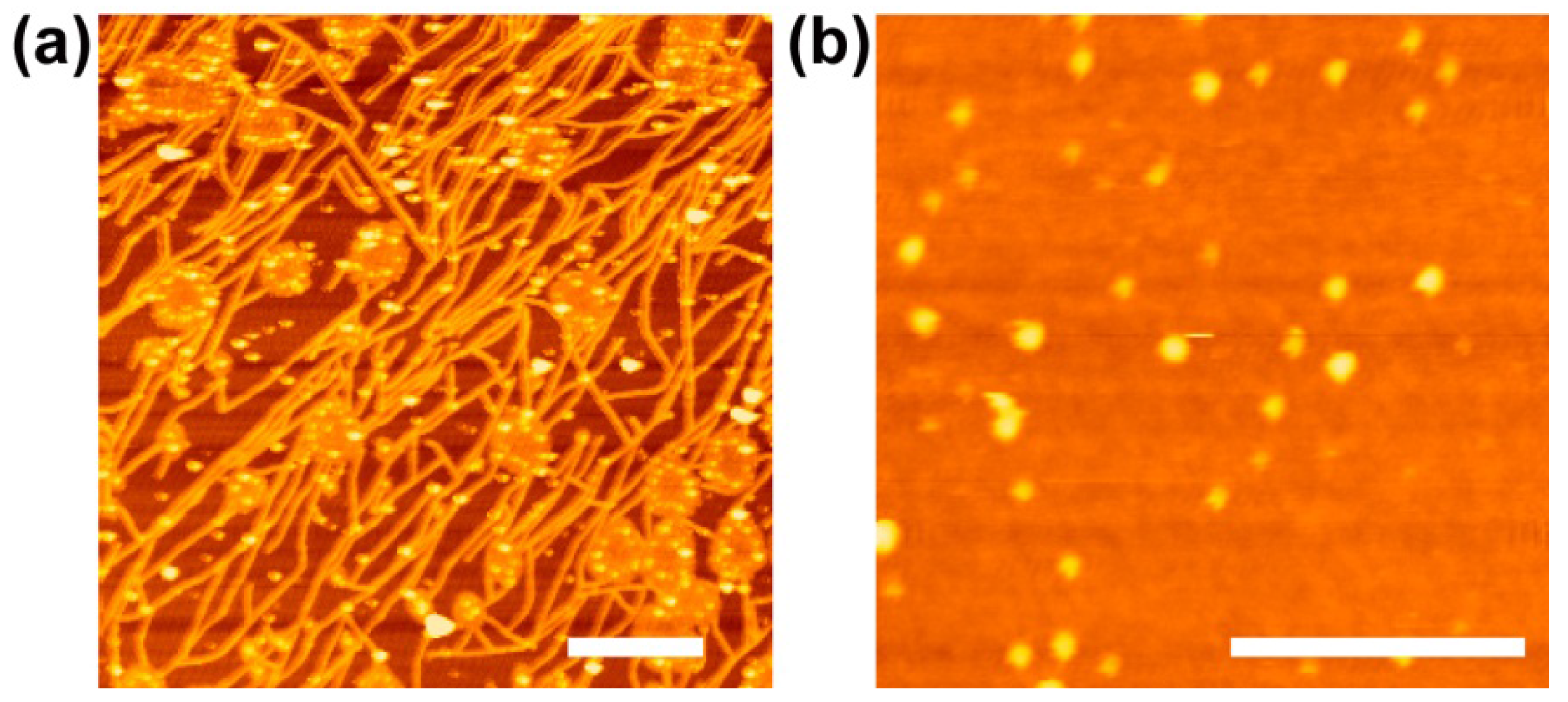
3. Substrate Effects on the Molecular Diffusion in SLB
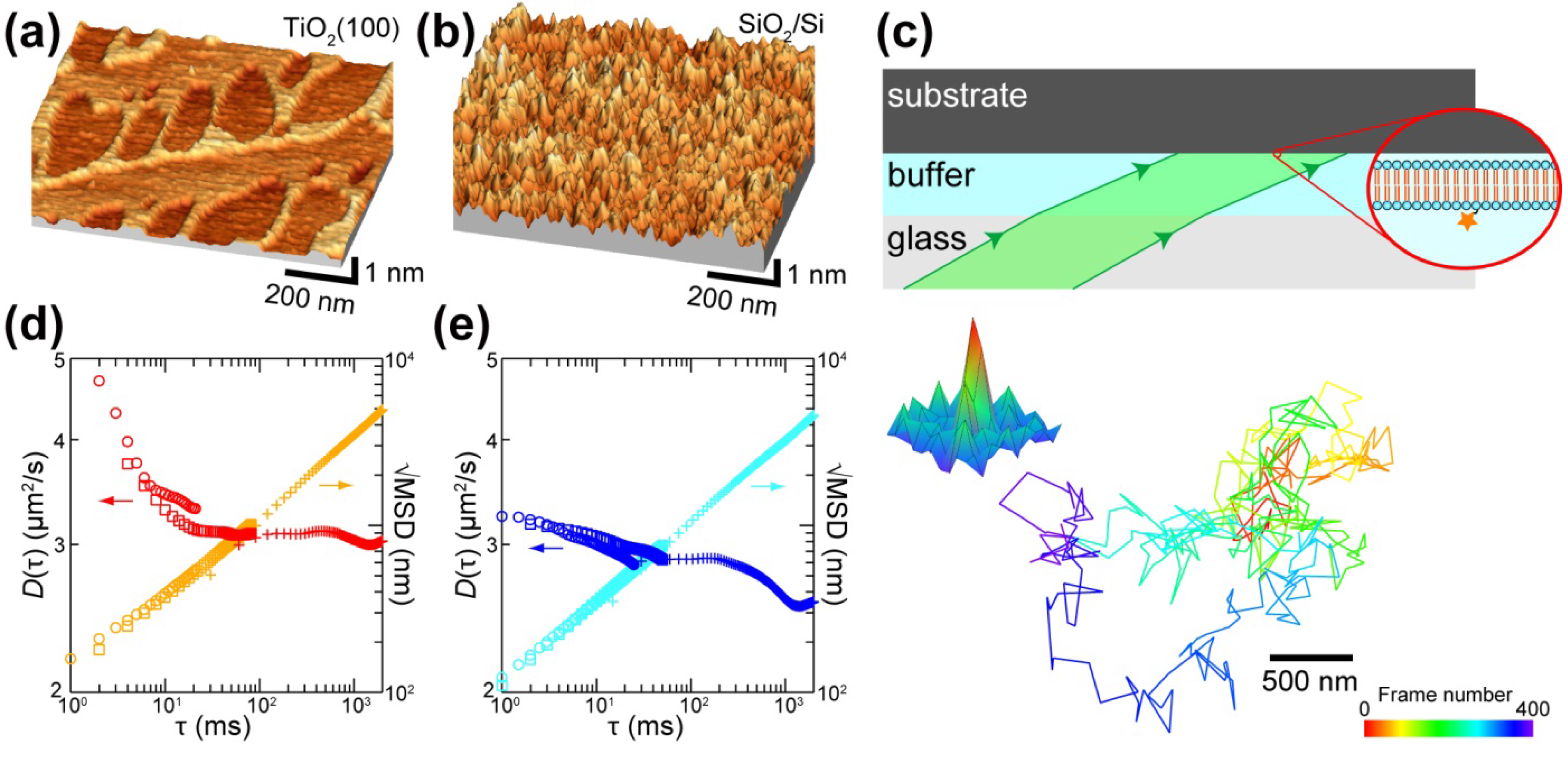
4. Substrate Effects on the Domain Formation and Reactivity of SLB
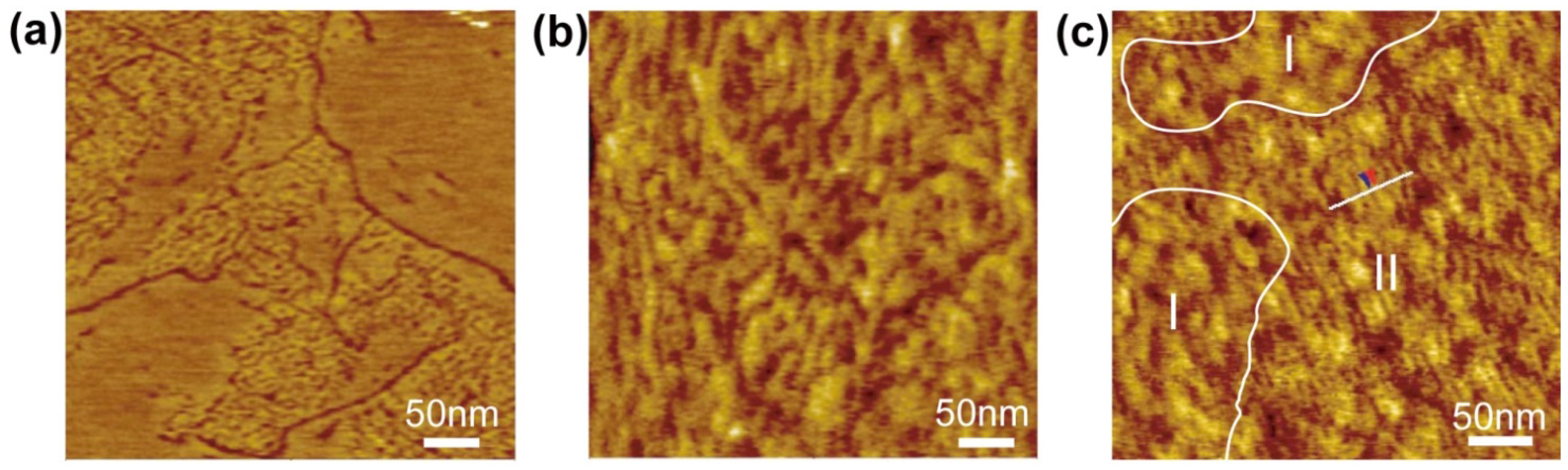
5. Outlook
Acknowledgments
References
- Simons, K.; Gerl, M.J. Revitalizing membrane rafts: New tools and insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.; Mouritsen, O.G.; Anderson, R.G.W. Lipid rafts: At a crossroad between cell biology and physics. Nat. Cell Biol. 2007, 9, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.Ø.; Mouritsen, O.G. Lipids do influence protein function-the hydrophobic matching hypothesis revisited. Biochim. Biophys. Acta 2004, 1666, 205–226. [Google Scholar]
- Kusumi, A.; Nakada, C.; Ritchie, K.; Murase, K.; Suzuki, K.; Murakoshi, H.; Kasai, R.S.; Kondo, J.; Fujiwara, T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: High-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.J.; Bayerl, T.M.; McDermott, D.C.; Adam, G.W.; Rennie, A.R.; Thomas, R.K.; Sackmann, E. Structure of an adsorbed dimyristoylphosphatidylcholine bilayer measured with specular reflection of neutrons. Biophys. J. 1991, 59, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Watkins, E.; Miller, C.; Mulder, D.; Kuhl, T.; Majewski, J. Structure and orientational texture of self-organizing lipid bilayers. Phys. Rev. Lett. 2009, 102, 1–4. [Google Scholar] [CrossRef]
- Ajo-Franklin, C.M.; Yoshina-Ishii, C.; Boxer, S.G. Probing the structure of supported membranes and tethered oligonucleotides by fluorescence interference contrast microscopy. Langmuir 2005, 21, 4976–4983. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.M.; Kiessling, V.; Tamm, L.K. Measuring lipid asymmetry in planar supported bilayers by fluorescence interference contrast microscopy. Langmuir 2005, 21, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, V.; Tamm, L.K. Measuring distances in supported bilayers by fluorescence interference-contrast microscopy: polymer supports and SNARE proteins. Biophys. J. 2003, 84, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, G.; Cremer, P.S. Investigations of water structure at the solid/liquid interface in the presence of supported lipid bilayers by vibrational sum frequency spectroscopy. Langmuir 2001, 17, 7255–7260. [Google Scholar] [CrossRef]
- Bayerl, T.M.; Bloom, M. Physical properties of single phospholipid bilayers adsorbed to micro glass beads. A new vesicular model system studied by 2H-nuclear magnetic resonance. Biophys. J. 1990, 58, 357–362. [Google Scholar]
- Goksu, E.I.; Vanegas, J.M.; Blanchette, C.D.; Lin, W.-C.; Longo, M.L. AFM for structure and dynamics of biomembranes. Biochim. Biophys. Acta 2009, 1788, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Giocondi, M.-C.; Yamamoto, D.; Lesniewska, E.; Milhiet, P.-E.; Ando, T.; Le Grimellec, C. Surface topography of membrane domains. Biochim. Biophys. Acta 2010, 1798, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; He, D. Recent progress in the application of atomic force microscopy for supported lipid bilayers. Chem. Eur. J. 2012, 18, 4148–4155. [Google Scholar] [CrossRef] [PubMed]
- Soumpasis, D.M. Theoretical analysis of fluorescence photobleaching recovery experiments. Biophys. J. 1983, 41, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.; Koppel, D.E.; Schlessinger, J.; Elson, E.; Webb, W.W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 1976, 16, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Machán, R.; Hof, M. Recent developments in fluorescence correlation spectroscopy for diffusion measurements in planar lipid membranes. Int. J. Mol. Sci. 2010, 11, 427–457. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Schütz, G.J.; Baumgartner, W.; Gruber, H.J.; Schindler, H. Imaging of single molecule diffusion. Proc. Natl. Acad. Sci. USA 1996, 93, 2926–2929. [Google Scholar] [CrossRef] [PubMed]
- Bayley, H.; Cremer, P.S. Stochastic sensors inspired by biology. Nature 2001, 413, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Groves, J.T. Micropatterning fluid lipid bilayers on solid supports. Science 1997, 275, 651–653. [Google Scholar] [CrossRef]
- Castellana, E.T.; Cremer, P.S. Solid supported lipid bilayers: From biophysical studies to sensor design. Surf. Sci. Rep. 2006, 61, 429–444. [Google Scholar] [CrossRef]
- Yamazaki, V.; Sirenko, O.; Schafer, R.J.; Nguyen, L.; Gutsmann, T.; Brade, L.; Groves, J.T. Cell membrane array fabrication and assay technology. BMC Biotechnol. 2005, 5. [Google Scholar] [CrossRef] [Green Version]
- Howland, M.C.; Sapuri-Butti, A.R.; Dixit, S.S.; Dattelbaum, A.M.; Shreve, A.P.; Parikh, A.N. Phospholipid morphologies on photochemically patterned silane monolayers. J. Am. Chem. Soc. 2005, 127, 6752–6765. [Google Scholar] [CrossRef] [PubMed]
- Tero, R.; Urisu, T.; Okawara, H.; Nagayama, K. Deposition of lipid bilayers on OH-density-controlled silicon dioxide surfaces. J. Vac. Sci. Technol. A 2005, 23, 751–754. [Google Scholar] [CrossRef]
- Tero, R.; Watanabe, H.; Urisu, T. Supported phospholipid bilayer formation on hydrophilicity-controlled silicon dioxide surfaces. Phys. Chem. Chem. Phys. 2006, 8, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Isono, T.; Ogino, T. Selective deposition of lipid membranes on locally anodic-oxidized silicon surface. e-J. Surf. Sci. Nanotech. 2011, 9, 357–362. [Google Scholar] [CrossRef]
- Okazaki, T.; Inaba, T.; Tatsu, Y.; Tero, R.; Urisu, T.; Morigaki, K. Polymerized lipid bilayers on a solid substrate: Morphologies and obstruction of lateral diffusion. Langmuir 2009, 25, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Nakashima, H.; Kashimura, Y.; Torimitsu, K. Novel “lipid-flow chip” configuration to determine donor-to-acceptor ratio-dependent fluorescence resonance energy transfer efficiency. Langmuir 2008, 24, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, M.R.; Bramble, J.P.; McMillan, D.G.G.; Krzeminski, L.; Han, X.; Johnson, B.R.G.; Bushby, R.J.; Olmsted, P.D.; Jeuken, L.J.C.; Marritt, S.J. Concentrating membrane proteins using asymmetric traps and AC electric fields. J. Am. Chem. Soc. 2011, 133, 6521–6524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabika, H.; Takimoto, B.; Murakoshi, K. Molecular separation in the lipid bilayer medium: Electrophoretic and self-spreading approaches. Anal. Bioanal. Chem. 2008, 391, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Sanii, B.; Smith, A.M.; Butti, R.; Brozell, A.M.; Parikh, A.N. Bending membranes on demand: Fluid phospholipid bilayers on topographically deformable substrates. Nano Lett. 2008, 8, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, J.; Jonas, S.; Frutos, A.; Kalal, P.; Fang, Y. Lipid microarrays. Biomed. Microdevices 2001, 157–164. [Google Scholar] [CrossRef]
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Appleyard, J. The main phase transition of mica-supported phosphatidylcholine membranes. J. Phys. Chem. B 2000, 104, 8097–8100. [Google Scholar] [CrossRef]
- Keller, D.; Larsen, N.; Møller, I.; Mouritsen, O. Decoupled phase transitions and grain-boundary melting in supported phospholipid bilayers. Phys. Rev. Lett. 2005, 94, 1–4. [Google Scholar] [CrossRef]
- Charrier, A.; Thibaudau, F. Main phase transitions in supported lipid single-bilayer. Biophys. J. 2005, 89, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.V.; Spurlin, T.A.; Gewirth, A.A. Direct visualization of asymmetric behavior in supported lipid bilayers at the gel-fluid phase transition. Biophys. J. 2005, 88, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Stanglmaier, S.; Hertrich, S.; Fritz, K.; Moulin, J.-F.; Haese-Seiller, M.; Rädler, J.O.; Nickel, B. Asymmetric distribution of anionic phospholipids in supported lipid bilayers. Langmuir 2012, 28, 10818–10821. [Google Scholar] [CrossRef] [PubMed]
- Shreve, A.P.; Howland, M.C.; Sapuri-Butti, A.R.; Allen, T.W.; Parikh, A.N. Evidence for leaflet-dependent redistribution of charged molecules in fluid supported phospholipid bilayers. Langmuir 2008, 24, 13250–13253. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, F.F.; Textor, M.; Reviakine, I. Asymmetric distribution of phosphatidyl serine in supported phospholipid bilayers on titanium dioxide. Langmuir 2006, 22, 3467–3473. [Google Scholar] [CrossRef] [PubMed]
- Brian, A.A.; McConnell, H.M. Allogeneic stimulation of cytotoxic T cells by supported planar membranes. Proc. Natl. Acad. Sci. USA 1984, 81, 6159–6163. [Google Scholar] [CrossRef] [PubMed]
- McConnell, H.M.; Watts, T.H.; Weis, R.M.; Brian, A.A. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim. Biophys. Acta 1986, 864, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Tamm, L.K.; McConnell, H.M. Supported phospholipid bilayers. Biophys. J. 1985, 47, 105–113. [Google Scholar] [CrossRef]
- Charitat, T.; Bellet-Amalric, E.; Fragneto, G.; Graner, F. Adsorbed and free lipid bilayers at the solid-liquid interface. Eur. Phys. J. B 1999, 8, 583–593. [Google Scholar] [CrossRef]
- Kalb, E.; Frey, S.; Tamm, L.K. Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim. Biophys. Acta 1992, 1103, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Rädler, J.; Strey, H.; Sackmann, E. Phenomenology and kinetics of lipid bilayer spreading on hydrophilic surfaces. Langmuir 1995, 11, 4539–4548. [Google Scholar] [CrossRef]
- Kim, S.H.; Haimovich-Caspi, L.; Omer, L.; Talmon, Y.; Franses, E.I. Effect of sonication and freezing-thawing on the aggregate size and dynamic surface tension of aqueous DPPC dispersions. J. Colloid Interface Sci. 2007, 311, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Lapinski, M.M.; Castro-Forero, A.; Greiner, A.J.; Ofoli, R.Y.; Blanchard, G.J. Comparison of liposomes formed by sonication and extrusion: Rotational and translational diffusion of an embedded chromophore. Langmuir 2007, 23, 11677–11683. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.C.; MacDonald, R.I.; Menco, B.P.M.; Takeshita, K.; Subbarao, N.K.; Hu, L. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta 1991, 1061, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, C.D.; Orme, C.A.; Ratto, T.V.; Longo, M.L. Quantifying growth of symmetric and asymmetric lipid bilayer domains. Langmuir 2008, 24, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Goksu, E.I.; Nellis, B.A.; Lin, W.-C.; Satcher, J.H.; Groves, J.T.; Risbud, S.H.; Longo, M.L. Effect of support corrugation on silica xerogel-supported phase-separated lipid bilayers. Langmuir 2009, 3713–3717. [Google Scholar] [CrossRef]
- Sumitomo, K.; Tamba, Y.; Shinozaki, Y.; Torimitsu, K. Confinement of fluorescent probes in microwells on Si substrates by sealing with lipid bilayers. Appl. Phys. Express 2010, 3. [Google Scholar] [CrossRef]
- Sumitomo, K.; McAllister, A.; Tamba, Y.; Kashimura, Y.; Tanaka, A.; Shinozaki, Y.; Torimitsu, K. Ca2+ ion transport through channels formed by α-hemolysin analyzed using a microwell array on a Si substrate. Biosens. Bioelectron. 2012, 31, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Rahman, M.M.; Zhang, Z.-L.; Misawa, N.; Tero, R.; Urisu, T. Supported lipid bilayer formation by the giant vesicle fusion induced by vesicle–surface electrostatic attractive interaction. Chem. Phys. Lett. 2006, 420, 569–573. [Google Scholar] [CrossRef]
- Rahman, M.M.; Nonogaki, Y.; Tero, R.; Kim, Y.-H.; Uno, H.; Zhang, Z.-L.; Yano, T.; Aoyama, M.; Sasaki, R.; Nagai, H.; et al. Giant vesicle fusion on microelectrodes fabricated by femtosecond laser ablation followed by synchrotron radiation etching. Jpn. J. Appl. Phys. 2005, 44, L1207–L1210. [Google Scholar]
- Egawa, H.; Furusawa, K. Liposome adhesion on mica surface studied by atomic force microscopy. Langmuir 1999, 15, 1660–1666. [Google Scholar] [CrossRef]
- Ratto, T.V.; Longo, M.L. Obstructed diffusion in phase-separated supported lipid bilayers: A combined atomic force microscopy and fluorescence recovery after photobleaching approach. Biophys. J. 2002, 83, 3380–3392. [Google Scholar] [CrossRef] [PubMed]
- Reviakine, I.; Brisson, A. Formation of supported phospholipid bilayers from unilamellar vesicles investigated by atomic force microscopy. Langmuir 2000, 16, 1806–1815. [Google Scholar] [CrossRef]
- Reimhult, E.; Höök, F.; Kasemo, B. Vesicle adsorption on SiO2 and TiO2: Dependence on vesicle size. J. Chem. Phys. 2002, 117, 7401–7404. [Google Scholar] [CrossRef]
- Reimhult, E.; Höök, F.; Kasemo, B. Intact vesicle adsorption and supported biomembrane formation from vesicles in solution: Influence of surface chemistry, vesicle size, temperature, and Osmotic pressure. Langmuir 2003, 19, 1681–1691. [Google Scholar] [CrossRef]
- Johnson, J.M.; Ha, T.; Chu, S.; Boxer, S.G. Early steps of supported bilayer formation probed by single vesicle fluorescence assays. Biophys. J. 2002, 83, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Tero, R.; Takizawa, M.; Li, Y.-J.; Yamazaki, M.; Urisu, T. Lipid membrane formation by vesicle fusion on silicon dioxide surfaces modified with alkyl self-assembled monolayer islands. Langmuir 2004, 20, 7526–7531. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.A.; Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J. 1998, 75, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Striebel, C.; Brecht, A.; Gauglitz, G. Characterization of biomembranes by spectral ellipsometry, surface plasmon resonance and interferometry with regard to biosensor application. Biosens. Bioelectron. 1994, 9, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Puu, G.; Gustafson, I. Planar lipid bilayers on solid supports from liposomes—Factors of importance for kinetics and stability. Biochim. Biophys. Acta 1997, 1327, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Jass, J.; Tjärnhage, T.; Puu, G. From liposomes to supported, planar bilayer structures on hydrophilic and hydrophobic surfaces: An atomic force microscopy study. Biophys. J. 2000, 79, 3153–3163. [Google Scholar] [CrossRef] [PubMed]
- Reimhult, E.; Zäch, M.; Höök, F.; Kasemo, B. A multitechnique study of liposome adsorption on Au and lipid bilayer formation on SiO2. Langmuir 2006, 22, 3313–3319. [Google Scholar] [CrossRef] [PubMed]
- Berquand, A.; Mazeran, P.; Pantigny, J.; Proux-Delrouyre, V.; Laval, J.; Bourdillon, C. Two-step formation of streptavidin-supported lipid bilayers by PEG-triggered vesicle fusion. Fluorescence and atomic force microscopy characterization. Langmuir 2003, 19, 1700–1707. [Google Scholar]
- Cho, N.-J.; Cho, S.-J.; Cheong, K.H.; Glenn, J.S.; Frank, C.W. Employing an amphipathic viral peptide to create a lipid bilayer on Au and TiO2. J. Am. Chem. Soc. 2007, 129, 10050–10051. [Google Scholar] [CrossRef] [PubMed]
- Sumino, A.; Dewa, T.; Takeuchi, T.; Sugiura, R.; Sasaki, N.; Misawa, N.; Tero, R.; Urisu, T.; Gardiner, A.T.; Cogdell, R.J.; et al. Construction and structural analysis of tethered lipid bilayer containing photosynthetic antenna proteins for functional analysis. Biomacromolecules 2011, 12, 2850–2858. [Google Scholar]
- Sugihara, K.; Jang, B.; Schneider, M.; Vörös, J.; Zambelli, T. A universal method for planar lipid bilayer formation by freeze and thaw. Soft Matter 2012, 8, 5525–5531. [Google Scholar] [CrossRef]
- Murray, D.H.; Tamm, L.K.; Kiessling, V. Supported double membranes. J. Struct. Biol. 2009, 168, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Achalkumar, A.S.; Cheetham, M.R.; Connell, S.D.A.; Johnson, B.R.G.; Bushby, R.J.; Evans, S.D. A self-assembly route for double bilayer lipid membrane formation. ChemPhysChem 2010, 11, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Kaasgaard, T.; Leidy, C.; Crowe, J.H.; Mouritsen, O.G.; Jørgensen, K. Temperature-controlled structure and kinetics of ripple phases in one- and two-component supported lipid bilayers. Biophys. J. 2003, 85, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Leidy, C.; Kaasgaard, T.; Crowe, J.H.; Mouritsen, O.G.; Jørgensen, K. Ripples and the formation of anisotropic lipid domains: imaging two-component supported double bilayers by atomic force microscopy. Biophys. J. 2002, 83, 2625–2633. [Google Scholar] [CrossRef] [PubMed]
- Giocondi, M.-C.; Le Grimellec, C. Temperature dependence of the surface topography in dimyristoylphosphatidylcholine/distearoylphosphatidylcholine multibilayers. Biophys. J. 2004, 86, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Kaizuka, Y.; Groves, J.T. Structure and dynamics of supported intermembrane junctions. Biophys. J. 2004, 86, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.P.; Groves, J.T. Topographical imaging of an intermembrane junction by combined fluorescence interference and energy transfer microscopies. J. Am. Chem. Soc. 2001, 123, 12414–12415. [Google Scholar] [CrossRef] [PubMed]
- Nagle, J.F.; Tristram-Nagle, S. Lipid bilayer structure. Curr. Opin. Struct. Biol. 2000, 10, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.; Jacobson, K.; Papahadjopoulos, D. Lateral diffusion in phospholipid multibilayers measured by fluorescence recovery after photobleaching. Biochemistry 1977, 16, 3936–3941. [Google Scholar] [CrossRef] [PubMed]
- Seifert, U. Configurations of fluid membranes and vesicles. Adv. Phys. 1997, 46, 13–137. [Google Scholar] [CrossRef]
- Rossetti, F.F.; Bally, M.; Michel, R.; Textor, M.; Reviakine, I. Interactions between titanium dioxide and phosphatidyl serine-containing liposomes: Formation and patterning of supported phospholipid bilayers on the surface of a medically relevant material. Langmuir 2005, 21, 6443–6450. [Google Scholar] [CrossRef] [PubMed]
- Reviakine, I.; Rossetti, F.F.; Morozov, A.N.; Textor, M. Investigating the properties of supported vesicular layers on titanium dioxide by quartz crystal microbalance with dissipation measurements. J. Chem. Phys. 2005, 122, 204711–204718. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.E.; Thompson, N.L. Formation and characterization of planar phospholipid bilayers supported on TiO2 and SrTiO3 single crystals. Langmuir 2000, 16, 10301–10308. [Google Scholar] [CrossRef]
- Oleson, T.A.; Sahai, N. Oxide-dependent adsorption of a model membrane phospholipid, dipalmitoylphosphatidylcholine: Bulk adsorption isotherms. Langmuir 2008, 24, 4865–4873. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Stevens, M.J.; Oleson, T.A.; Last, J.A.; Sahai, N. Role of oxide surface chemistry and phospholipid phase on adsorption and self-assembly: Isotherms and atomic force microscopy. J. Phys. Chem. C 2009, 113, 2187–2196. [Google Scholar] [CrossRef]
- Jenkins, A.T.A.; Boden, N.; Bushby, R.J.; Evans, S.D.; Knowles, P.F.; Miles, R.E.; Ogier, S.D.; Schönherr, H.; Vancso, G.J. Microcontact printing of lipophilic self-assembled monolayers for the attachment of biomimetic lipid bilayers to surfaces. J. Am. Chem. Soc. 1999, 121, 5274–5280. [Google Scholar] [CrossRef]
- Jenkins, A.T.A.; Bushby, R.J.; Evans, S.D.; Knoll, W.; Offenhäusser, A.; Ogier, S.D. Lipid vesicle fusion on μCP patterned self-assembled monolayers: Effect of pattern geometry on bilayer formation. Langmuir 2002, 18, 3176–3180. [Google Scholar] [CrossRef]
- Isono, T.; Tanaka, H.; Ogino, T. Effect of chemical modification of the substrate surface on supported lipid bilayer formation. e-J. Surf. Sci. Nanotech. 2007, 5, 99–102. [Google Scholar] [CrossRef]
- Cha, T.; Guo, A.; Zhu, X.-Y. Formation of supported phospholipid bilayers on molecular surfaces: Role of surface charge density and electrostatic interaction. Biophys. J. 2006, 90, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Cassier, T.; Sinner, A.; Offenhäuser, A.; Möhwald, H. Homogeneity, electrical resistivity and lateral diffusion of lipid bilayers coupled to polyelectrolyte multilayers. Colloids Surf. B 1999, 15, 215–225. [Google Scholar] [CrossRef]
- Chuang, I.-S.; Maciel, G.E. A detailed model of local structure and silanol hydrogen bonding of silica gel surfaces. J. Phys. Chem. B 1997, 101, 3052–3064. [Google Scholar] [CrossRef]
- Tero, R.; Ujihara, T.; Urisu, T. Lipid bilayer membrane with atomic step structure: Supported bilayer on a step-and-terrace TiO2(100) surface. Langmuir 2008, 24, 11567–11576. [Google Scholar] [CrossRef] [PubMed]
- Tero, R.; Ujihara, T.; Urisu, T. Shape Transformation of adsorbed vesicles on oxide surfaces: Effect of substrate material and photo-irradiation. Trans. Mater. Res. Soc. Jpn. 2009, 34, 183–188. [Google Scholar]
- Nabika, H.; Fukasawa, A.; Murakoshi, K. Control of the structure of self-spreading lipid membrane by changing electrolyte concentration. Langmuir 2006, 22, 10927–10931. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, T.J.; Simon, S.A. Hydration and steric pressures between phospholipid bilayers. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 27–51. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.; Wennerström, H. Role of hydration and water structure in biological and colloidal interactions. Nature 1996, 379, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Madathingal, R.R.; Wunder, S.L.; Chen, Y.; Bothun, G. Hydration repulsion effects on the formation of supported lipid bilayers. Soft Matter 2011, 7, 1936–1947. [Google Scholar] [CrossRef]
- Tero, R.; Ujihara, T.; Urisu, T. Supported lipid bilayer membranes on SiO2 and TiO2: Substrate effects on membrane formation and shape transformation. Proc. SPIE 2007, 6769. [Google Scholar] [CrossRef]
- Von Tscharner, V.; McConnell, H.M. Physical properties of lipid monolayers on alkylated planar glass surfaces. Biophys. J. 1981, 36, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Lingler, S.; Rubinstein, I.; Knoll, W.; Offenhäusser, A. Fusion of small unilamellar lipid vesicles to alkanethiol and thiolipid self-assembled monolayers on gold. Langmuir 1997, 13, 7085–7091. [Google Scholar] [CrossRef]
- Lenz, P.; Ajo-Franklin, C.M.; Boxer, S.G. Patterned supported lipid bilayers and monolayers on poly(dimethylsiloxane). Langmuir 2004, 20, 11092–11099. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Tsuzuki, K.; Iwasa, S.; Ishikawa, R.; Sandhu, A.; Tero, R. Fabrication of supported lipid bilayer on graphene oxide. J. Phys. Conf. Ser. 2012, 352. [Google Scholar] [CrossRef]
- Tsuzuki, K.; Okamoto, Y.; Iwasa, S.; Ishikawa, R.; Sandhu, A.; Tero, R. Reduced graphene oxide as the support for lipid bilayer membrane. J. Phys. Conf. Ser. 2012, 352. [Google Scholar] [CrossRef]
- Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the local chemical structure of graphene oxide and reduced graphene oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar] [CrossRef] [PubMed]
- Cote, L.J.; Kim, J.; Tung, V.C.; Luo, J.; Kim, F.; Huang, J. Graphene oxide as surfactant sheets. Pure Appl. Chem. 2011, 83, 95–110. [Google Scholar]
- Furukawa, K.; Hibino, H. Self-spreading of supported lipid bilayer on SiO2 surface bearing graphene oxide. Chem. Lett. 2012, 41, 1259–1261. [Google Scholar] [CrossRef]
- Przybylo, M.; Sýkora, J.; Humpolíckova, J.; Benda, A.; Zan, A.; Hof, M. Lipid diffusion in giant unilamellar vesicles is more than 2 times faster than in supported phospholipid bilayers under identical conditions. Langmuir 2006, 22, 9096–9099. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Har, J.Y.; Sankaran, J.; Hong, Y.; Kannan, B.; Wohland, T. Molecular diffusion measurement in lipid bilayers over wide concentration ranges: A comparative study. ChemPhysChem 2008, 9, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, B.; Nabika, H.; Murakoshi, K. Single molecular observation of hop diffusion in a lipid bilayer at metallic nanogates. J. Phys. Chem. C 2009, 113, 3127–3132. [Google Scholar] [CrossRef]
- Jung, M.; Vogel, N.; Köper, I. Nanoscale patterning of solid-supported membranes by integrated diffusion barriers. Langmuir 2011, 27, 7008–7015. [Google Scholar] [CrossRef] [PubMed]
- Motegi, T.; Nabika, H.; Murakoshi, K. Enhanced brownian ratchet molecular separation using a self-spreading lipid bilayer. Langmuir 2012, 28, 6656–6661. [Google Scholar] [PubMed]
- Tero, R.; Sazaki, G.; Ujihara, T.; Urisu, T. Anomalous diffusion in supported lipid bilayers induced by oxide surface nanostructures. Langmuir 2011, 27, 9662–9665. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D. Total internal reflection fluorescence microscopy in cell biology. Traffic 2001, 2, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Sazaki, G.; Okada, M.; Matsui, T.; Watanabe, T.; Higuchi, H.; Tsukamoto, K.; Nakajima, K. Single-molecule visualization of diffusion at the solution−crystal interface. Cryst. Growth Des. 2008, 8, 2024–2031. [Google Scholar] [CrossRef]
- Knoll, W.; Yu, F.; Neumann, T.; Schiller, S.; Naumann, R. Supramolecular functional interfacial architectures for biosensor applications. Phys. Chem. Chem. Phys. 2003, 5, 5169–5175. [Google Scholar] [CrossRef]
- Rossi, C.; Chopineau, J. Biomimetic tethered lipid membranes designed for membrane-protein interaction studies. Eur. Biophys. J. 2007, 36, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Dewa, T.; Sugiura, R.; Suemori, Y.; Sugimoto, M.; Takeuchi, T.; Hiro, A.; Iida, K.; Gardiner, A.T.; Cogdell, R.J.; Nango, M. Lateral organization of a membrane protein in a supported binary lipid domain: direct observation of the organization of bacterial light-harvesting complex 2 by total internal reflection fluorescence microscopy. Langmuir 2006, 22, 5412–5418. [Google Scholar] [CrossRef] [PubMed]
- Sumino, A.; Dewa, T.; Kondo, M.; Morii, T.; Hashimoto, H.; Gardiner, A.T.; Cogdell, R.J.; Nango, M. Selective assembly of photosynthetic antenna proteins into a domain-structured lipid bilayer for the construction of artificial photosynthetic antenna systems: Structural analysis of the assembly using surface plasmon resonance and atomic force microscopy. Langmuir 2011, 27, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Tero, R.; Misawa, N.; Watanabe, H.; Yamamura, S.; Nambu, S.; Nonogaki, Y.; Urisu, T. Fabrication of avidin single molecular layer on silicon oxide surfaces and formation of tethered lipid bilayer membranes. e-J. Surf. Sci. Nanotech. 2005, 3, 237–243. [Google Scholar] [CrossRef]
- Misawa, N.; Yamamura, S.; Yonghoon, K.; Tero, R.; Nonogaki, Y.; Urisu, T. Orientation of avidin molecules immobilized on COOH-modified SiO2/Si(100) surfaces. Chem. Phys. Lett. 2006, 419, 86–90. [Google Scholar] [CrossRef]
- Sumino, A.; Dewa, T.; Sasaki, N.; Watanabe, N.; Kondo, M.; Morii, T.; Hashimoto, H.; Nango, M. Reconstitution and AFM observation of photosynthetic membrane protein assembly in planar lipid bilayers. e-J. Surf. Sci. Nanotech. 2011, 9, 15–20. [Google Scholar] [CrossRef]
- Binder, W.H.; Barragan, V.; Menger, F.M. Domains and rafts in lipid membranes. Angew. Chem. Int. Ed. 2003, 42, 5802–5827. [Google Scholar] [CrossRef]
- Almeida, P.F.F. Thermodynamics of lipid interactions in complex bilayers. Biochim. Biophys. Acta 2009, 1788, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Feigenson, G.W. Phase diagrams and lipid domains in multicomponent lipid bilayer mixtures. Biochim. Biophys. Acta 2009, 1788, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.-Y.; Jeong, C.; Lee, S.-W.; Kim, J.H.; Choi, M.C.; Kim, S.-J.; Kim, M.W.; Lee, S.-D. Topographic control of lipid-raft reconstitution in model membranes. Nat. Mater. 2006, 5, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Yu, C.; Groves, J.T. Curvature-modulated phase separation in lipid bilayer membranes. Langmuir 2006, 22, 5095–5099. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Tatsu, Y.; Morigaki, K. Phase separation of lipid microdomains controlled by polymerized lipid bilayer matrices. Langmuir 2010, 26, 4126–4129. [Google Scholar] [CrossRef] [PubMed]
- Sapuri-Butti, A.R.; Li, Q.; Groves, J.T.; Parikh, A.N. Nonequilibrium patterns of cholesterol-rich chemical heterogenieties within single fluid supported phospholipid bilayer membranes. Langmuir 2006, 22, 5374–5384. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Tero, R.; Misawa, N.; Yamamura, S.; Wan, L.; Urisu, T. AFM characterization of gramicidin-A in tethered lipid membrane on silicon surface. Chem. Phys. Lett. 2006, 429, 244–249. [Google Scholar] [CrossRef]
- Kelkar, D.A.; Chattopadhyay, A. The gramicidin ion channel: A model membrane protein. Biochim. Biophys. Acta 2007, 1768, 2011–2025. [Google Scholar] [CrossRef] [PubMed]
- Woolley, A.G.; Wallace, B.A. Model ion channels: Gramicidin and alamethicin. J. Membr. Biol. 1992, 129, 109–136. [Google Scholar] [PubMed]
- Antoinette Killian, J. Gramicidin and gramicidin-lipid interactions. Biochim. Biophys. Acta 1992, 1113, 391–425. [Google Scholar] [CrossRef] [PubMed]
- Mou, J.; Czajkowsky, D.M.; Shao, Z. Gramicidin a aggregation in supported gel state phosphatidylcholine bilayers. Biochemistry 1996, 35, 3222–3226. [Google Scholar] [CrossRef] [PubMed]
- Diociaiuti, M.; Bordi, F.; Motta, A.; Carosi, A.; Molinari, A.; Arancia, G.; Coluzza, C. Aggregation of gramicidin A in phospholipid Langmuir-Blodgett monolayers. Biophys. J. 2002, 82, 3198–3206. [Google Scholar] [CrossRef] [PubMed]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta 2000, 1469, 159–195. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Kimura, N.; Yamaguchi, H.; Hasegawa, K.; Yokoseki, T.; Shibata, M.; Yamamoto, N.; Michikawa, M.; Yoshikawa, Y.; Terao, K.; et al. A seed for Alzheimer amyloid in the brain. J. Neurosci. 2004, 24, 4894–4902. [Google Scholar]
- Yanagisawa, K. Role of gangliosides in Alzheimer’s disease. Biochim. Biophys. Acta 2007, 1768, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Shang, Z.; Imai, Y.; Hoshino, T.; Tero, R.; Tanaka, M.; Yamamoto, N.; Yanagisawa, K.; Urisu, T. Surface-induced phase separation of a sphingomyelin/cholesterol/ganglioside GM1-planar bilayer on mica surfaces and microdomain molecular conformation that accelerates Abeta oligomerization. Biochim. Biophys. Acta 2010, 1798, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Tero, R.; Imai, Y.; Hoshino, T.; Urisu, T. The morphology of GM1x/SM0.6−x/Chol0.4 planar bilayers supported on SiO2 surfaces. Chem. Phys. Lett. 2008, 460, 289–294. [Google Scholar]
- Forstner, M.B.; Yee, C.K.; Parikh, A.N.; Groves, J.T. Lipid lateral mobility and membrane phase structure modulation by protein binding. J. Am. Chem. Soc. 2006, 128, 15221–15227. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yang, T.; Kataoka, S.; Zhang, Y.; Diaz, A.J.; Cremer, P.S. GM1 clustering inhibits cholera toxin binding in supported phospholipid membranes. J. Am. Chem. Soc. 2007, 129, 5954–5961. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Mao, Y.; Tero, R.; Liu, X.; Hoshino, T.; Tanaka, M.; Urisu, T. Clustering effects of GM1 and formation mechanisms of interdigitated liquid disordered domains in GM1/SM/CHOL-supported planar bilayers on mica surfaces. Chem. Phys. Lett. 2010, 497, 108–114. [Google Scholar] [CrossRef]
- Fukuma, T. Water distribution at solid/liquid interfaces visualized by frequency modulation atomic force microscopy. Sci. Technol. Adv. Mater. 2010, 11. [Google Scholar] [CrossRef]
- Fukuma, T.; Higgins, M.J.; Jarvis, S.P. Direct imaging of individual intrinsic hydration layers on lipid bilayers at angstrom resolution. Biophys. J. 2007, 92, 3603–3609. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.M.; Magenau, A.; Williamson, D.; Gaus, K. The lipid raft hypothesis revisited—New insights on raft composition and function from super-resolution fluorescence microscopy. Bioessays 2012, 739–747. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tero, R. Substrate Effects on the Formation Process, Structure and Physicochemical Properties of Supported Lipid Bilayers. Materials 2012, 5, 2658-2680. https://doi.org/10.3390/ma5122658
Tero R. Substrate Effects on the Formation Process, Structure and Physicochemical Properties of Supported Lipid Bilayers. Materials. 2012; 5(12):2658-2680. https://doi.org/10.3390/ma5122658
Chicago/Turabian StyleTero, Ryugo. 2012. "Substrate Effects on the Formation Process, Structure and Physicochemical Properties of Supported Lipid Bilayers" Materials 5, no. 12: 2658-2680. https://doi.org/10.3390/ma5122658




