Oriented Collagen Scaffolds for Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
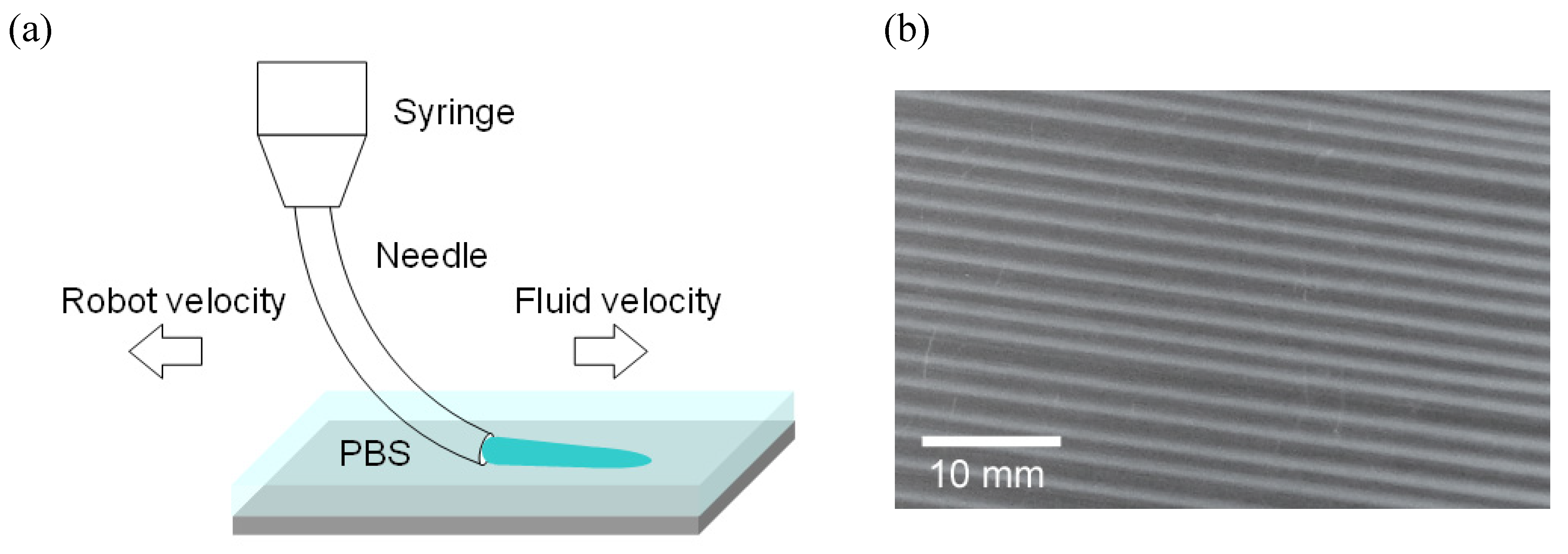
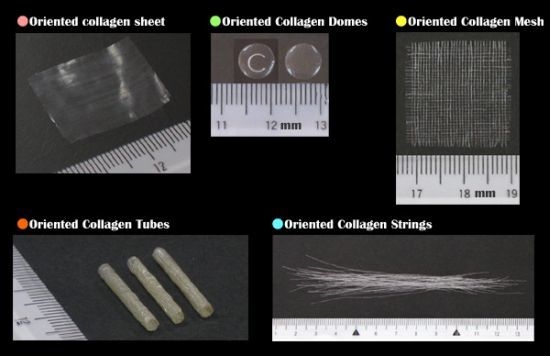
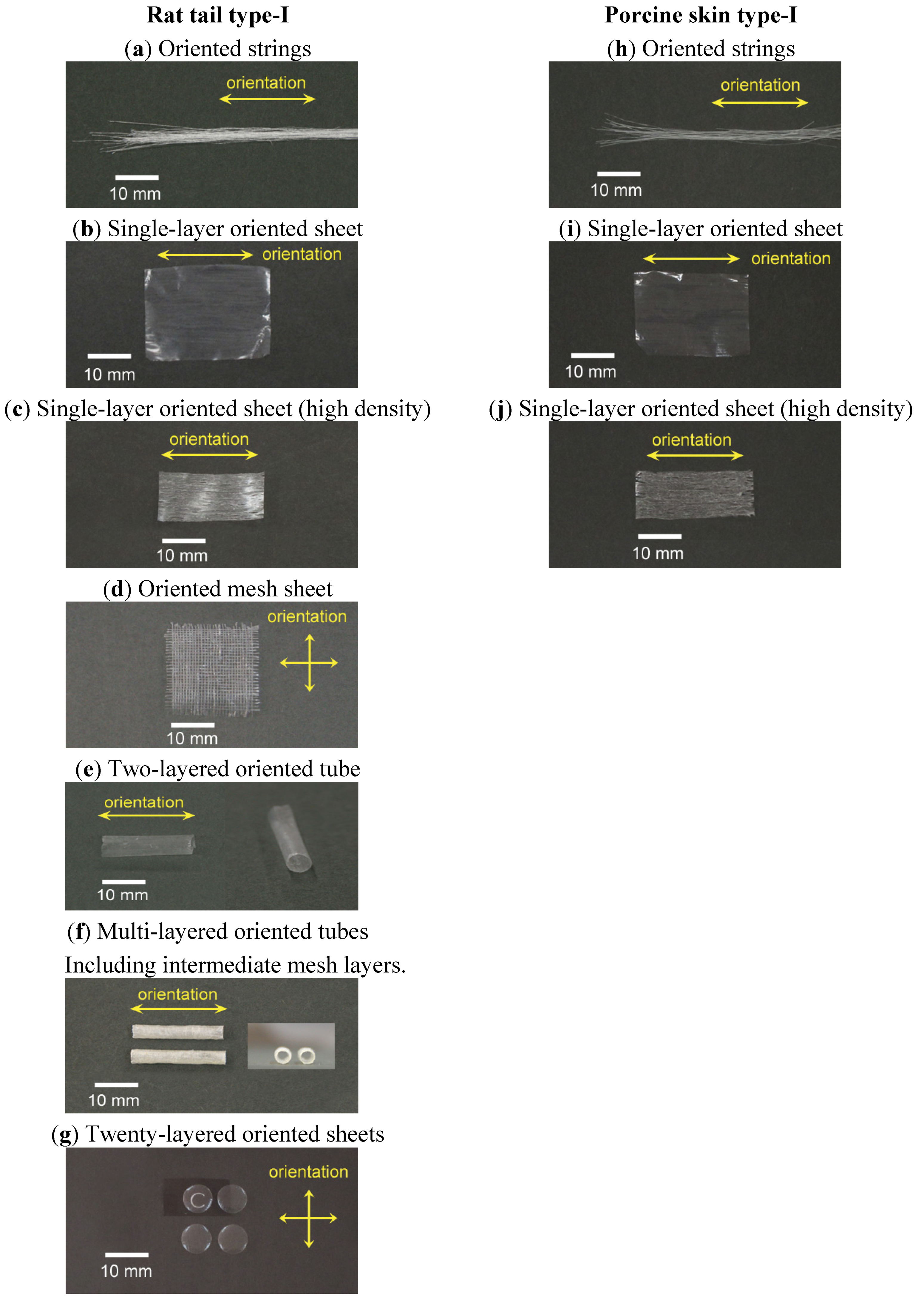
3. Results and Discussion
3.1. Three-Dimensional Oriented Collagen Scaffolds
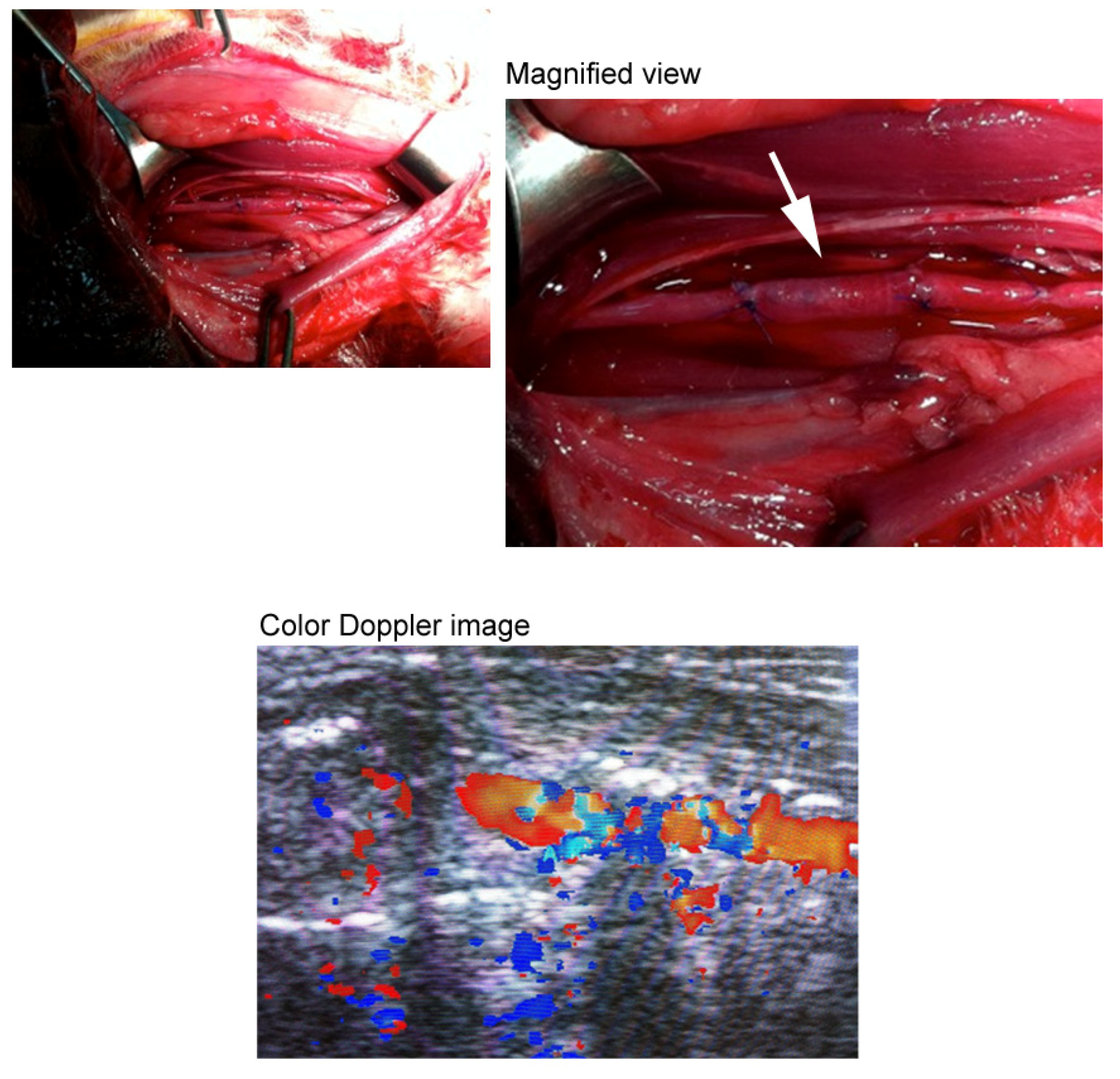
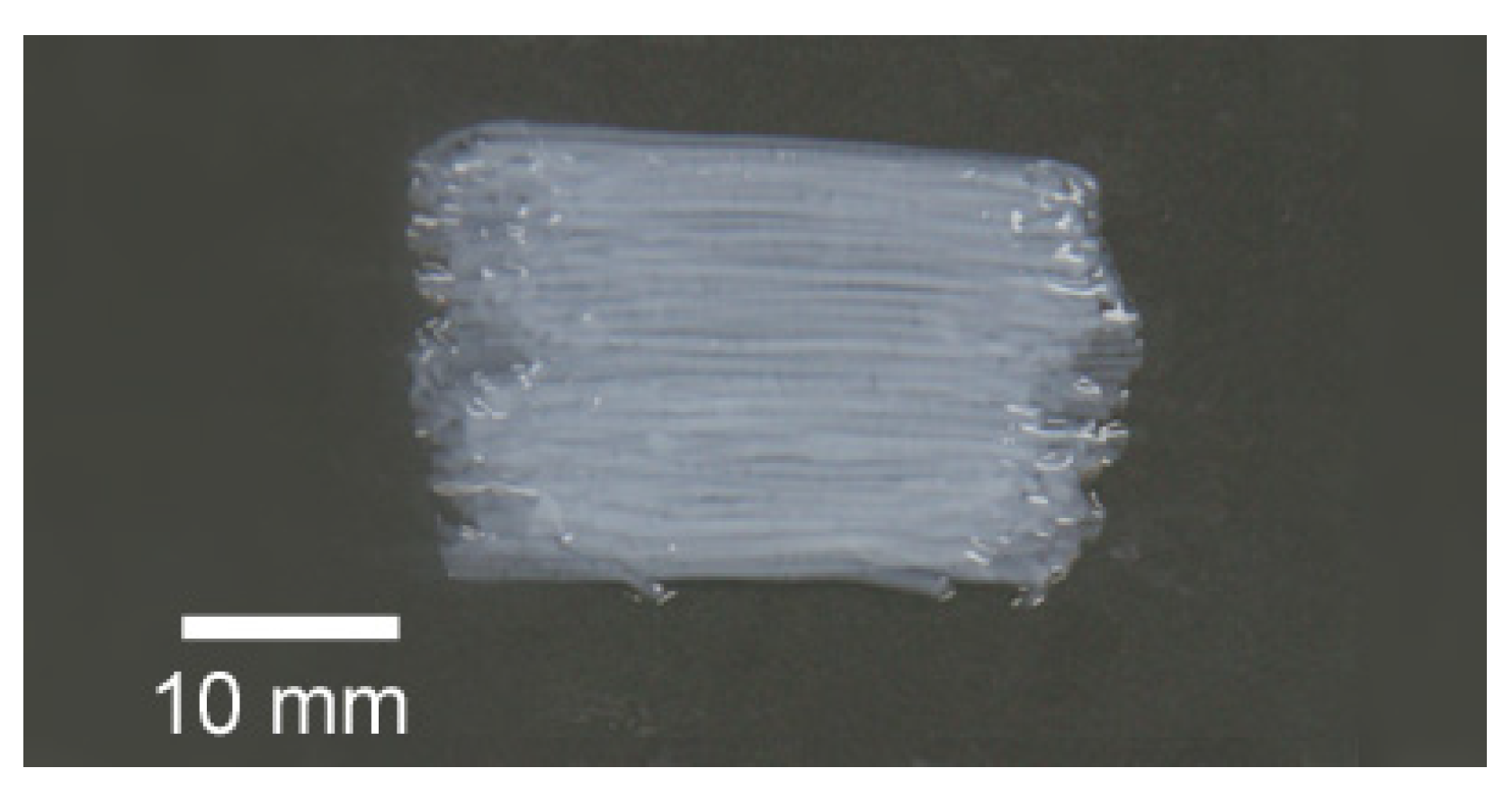
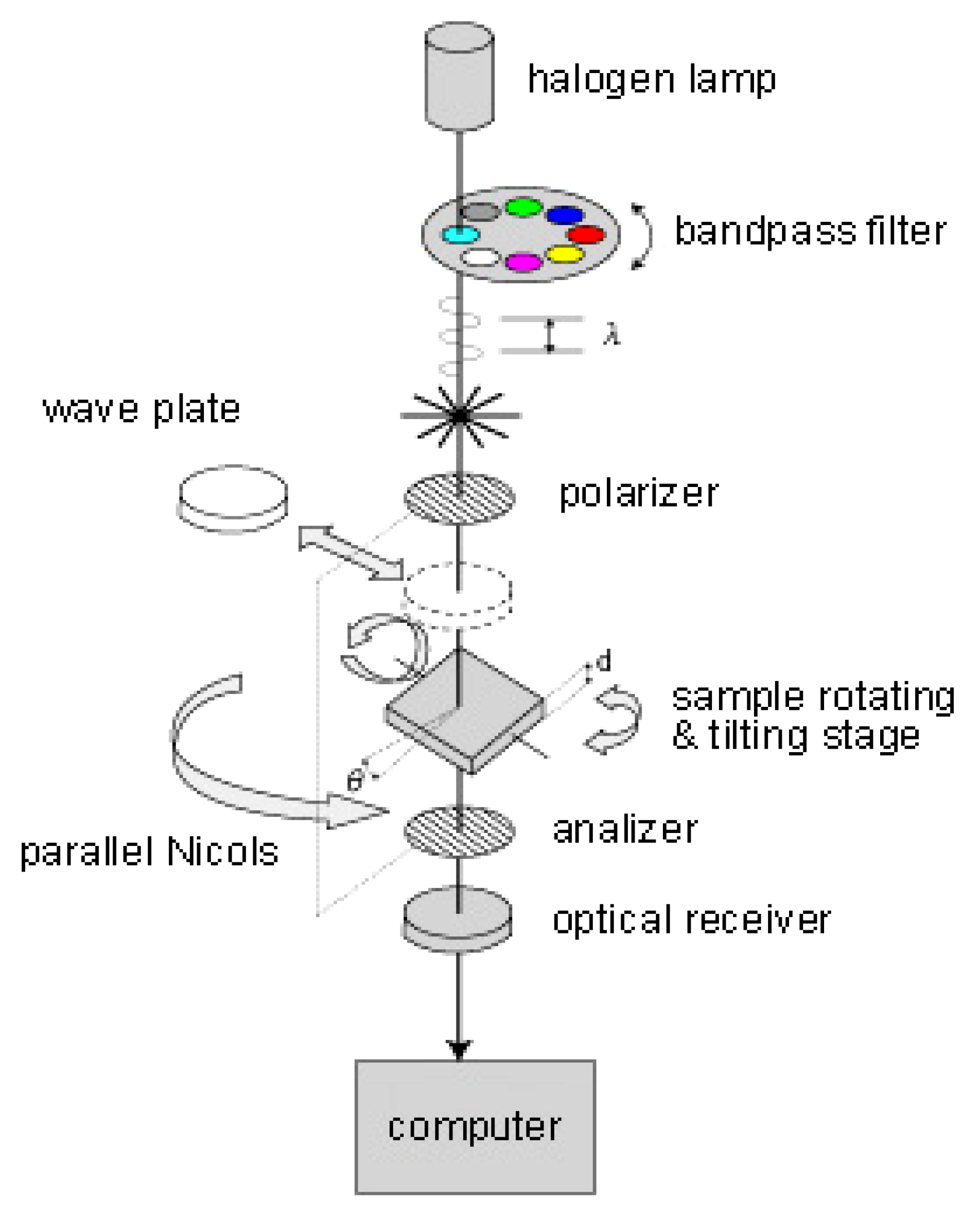
3.2. Birefringence Measurements
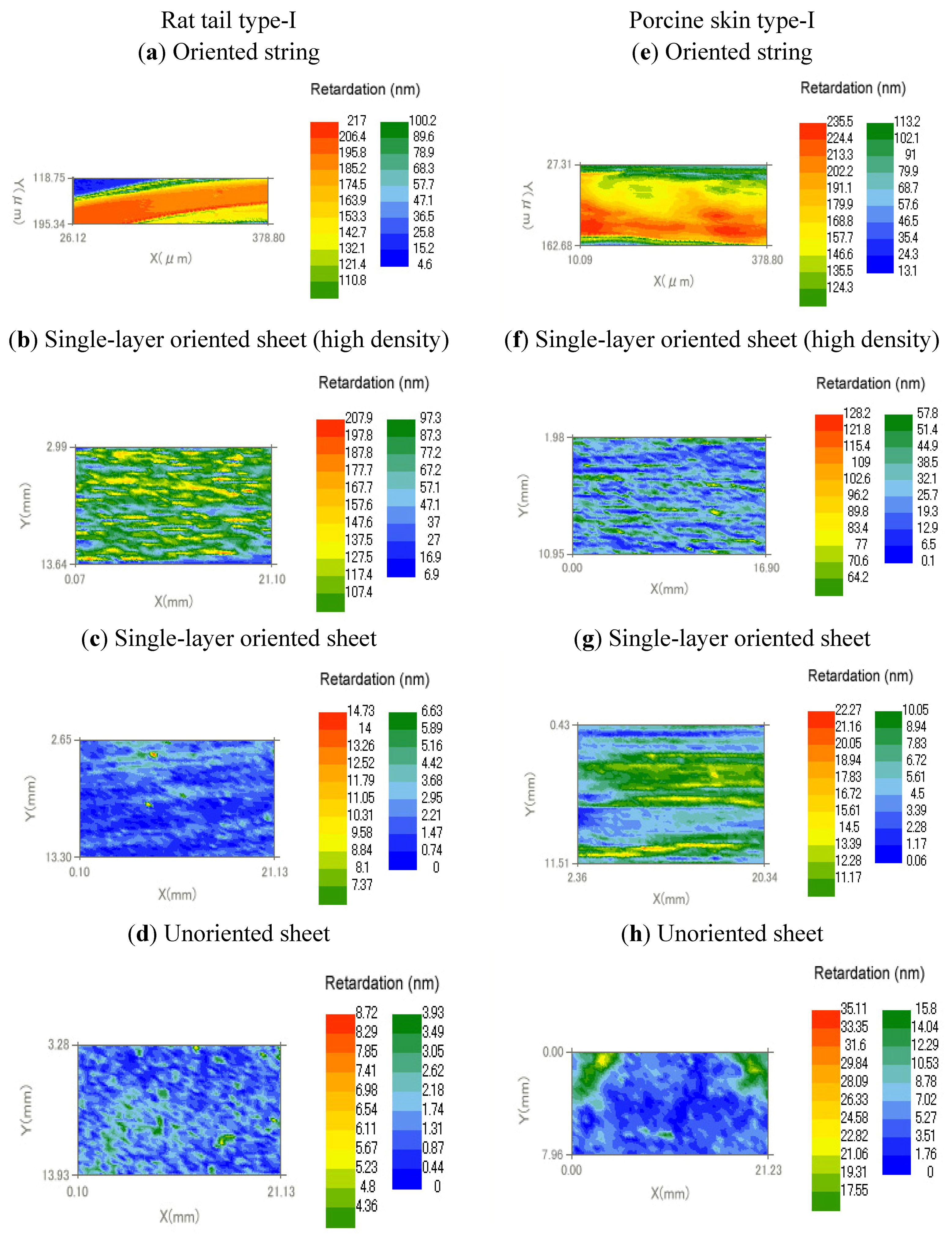
| Type | Collagen | Retardation (nm) | Thickness (μm) | Birefringence (×103) | |
|---|---|---|---|---|---|
| (a) | Oriented string | Rat tail type-I | 195 | 57 | 3.4 |
| (b) | Oriented string | Porcine skin type-I | 213 | 92 | 2.3 |
| (c) | Single-layer oriented sheet (high density) | Rat tail type-I | 127 | 62 | 2.0 |
| (d) | Single-layer oriented sheet (high density) | Porcine skin type-I | 83.4 | 55 | 1.5 |
| (e) | Single-layer oriented sheet | Rat tail type-I | 3.6 | 16 | 0.2 |
| (f) | Single-layer oriented sheet | Porcine skin type-I | 13 | 11 | 1.2 |
| (g) | Unoriented sheet | Rat tail type-I | 1.7 | 12 | 0.1 |
| (h) | Unoriented sheet | Porcine skin type-I | 5.2 | 21 | 0.3 |
4. Conclusions
Acknowledgments
References and Notes
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Ehara, A.; Ogata, K.; Imazato, S.; Ebisu, S.; Nakano, T.; Umakoshi, Y. Effects of α-TCP and TetCP on MC3T3-E1 proliferation, differentiation and mineralization. Biomaterials 2003, 24, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Harley, B.A.; Lynn, A.K.; Wissner-Gross, Z.; Bonfield, W.; Yannas, I.V.; Gibson, L.J. Design of a multiphase osteochondral scaffold. II. Fabrication of a mineralized collagen-glycosaminoglycan scaffold. J. Biomed. Mater. Res. A 2010, 92, 1066–1077. [Google Scholar] [PubMed]
- Cook, J.L.; Fox, D.B.; Malaviya, P.; Tomlinson, J.L.; Farr, J.; Kuroki, K.; Cook, C.R. Evaluation of small intestinal submucosa grafts for meniscal regeneration in a clinically relevant posterior meniscectomy model in dogs. J. Knee Surg. 2006, 19, 159–167. [Google Scholar] [PubMed]
- Yokota, T.; Ichikawa, H.; Matsumiya, G.; Kuratani, T.; Sakaguchi, T.; Iwai, S.; Shirakawa, Y.; Torikai, K.; Saito, A.; Uchimura, E.; Kawaguchi, N.; Matsuura, N.; Sawa, Y. In situ tissue regeneration using a novel tissue-engineered, small-caliber vascular graft without cell seeding. J. Thorac. Cardiovasc. Surg. 2008, 136, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.S.; Anderson, C.M.; Fuller, G.G. Designing a tubular matrix of oriented collagen fibrils for tissue engineering. Acta Biomaterialia 2011, 7, 2448–2458. [Google Scholar] [CrossRef]
- Yost, M.J.; Baicu, C.F.; Stonerock, C.E.; Goodwin, R.L.; Price, R.L.; Davis, J.M; Evans, H.; Watson, P.D.; Gore, C.M; Sweet, J.; et al. A novel tubular scaffold for cardiovascular tissue engineering. Tissue Engineering 2004, 10, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Zeugolis, D.I.; Paul, R.G.; Attenburrow, G. Engineering extruded collagen fibers for biomedical applications. J. Appl. Polym. Sci. 2008, 108, 2886–2894. [Google Scholar] [CrossRef]
- Helary, C.; Ovtracht, L.; Coulomb, B.; Godeau, G.; Giraud-Guille, M. Dense fibrillar collagen matrices: A model to study myofibroblast behavior during wound healing. Biomaterials 2006, 27, 6411–6417. [Google Scholar] [CrossRef]
- Berthoda, F.; Germaina, L.; Lia, H.; Xua, W.; Damourb, O.; Augera, F.A. Collagen fibril network and elastic system remodeling in a reconstructed skin transplanted on nude mice. Matrix Biology 2001, 20, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Márqueza, S.P.; Martíneza, V.S.; Ambrosea, W.M.; Wanga, J.; Gantxeguib, N.G.; Scheinc, O.; Elisseeffa, J. Decellularization of bovine corneas for tissueengineering applications. Acta Biomaterialia 2009, 5, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Buillesa, N.; Janin-Manificata, H.; Malbouyresb, M.; Justina, V.; Rovèrea, M.; Pellegrinic, G.; Torbetb, J.; Hulmesb, D.J.S.; Burillona, C.; Damoura, O.; et al. Use of magnetically oriented orthogonal collagen scaffolds for hemi-corneal reconstruction and regeneration. Biomaterials 2010, 31, 8313–8322. [Google Scholar] [CrossRef] [PubMed]
- Duncana, T.J.; Tanakaa, Y.; Shia, D.; Kubota, A.; Quantock, A.J; Nishida, K. Flow-manipulated, crosslinked collagen gels for use as corneal equivalents. Biomaterials 2010, 31, 8996–9005. [Google Scholar] [CrossRef] [PubMed]
- Mikami, H.; Kuwahara, G.; Nakamura, N.; Kondo, M.; Tanaka, M.; Yamato, M.; Kodama, S. Two-Layered Tissue-Engineered Urethra Using Oral Epithelial and Muscle-derived Cells. In Presented at the TERMIS-EU 2011 Annual Meeting, Granada, Spain; 2011. 45.O4. [Google Scholar]
- Atala, A. Bioengineered tissues for urogenital repair in children. PediatricResearch 2008, 63, 569–575. [Google Scholar]
- Rosner, B.I.; Siegel, R.A.; Grosberg, A.; Tranquillo, R.T. Rational design of contact guiding, neurotrophic matrices for peripheral nerve regeneration. Ann. Biomed. Eng. 2003, 31, 1383–1401. [Google Scholar] [CrossRef] [PubMed]
- Albu, M.G.; Titorencu, I.; Ghica, M.V. Collagen-based drug delivery systems for tissue engineering. In Biomaterials Applications for Nanomedicine; Rosario, P., Ed.; InTech: Rijeka, Croatia, 2011; pp. 333–358. [Google Scholar]
- Riggs, C.; Vaughan, L.; Evans, G.; Lanyon, L.; Boyde, A. Mechanical implications of collagen fibre orientation in cortical bone of the equine radius. Anat Embryol 1993, 187, 239–248. [Google Scholar] [PubMed]
- Jones, S.J.; Boyde, A.; Pawley, J.B. Osteoblasts and collagen orientation. Cell Tiss. Res. 1975, 159, 73–80. [Google Scholar] [CrossRef]
- Popescu, D.; Lems, R.; Rossi, N.; Yeoh, C.; Loos, J.; Holder, S.; Bouten, C.; Sommerdijk, N. The patterning and alignment of muscles cells using the selective adhesion of poly(oligoethylene glycol methyl ether methacraylate)-based ABA block copolymers. Adv. Mater. 2005, 17, 2324–2329. [Google Scholar] [CrossRef]
- Thompson, D.M.; Buettner, H.M. Neurite outgrowth is directed by Schwann cell alignment in the absence of other guidance cues. Ann. Biomed. Eng. 2006, 34, 669–676. [Google Scholar] [CrossRef]
- Thompson, D.M.; Buettner, H.M. Oriented Schwann cell monolayers for directed neurite outgrowth. Ann. Biomed. Eng. 2004, 32, 1121–1131. [Google Scholar]
- Wilson, D.L.; Martin, R.; Hong, S.; Cronin-Golomb, M.; Mirkin, C.A.; Kaplan, D.L. Surface organization and nanopatterning of collagen by dip-pen lithography. Proc. Natl. Acad. Sci. USA 2001, 98, 13660–13664. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.P.; Nash, L.; Hu, X.W.; Haffegee, H.; Ho, M.-W.J. In vitro formation by reverse dialysis of collagen gels containing highly oriented arrays of fibrils. Biomed. Mater. Res. 1998, 41, 185–191. [Google Scholar] [CrossRef]
- Torbet, J.; Malbouyres, M.; Builles, N.; Justin, V.; Roulet, M.; Damour, O.; Oldberg, A.; Ruggiero, F.; Hulmes, D. Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. J. S. Biomaterials. 2007, 28, 4268–4276. [Google Scholar] [CrossRef]
- Dickenson, R.B.; Guido, S.; Tranquillo, R.T. Biased cell migration of fibroblasts exhibiting contact guidance in oriented collagen gels. Ann. Biomed. Eng. 1994, 22, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Kaufman, L. Flow and magnetic field induced collagen alignment. J. Biomaterials 2007, 28, 1105–1114. [Google Scholar] [CrossRef]
- Zhong, S.; Teo, W.E.; Zhu, X.; Beuerman, R.W.; Ramakrishna, S.; Yung, L.Y.L. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J. Biomed. Mater. Res. A 2006, 79A, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, J.E.; Fuller, G.G. Liquid Crystalline Collagen: A self-assembled morphology for the orientation of mammalian cells. Langmuir 2009, 25, 3200–3206. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Isobe, Y.; Kosaka, T.; Kuwahara, G.; Mikami, H.; Saku, T.; Kodama, S. Oriented Collagen Scaffolds for Tissue Engineering. Materials 2012, 5, 501-511. https://doi.org/10.3390/ma5030501
Isobe Y, Kosaka T, Kuwahara G, Mikami H, Saku T, Kodama S. Oriented Collagen Scaffolds for Tissue Engineering. Materials. 2012; 5(3):501-511. https://doi.org/10.3390/ma5030501
Chicago/Turabian StyleIsobe, Yoshihiro, Toru Kosaka, Go Kuwahara, Hiroshi Mikami, Taro Saku, and Shohta Kodama. 2012. "Oriented Collagen Scaffolds for Tissue Engineering" Materials 5, no. 3: 501-511. https://doi.org/10.3390/ma5030501