An Ultrasensitive Electrochemical Immunosensor for Alpha-Fetoprotein Using an Envision Complex-Antibody Copolymer as a Sensitive Label
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Characterization of the Envision Nanocomposite
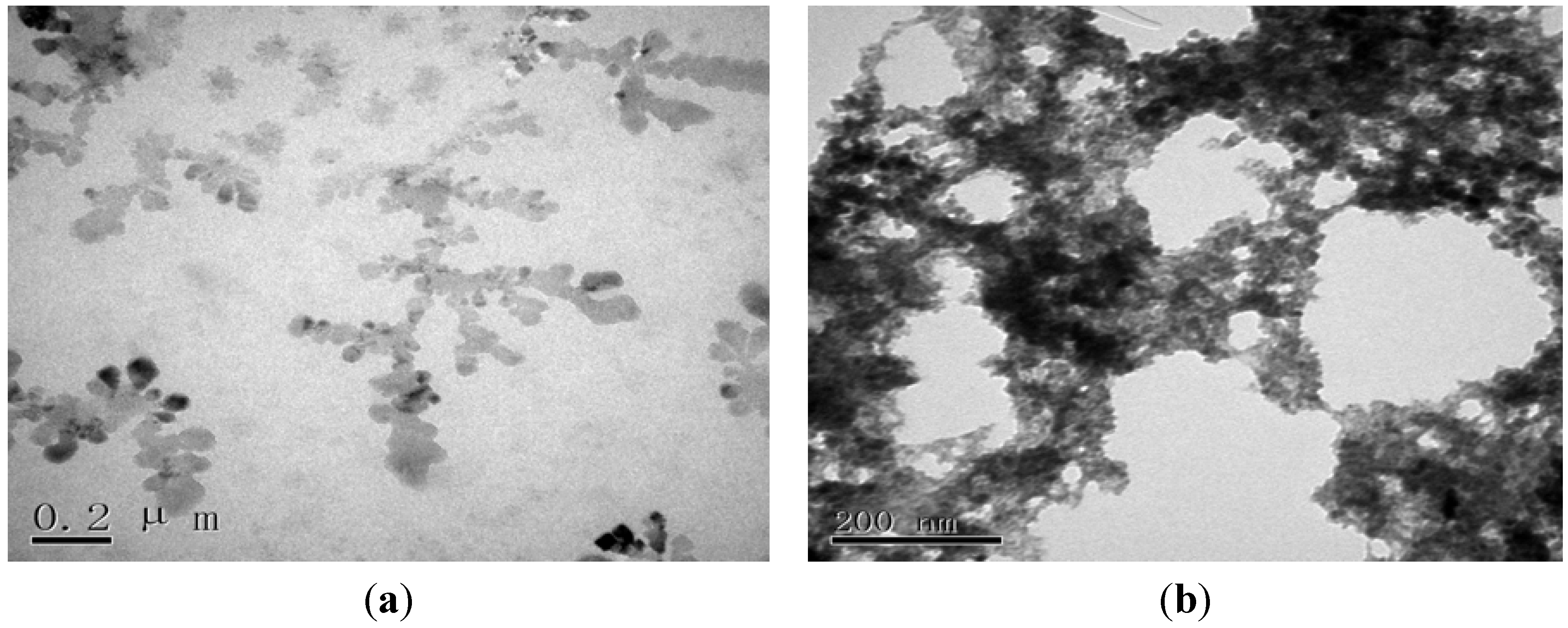
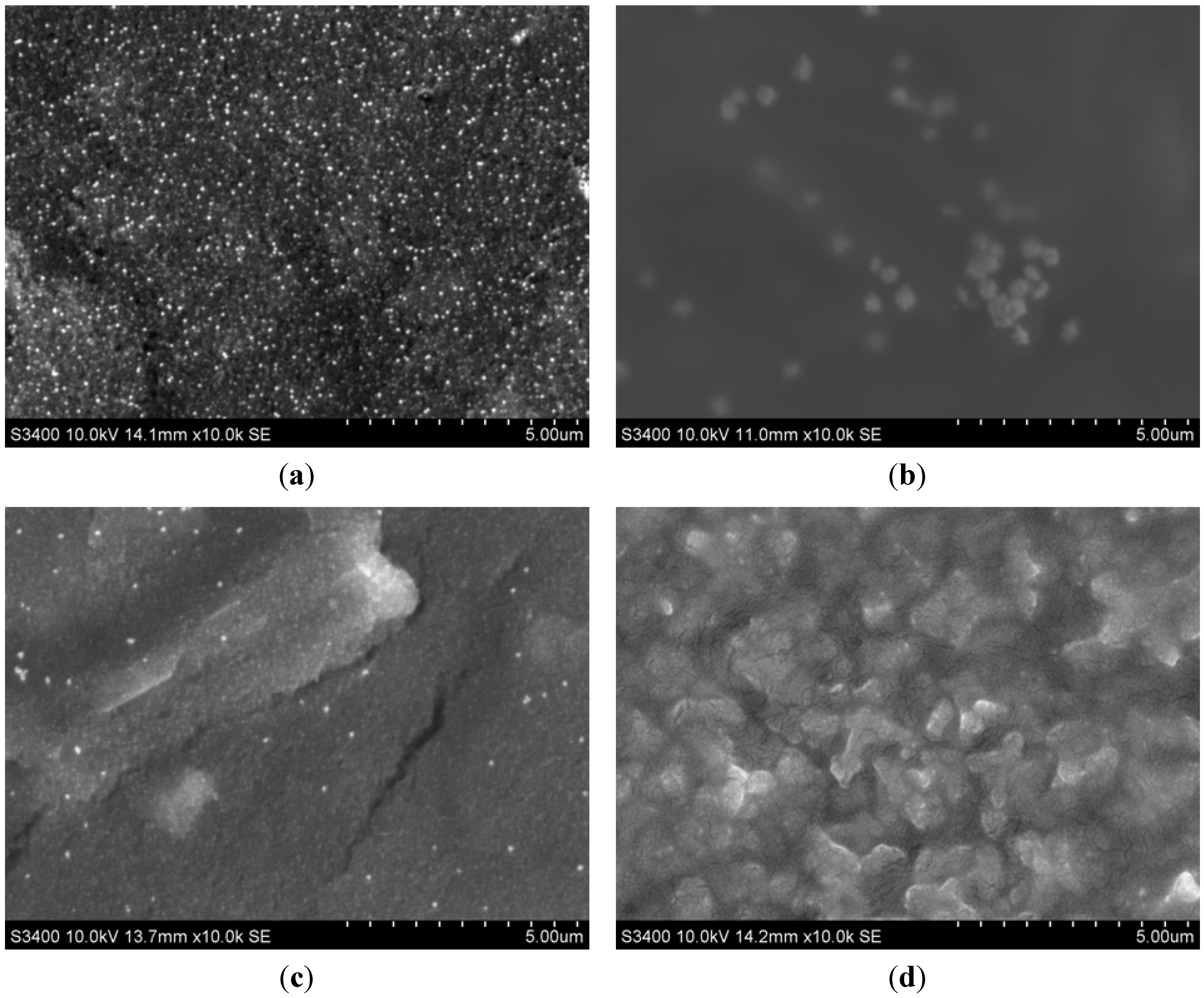
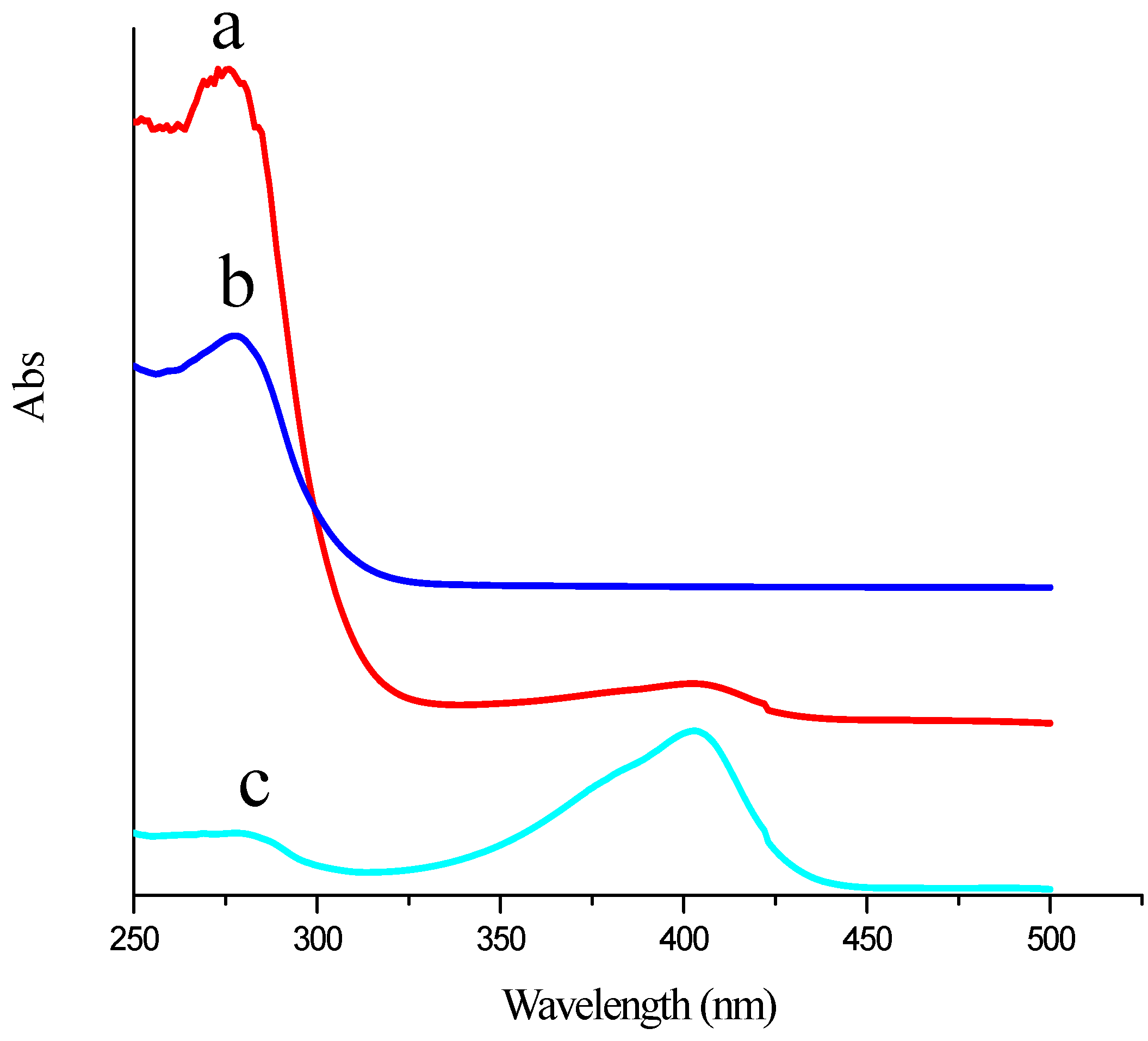
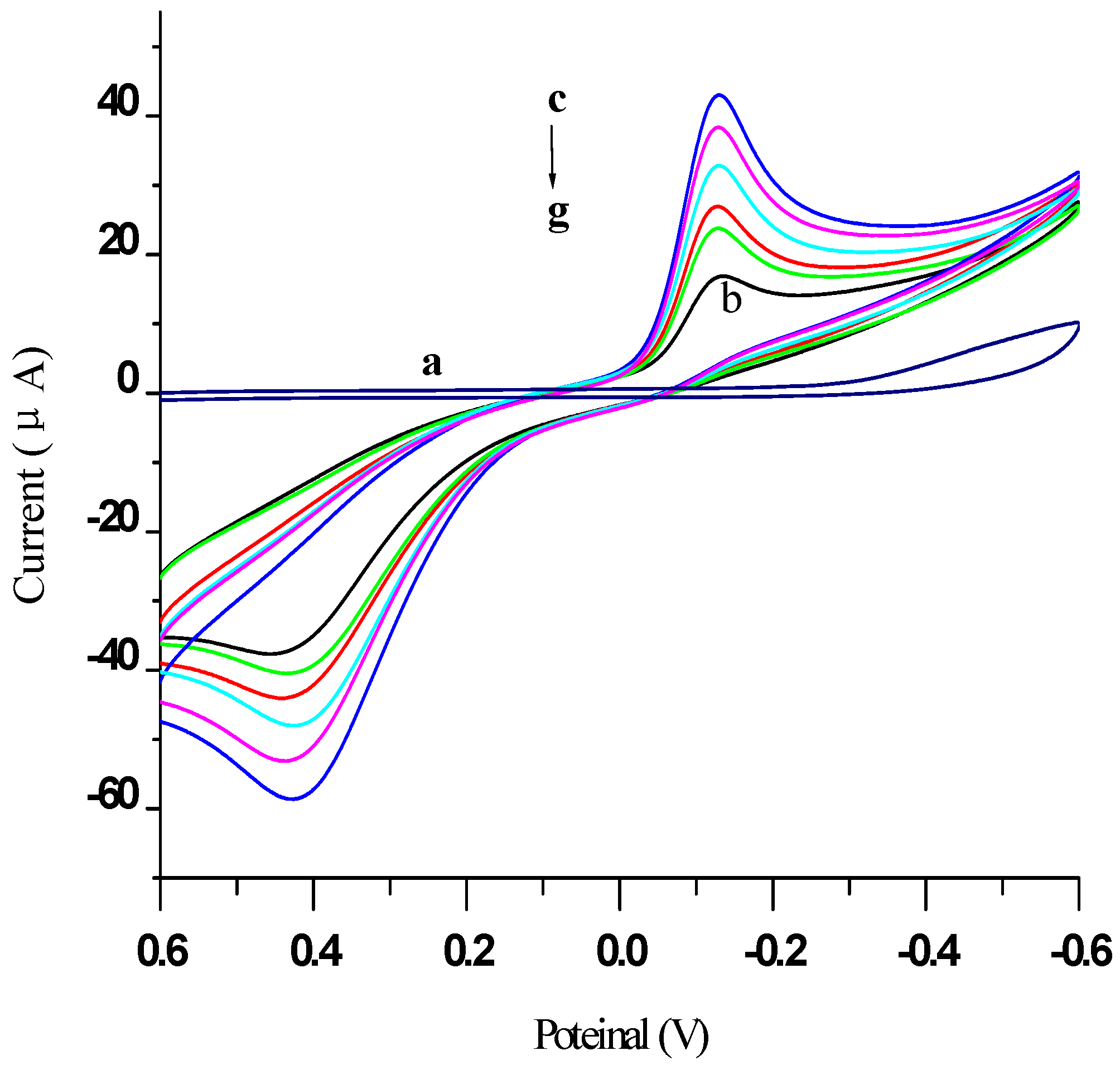
2.2. Electrochemical Characterization of the Immunosensor
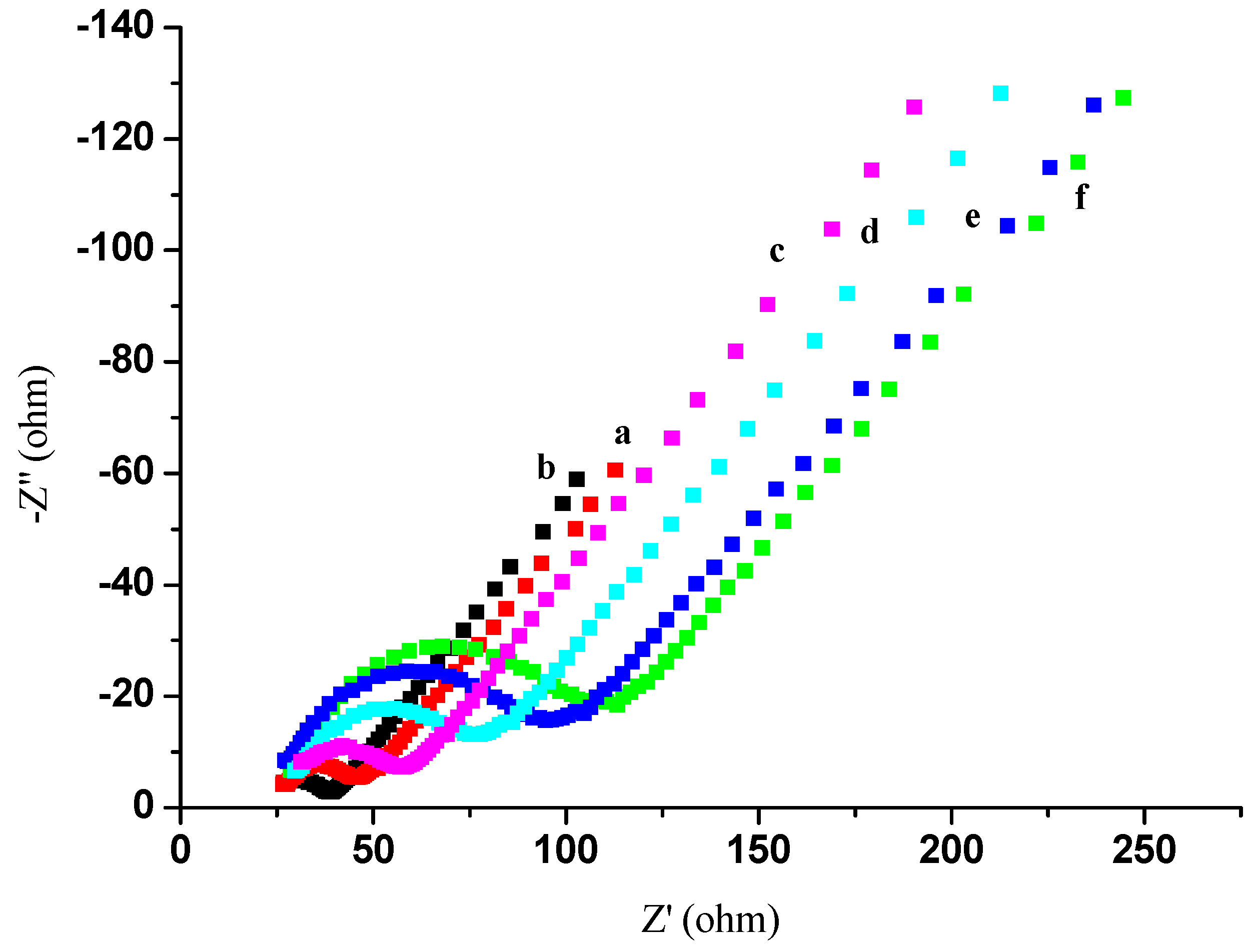
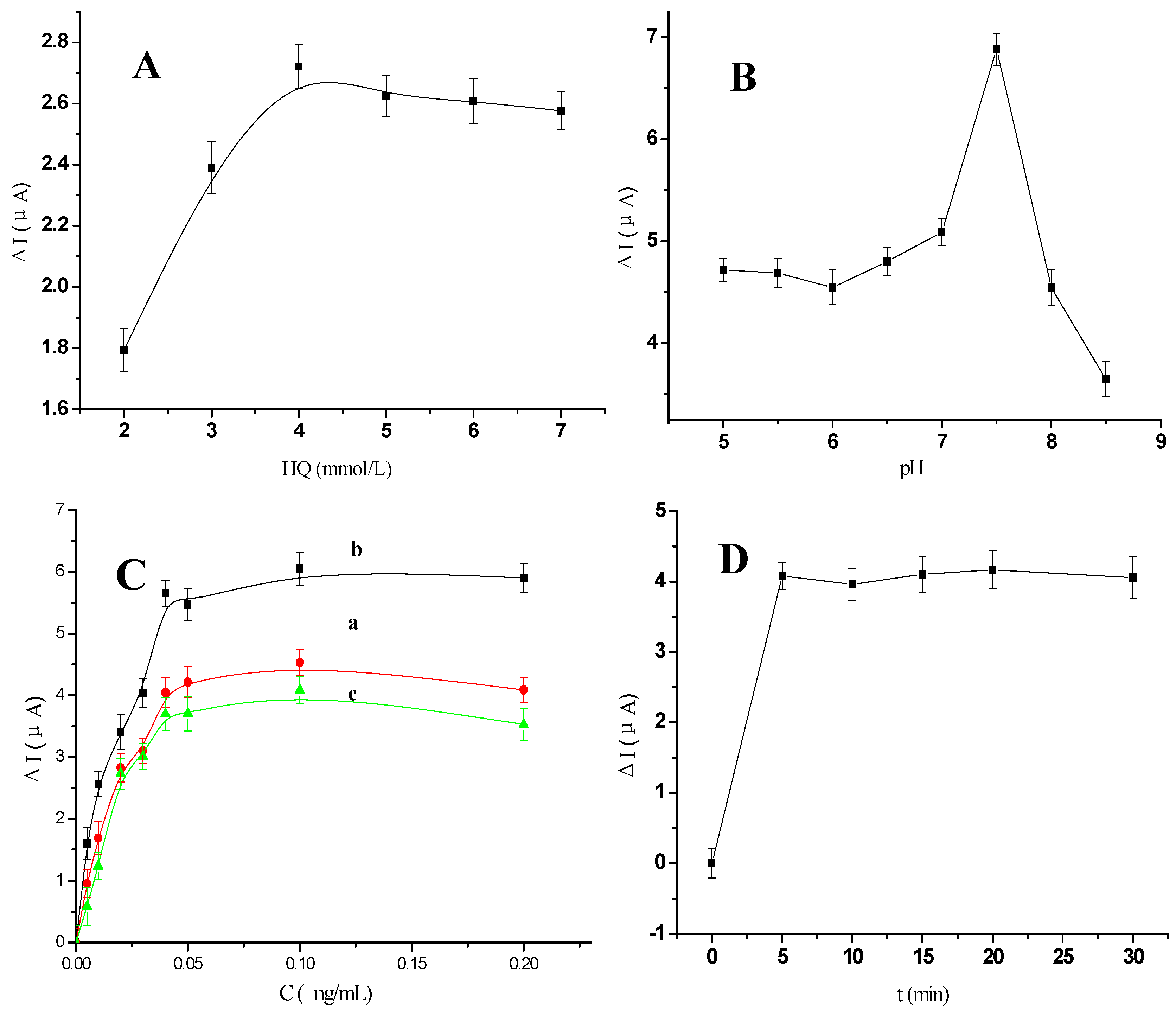
2.3. Optimization of Experimental Conditions
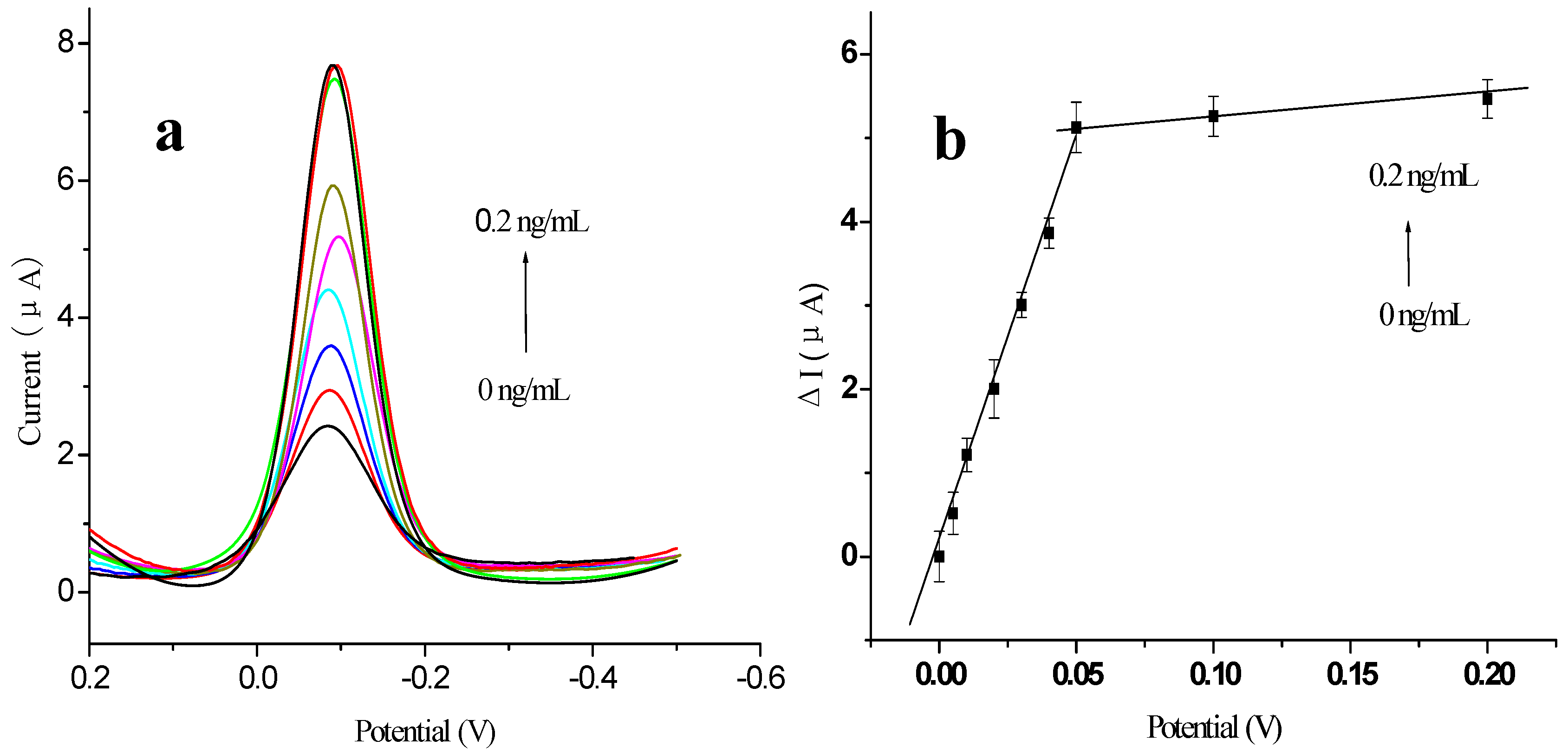
2.4. Characterization of Signal Amplification by EVC-AFP Ab2 Using Different Labels

2.5. Calibration Curve of the Immunosensor
| Assay method | Linear range (ng·mL−1) | LOD (ng·mL−1) | Signal antibody | Reference |
|---|---|---|---|---|
| ELISA | 0.7–15 | 0.2 | HRP labeled AFP Ab2 | The used Kit’s instructions |
| Electrochemical immunoassay | 0.1–30.0 | 0.018 | label-free | [35] |
| 0.02–3.5 | 0.096 | DNA modified gold nanoparticles | [36] | |
| Chemiluminescence | 0.01–100 | 0.005 | SiO2/HRP–antibody | [37] |
| Electrochemiluminescence immunoassay | 0.05–50 | 0.03 | Ru-silica@Au–antibody | [7] |
| Electrochemical ELISA | 0.005–0.2 | 0.002 | EVC-AFP Ab2 | This work |
2.6. Regeneration of the Immunosensor
| Serum samples | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| ELISA (ng/mL) | ND | 10 | 20 | 40 | ND | ND |
| Immunosensor (ng/mL) | 2.1 | 10.5 | 21 | 42 | 0.2 | 0.1 |
| Relative deviation (%) | 2.3 | 4.5 | 2.4 | 3.1 | 3.2 | 3.2 |
3. Experimental Section
3.1. Apparatus and Regents
3.2. Preparation of Envision-AFP Antibody Copolymer (EVC-AFP Ab2)
3.3. Preparation of Thiolated Protein G Modified Au Electrode
3.4. Fabrication of the Immunosensor
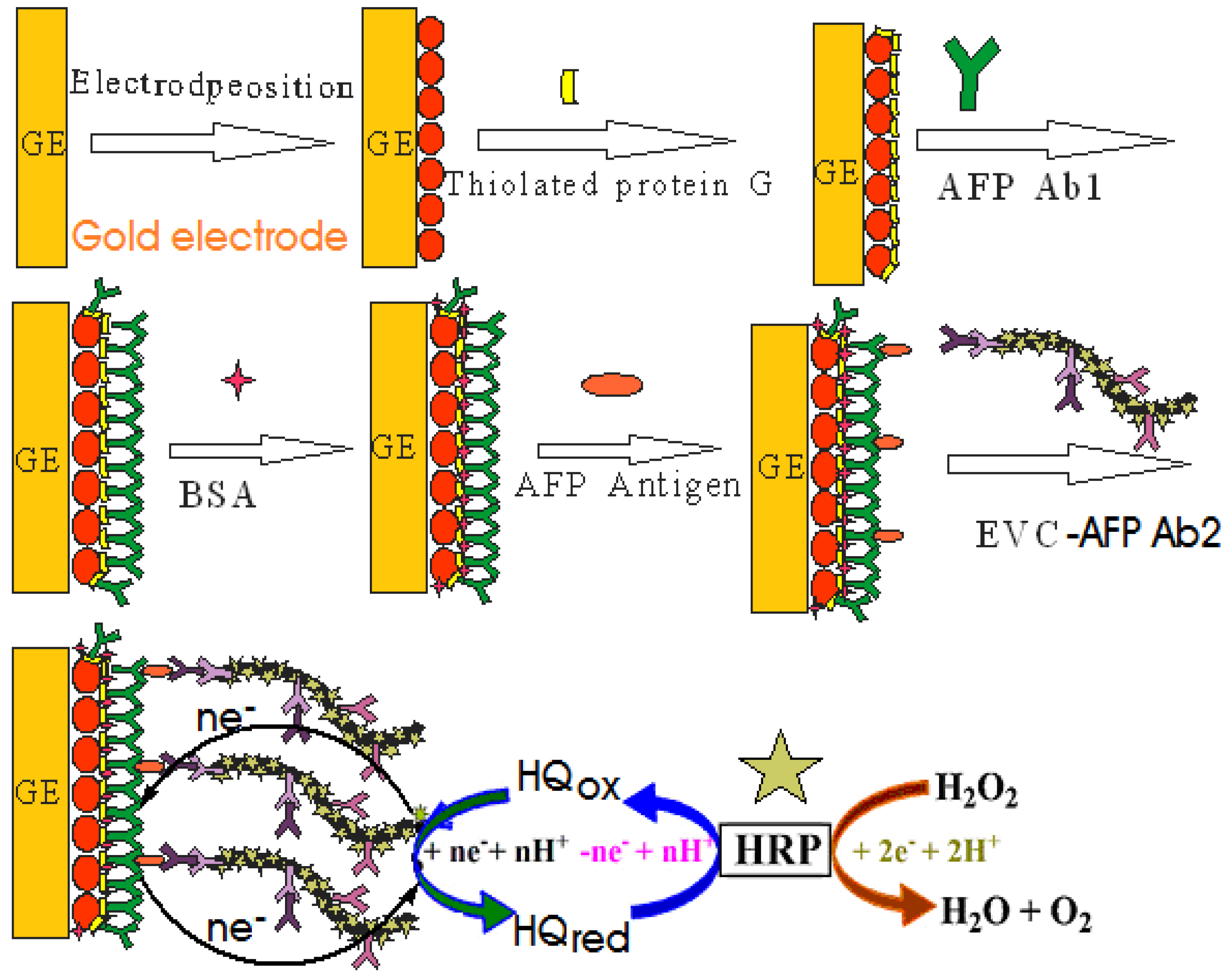
3.5. Electrochemical Measurements of AFP
4. Conclusions
Acknowledgements
References
- Gitlin, D.; Perricelli, A.; Gitlin, G.M. Synthesis of α-fetoprotein by liver, yolk sac, and gastrointestinal tract of the human conceptus. Cancer Res. 1972, 32, 979–982. [Google Scholar]
- Won, Y.S.; Lee, S.W. Targeted retardation of hepatocarcinoma cells by specific replacement of alpha-fetoprotein RNA. J. Biotechnol. 2007, 129, 614–619. [Google Scholar] [CrossRef]
- Badera, D.; Riskina, A.; Vafsia, O.; Tamir, A.; Peskin, B.; Israel, N.; Merksamer, R.; Dar, H.; David, M. Alpha-fetoprotein in the early neonatal period—A large study and review of the literature. Clin. Chim. Acta 2004, 349, 15–23. [Google Scholar] [CrossRef]
- Tatsuta, M.; Yamamura, H.; Lishi, H.; Kasugai, H.; Okuda, S. Value of serum alpha-fetoprotein and ferritin in the diagnosis of hepatocellular carcinoma. Oncology 1986, 43, 306–310. [Google Scholar] [CrossRef]
- Jia, C.P.; Zhong, X.Q.; Hua, B.; Liu, M.Y.; Jing, F.X.; Lou, X.H.; Yao, S.H.; Xiang, J.Q.; Jin, Q.H.; Zhao, J.L. Nano-ELISA for highly sensitive protein detection. Biosens. Bioelectron. 2009, 24, 2836–2841. [Google Scholar] [CrossRef]
- Wang, H.; Sun, D.; Tan, Z.; Gong, W.; Wang, L. Electrochemiluminescence immunosensor for α-Fetoprotein using Ru(bpy)32+-encapsulated liposome as labels. Colloids Surf. B Interfaces 2011, 84, 515–519. [Google Scholar] [CrossRef]
- Yuan, S.; Yuan, R.; Chai, Y.Q.; Mao, L.; Yang, X.; Yuan, Y.; Niu, H. Sandwich-type electrochemiluminescence immunosensor based on Ru-silica@Au composite nanoparticles labeled anti-AFP. Talanta 2010, 82, 1468–1471. [Google Scholar] [CrossRef]
- Fu, Z.F.; Hao, C.; Fei, X.; Ju, H. Flow-injection chemiluminescent immunoassay for α-fetoprotein based on epoxysilane modified glass microbeads. J. Immunol Methods 2006, 312, 61–67. [Google Scholar] [CrossRef]
- Bi, S.; Yan, Y.; Yang, X.; Zhang, S. Gold nanolabels for new enhanced chemiluminescence immunoassay of alpha-fetoprotein based on magnetic beads. Chem. Eur. J. 2009, 15, 4704–4709. [Google Scholar] [CrossRef]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Teramura, Y.J.; Iwata, H. Label-free immunosensing for α-fetoprotein in human plasma using surface plasmon resonance. Anal. Biochem. 2007, 365, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, M.; Wen, Q.; et al. Rapid and simultaneous quantification of 4 urinary proteins by piezoelectric quartz crystal microbalance immunosensor array. Clin. Chem. 2006, 52, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.F.; Hsu, W.L.; Hwang, J.M.; Chen, C.Y. Determination of alpha-fetoprotein in human serum by a quartz crystal microbalance-based immunosensor. Clin. Chem. 2002, 48, 913–918. [Google Scholar] [PubMed]
- Zhang, Y.X.; Zhang, Z.J.; Yang, F. A sensitive immunoassay for determination of hepatitis B surface antigen and antibody in human serum using capillary electrophoresis with chemiluminescence detection. J. Chromatogr. B 2007, 857, 100–107. [Google Scholar] [CrossRef]
- Wang, J.; Ibanez, A.; Chatrathi, M.P.; Escarpa, A. Electrochemical enzyme immunoassays on microchip platforms. Anal. Chem. 2001, 73, 5323–5327. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, M.; Yamamura, H.; Lishi, H.; Kasugai, H.; Okuda, S. Value of serum alpha-fetoprotein and ferritin in the diagnosis of hepatocellular carcinoma. Oncology 1986, 43, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Chen, C.L.; Liu, S.Q. Enzyme-functionalized silica nanoparticles as sensitive labels in biosensing. Anal. Chem. 2009, 81, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Zhang, S.; Zhang, M.; Zhang, L. Progress of electrochemical immunoassay. Chin. J. Anal. Chem. 1995, 23, 1211–1217. [Google Scholar]
- Zheng, T.T.; Zhang, R.; Zou, L.; Zhu, J.J. A label-free cytosensor for the enhanced electrochemical detection of cancer cells using polydopamine-coated carbon nanotubes. Analyst 2012, 137, 1316–1318. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Zou, Z.; Shin, Y.S; Wang, J.; Wu, H.; Engelhard, M.H.; Liu, J.; Aksay, I.A.; Lin, Y.H. Sensitive immunosensor for cancer biomarker based on dual signal amplification strategy of graphene sheets and multienzyme functionalized carbon nanospheres. Anal. Chem. 2010, 82, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Park, H.K.; Jung, Y.; Kim, J.K.; Jung, S.O.; Chung, B.H. Direct immobilization of protein G variants with various numbers of cysteine residues on a gold surface. Anal. Chem. 2007, 79, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.P.; Ren, J.J. In Situ amplified electrochemical immunoassay for carcinoembryonic antigen using horseradish peroxidase-encapsulated nanogold hollow microspheres as labels. Anal. Chem. 2008, 80, 8064–8070. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.M.; Stuart, M.C.; Wong, D.K. Self-assembled layer of thiolated protein G as an immunosensor scaffold. Anal. Chem. 2007, 79, 350–354. [Google Scholar] [CrossRef] [PubMed]
- De la Escosura-Muñiz, A.; Sánchez-Espinel, C.; Díaz-Freitas, B.; González-Fernández, A.; Maltez-da Costa, M.; Merkoçj, A. Rapid identification and quantification of tumor cells using an electrocatalytic method based on gold nanoparticles. Anal. Chem. 2009, 81, 10268–10274. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chiu, N.F.; Lin, D.S.; Chu-Su, Y.; Liang, Y.H.; Lin, C.W. High-sensitivity detection of carbohydrate antigen 15-3 using a gold/zinc oxide thin film surface plasmon resonance-based biosensor. Anal. Chem. 2010, 82, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, S.; Jiang, L.P.; Hou, W.; Shen, Q.; Zhu, J.J. Graphene-CdS nanocomposites: Facile one-step synthesis and enhanced photoelectrochemical cytosensing. Chem. Eur. J. 2012, 18, 4974–4981. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, J.; Song, G.; Zhu, J.J. Fabrication of silica/PDMS hybrid nanoparticles by a novel solvent adjustment route. J. Nanosci. Nanotechnol. 2012, 12, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.P.; Yuan, R.; Chai, Y.Q. Magnetic core-shell Fe3O4@Ag nanoparticles coated carbon paste interface for studies of carcinoembryonic antigen in clinical immunoassay. J. Phys. Chem. B. 2006, 110, 11640–11646. [Google Scholar] [CrossRef] [PubMed]
- Kämmerer, U.; Kapp, M.; Gassel, A.M.; Richter, T.; Tank, C.; Dietl, J.; Ruck, P. A new rapid immunohistochemical staining technique using the Envision antibody complex. J. Histochem. Cytochem. 2001, 49, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Zhang, J.; Li, Y.; Jiang, L.; Zhu, J.-J. Fabrication of a novel impedance cell sensor based on the polystyrene/polyaniline/Au nanocomposite. Talanta 2009, 80, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Duckworth, P.A.; Wong, K.Y. Square wave voltammetry versus electrochemical impedance spectroscopy as a rapid detection technique at electrochemical immunosensors. Biosens. Bioelectron. 2010, 25, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.-D.; Wang, R.; Liang, R.P.; Xia, X.H. Electrochemically deposited nanocomposite film of CS-Fc/Au NPs/GOx for glucose biosensor application. Biosens. Bioelectron. 2009, 24, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.D.; Liang, R.P.; Wang, R.; Fan, L.X.; Chen, Y.W.; Xia, X.H. A label-free amperometric immunosensor based on biocompatible conductive redox chitosan-ferrocene/gold nanoparticles matrix. Biosens. Bioelectron. 2009, 25, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Y.; Ji, P.Y.; Jiang, L.P.; Zhu, J.J. Synthesis of Ni@NiO core-shell nanospheres and application for fabrication of electrochemical biosensor. Chin. J. Inorg. Chem. 2012, 28, 1251–1258. [Google Scholar]
- Liang, R.P.; Wang, Z.X.; Zhang, L.; Qiu, J.D. A label-free amperometric immunosensor for alpha-fetoprotein determination based on highly ordered porous multiwalled carbon nanotubes/silica nanoparticles array platform. Sensor. Actuator. B Chem. 2012, 166–167, 569–575. [Google Scholar]
- Ding, C.F.; Zhang, Q.; Zhang, S.S. An electrochemical immunoassay for protein based on bio bar code method. Biosens. Bioelectron. 2009, 24, 2434–2440. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhao, Y.; Wei, Z.; Wang, W. Chemiluminescence immunoassay based on dual signal amplification strategy of Au/mesoporous silica and multienzyme functionalized mesoporous silica. Mater. Sci. Eng. B 2011, 176, 1474–1478. [Google Scholar] [CrossRef]
- Tang, J.; Hu, R.; Wu, Z.S.; Shen, G.L.; Yu, R.Q. A highly sensitive electrochemical immunosensor based on coral-shaped AuNPs with CHITs inorganic-organic hybrid film. Talanta 2011, 85, 117–122. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xiong, P.; Gan, N.; Cao, Y.; Hu, F.; Li, T.; Zheng, L. An Ultrasensitive Electrochemical Immunosensor for Alpha-Fetoprotein Using an Envision Complex-Antibody Copolymer as a Sensitive Label. Materials 2012, 5, 2757-2772. https://doi.org/10.3390/ma5122757
Xiong P, Gan N, Cao Y, Hu F, Li T, Zheng L. An Ultrasensitive Electrochemical Immunosensor for Alpha-Fetoprotein Using an Envision Complex-Antibody Copolymer as a Sensitive Label. Materials. 2012; 5(12):2757-2772. https://doi.org/10.3390/ma5122757
Chicago/Turabian StyleXiong, Ping, Ning Gan, Yuting Cao, Futao Hu, Tianhua Li, and Lei Zheng. 2012. "An Ultrasensitive Electrochemical Immunosensor for Alpha-Fetoprotein Using an Envision Complex-Antibody Copolymer as a Sensitive Label" Materials 5, no. 12: 2757-2772. https://doi.org/10.3390/ma5122757




