Effect of Alkyl Chain Length on Carboxylic Acid SAMs on Ti-6Al-4V
Abstract
:1. Introduction
2. Results and Discussion

| Organic acid | Carbons in molecule | Oxide surface | -CH2- stretches (asymm, symm) | Binding mode (-dentate) |
|---|---|---|---|---|
| ODA | 18 | Ti-6Al-4V | - | - |
| ECA | 20 | Ti-6Al-4V | 2921, 2850 | - |
| DCA | 22 | Ti-6Al-4V | 2922, 2850 | - |
| TCA | 24 | Ti-6Al-4V | 2918, 2848 | - |
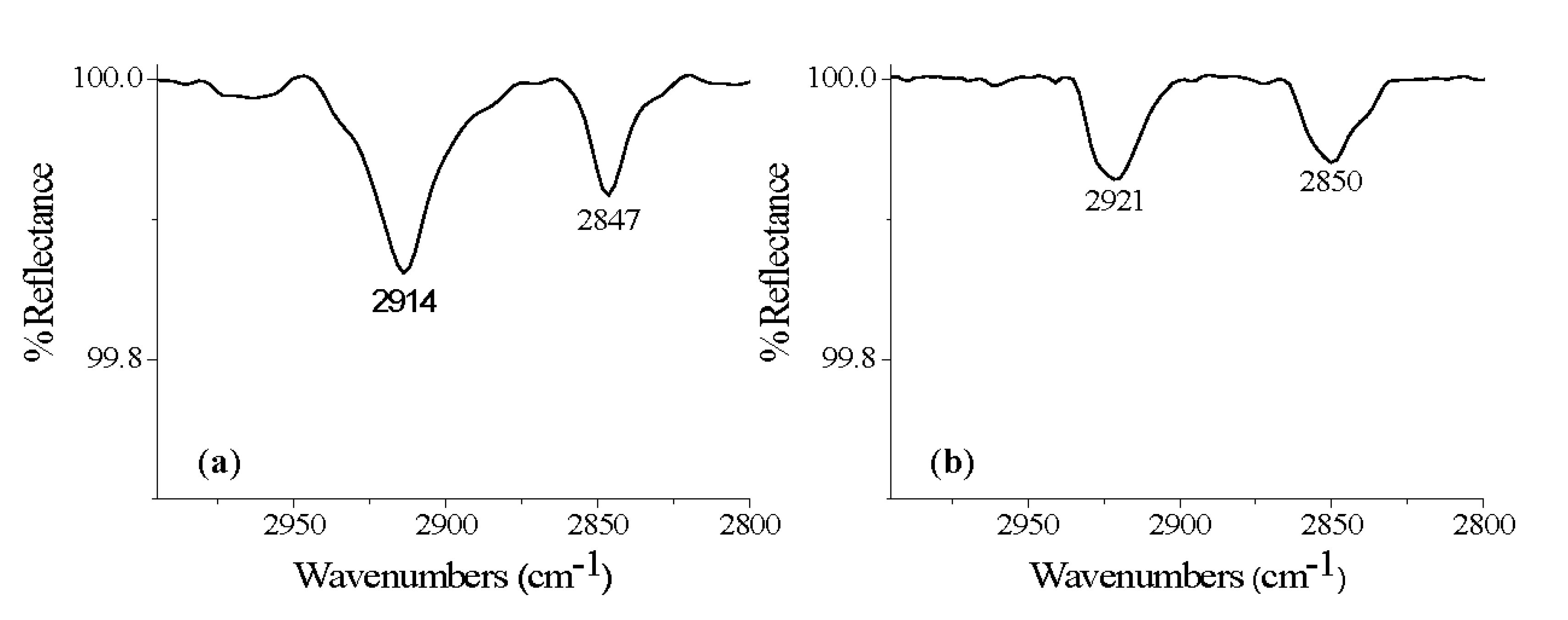
| HCA | 26 | Ti-6Al-4V | 2914, 2847 | - |
| OCA | 28 | Ti-6Al-4V | 2913, 2846 | Bi |
| - | - | Ti | 2914, 2847 | Bi |
| - | - | Al | 2914, 2848 | Bi |
| - | - | V | - | - |
| TAA | 30 | Ti-6Al-4V | 2913, 2847 | Bi |
| - | - | Ti | 2912, 2847 | Bi |
| - | - | Al | 2917, 2849 | Bi |
| - | - | V | 2923, 2852 | Bi |
3. Experimental
3.1. Materials
3.2. Coupon Preparation
3.3. SAM Formation Protocols
3.4. Protocols Not Resulting in SAM Formation
Octadecanoic (18 Carbons) Acid
| Protocol | Organic acid | Method used | Solution conc. (mM) | Cooling method | Sprays | Solvent removal | Oven time (min) | Oven temp. (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | ODA-TAA | TLC Spray | 1 | Ice, 1 hr. | 3 | Oven | 30 | 100 |
| 2 | ODA-HCA | TLC Spray | 1 | Ice, 1 hr. | 3 | Oven | 30 | 120 |
| 3 | ODA-HCA | TLC Spray | 1 | Ice, 1 hr. | 5 | Oven | 45 | 100 |
| 4 | ODA-HCA | TLC Spray | 1 | Ice, 1 hr. | 3 | Ambient | 30 | 100 |
| 5 | ODA-HCA | TLC Spray | 1 | Ice, 1 hr. | 5 | 0.1 torr vac line | 30 | 100 |
| 6 | ODA-HCA | Solution (50°C, 3hr) | 2 | Ice, 1 hr. | - | Oven | - | 100 |
3.5. Protocols Resulting in SAM Formation
3.5.1. Eicosanoic (20 Carbons) Acid
3.5.2. Docosanoic (22 Carbons) Acid
3.5.3. Tetracosanoic (24 Carbons) and Hexacosanoic (26 Carbons) Acids
3.5.4. Octacosanoic (28 Carbons) and Triacontanoic (30 Carbons) Acids
3.6. Infrared Spectroscopy
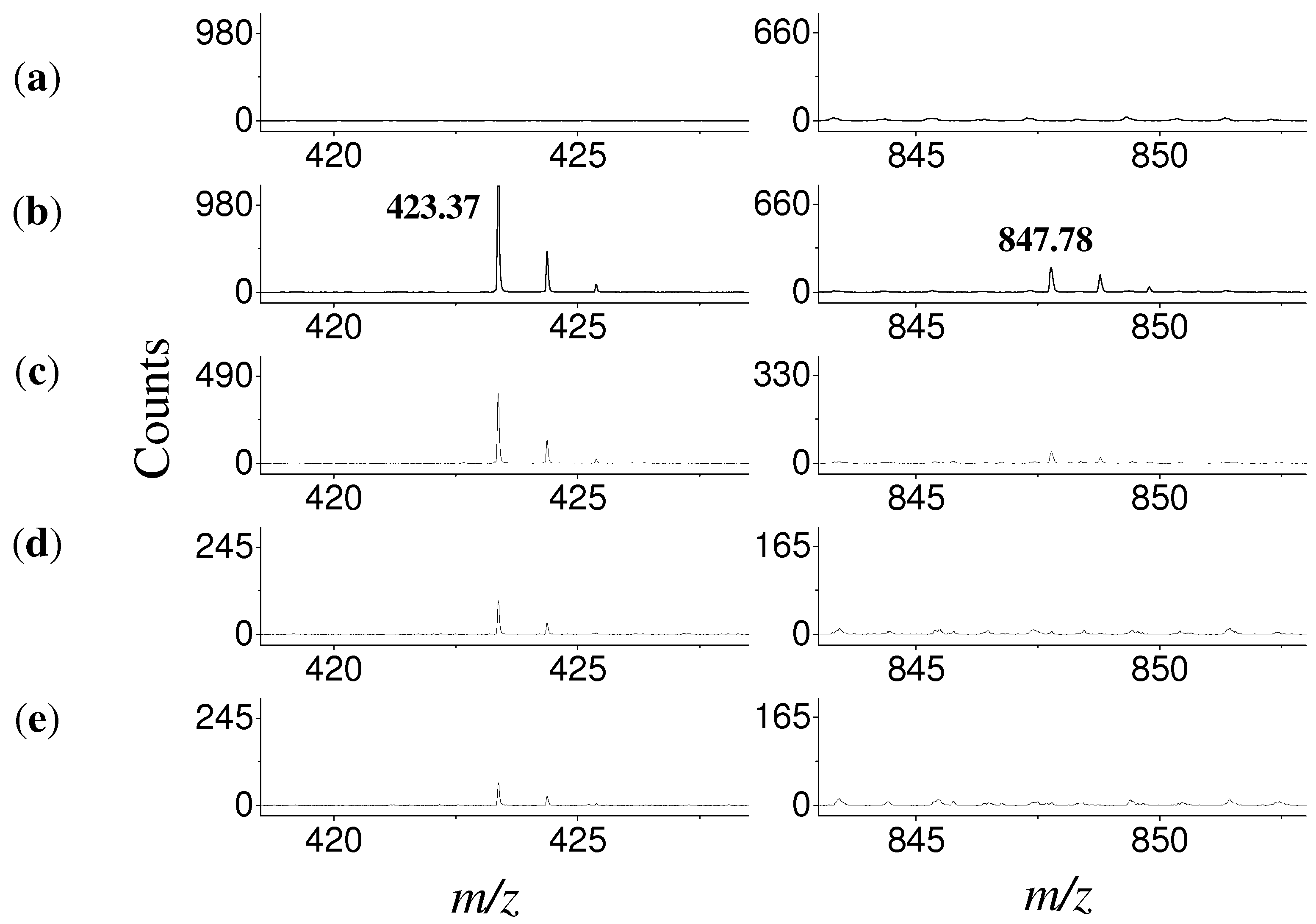
3.7. Mass Spectrometry
3.8. Contact Angle Analysis
3.9. SAM Stability Assessment
4. Conclusions
Supplementary material
Supplementary File 1Acknowledgments
References
- Dong, H.; Bell, T. Enhanced wear resistance of titanium surfaces by a new thermal oxidation treatment. Wear 2000, 238, 131–137. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Oxidation of Ti-6Al-4V alloy. J. Alloys Compd. 2009, 472, 241–246. [Google Scholar] [CrossRef]
- Krishna, S.R.D.; Brama, Y.L.; Sun, Y. Thick rutile layer on titanium for tribological applications. Tribol. Int. 2007, 40, 329–334. [Google Scholar] [CrossRef]
- Su, Y.; Wang, L.; Luo, L.; Jiang, X.; Guo, J.; Fu, H. Deoxidation of titanium alloy using hydrogen. Int. J. Hydrog. Energy 2009, 34, 8958–8963. [Google Scholar] [CrossRef]
- Boyer, R.R. An overview on the use of titanium in the aerospace industry. Mater. Sci Eng. A 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Mitsuo, N. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Mani, G.; Johnson, D.M.; Marton, D.; Feldman, M.D.; Patel, D.; Ayon, A.A.; Agrawal, C.M. Drug delivery from gold and titanium surfaces using self-assembled monolayers. Biomaterials 2008, 29, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liang, C.; Huang, N. Studies on corrosion inhibition of copper by alkanethiol SAMs prepared in aqueous micellar solution. Mater. Corros. 2010, 61, 332–337. [Google Scholar] [CrossRef]
- Ishizaki, T.; Okido, M.; Masuda, Y.; Saito, N.; Sakamoto, M. Corrosion resistant performances of alkanoic and phosphonic acids derived self-assembled monolayers on magnesium alloy AZ31 by vapor-phase method. Langmuir 2011, 27, 6009–6017. [Google Scholar] [CrossRef] [PubMed]
- Alagta, A.; Felhösi, I.; Bertoti, I.; Kálmán, E. Corrosion protection properties of hydroxamic acid self-assembled monolayer on carbon steel. Corros. Sci. 2008, 50, 1644–1649. [Google Scholar] [CrossRef]
- Kruszewski, K.M.; Renk, E.R.; Gawalt, E.S. Self-assembly of organic acid molecules on the metal oxide surface of a cupronickel alloy. Thin Solid Films 2012, 520, 4326–4331. [Google Scholar] [CrossRef]
- Kruszewski, K.M.; Gawalt, E.S. Perfluorocarbon thin films and polymer brushes on stainless steel 316 L for the control of interfacial properties. Langmuir 2011, 27, 8120–8125. [Google Scholar] [CrossRef] [PubMed]
- Adden, N.; Gamble, L.J.; Castner, D.G.; Hoffmann, A.; Gross, G.; Menzel, H. Phosphonic acid monolayers for binding of bioactive molecules to titanium surfaces. Langmuir 2006, 22, 8197–8204. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Dickinson, L.; Grozinger, C.; Morin, F.G.; Reven, L. Self-assembled monolayers of alkylphosphonic acids on metal oxides. Langmuir 1996, 12, 6429–6435. [Google Scholar] [CrossRef]
- Kanta, A.; Sedev, R.; Ralston, J. The formation and stability of self-assembled monolayers of octadecylphosphonic acid on titania. Colloid Surf. A 2006, 291, 51–58. [Google Scholar] [CrossRef]
- Schwartz, J.; Avaltroni, M.J.; Danahy, M.P.; Silverman, B.M.; Hanson, E.L.; Schwarzbauer, J.E.; Midwood, K.S.; Gawalt, E.S. Cell attachment and spreading on metal implant materials. Mater. Sci. Eng. C 2003, 23, 395–400. [Google Scholar] [CrossRef]
- Silverman, B.M.; Wieghaus, K.A.; Schwartz, J. Comparative properties of siloxane vs. phosphonate monolayers on a key titanium alloy. Langmuir 2004, 21, 225–228. [Google Scholar] [CrossRef]
- Zorn, G.; Gotman, I.; Gutmanas, E.Y.; Adadi, R.; Salitra, G.; Sukenik, C.N. Surface modification of Ti45Nb alloy with an alkylphosphonic acid self-assembled monolayer. Chem. Mater. 2005, 17, 4218–4226. [Google Scholar] [CrossRef]
- Quiñones, R.; Raman, A.; Gawalt, E.S. Functionalization of nickel oxide using alkylphosphonic acid self-assembled monolayers. Thin Solid Films 2008, 516, 8774–8781. [Google Scholar] [CrossRef]
- Raman, A.; Dubey, M.; Gouzman, I.; Gawalt, E.S. Formation of self-assembled monolayers of alkylphosphonic acid on the native oxide surface of SS316L. Langmuir 2006, 22, 6469–6472. [Google Scholar] [CrossRef] [PubMed]
- Raman, A.; Quiñones, R.; Barriger, L.; Eastman, R.; Parsi, A.; Gawalt, E.S. Understanding organic film behavior on alloy and metal oxides. Langmuir 2010, 26, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.; Punchihewa, S.; Infelta, P.; Gratzel, M. Surface complexation of colloidal semiconductors strongly enhances interfacial electron-transer rates. Langmuir 1991, 7, 3012–3018. [Google Scholar] [CrossRef]
- Mann, J.R.; Nevins, J.S.; Soja, G.R.; Wells, D.D.; Levy, S.C.; Marsh, D.A.; Watson, D.F. Influence of solvation and the structure of adsorbates on the kinetics and mechanism of dimerization-induced compositional changes of mixed monolayers on TiO2. Langmuir 2009, 25, 12217–12228. [Google Scholar] [CrossRef] [PubMed]
- Uetsuka, H.; Sasahara, A.; Yamakata, A.; Onishi, H. Microscopic identifiaction of a bimolecular reaction intermediate. J. Phys. Chem. B 2002, 11549–11552. [Google Scholar] [CrossRef]
- Aronoff, Y.G.; Chen, B.; Lu, G.; Seto, C.; Schwartz, J.; Bernasek, S.L. Stabilization of self-assembled monolayers of carboxylic acids on native oxides of metals. J. Am. Chem. Soc. 1997, 119, 259–262. [Google Scholar] [CrossRef]
- Badia, A.; Lennox, R.B.; Reven, L. A dynamic view of self-assembled monolayers. Acc. Chem. Res. 2000, 33, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.; Leggett, G.J. Influence of tail-group hydrogen bonding on the stabilities of self-assembled monolayers of alkylthiols on gold. Langmuir 1999, 15, 1024–1032. [Google Scholar] [CrossRef]
- Tao, Y.T. Structural comparison of self-assembled monolayers of n-alkanoic acids on the surfaces of silver, copper, and aluminum. J. Am. Chem. Soc. 1993, 115, 4350–4358. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Gawalt, E.S.; Avaltroni, M.J.; Koch, N.; Schwartz, J. Self-assembly and bonding of alkanephosphonic acids on the native oxide surface of titanium. Langmuir 2001, 17, 5736–5738. [Google Scholar] [CrossRef]
- D’Andre, S.C.; Fadeev, A.Y. Covalent surface modification of calcium hydroxyapatite using n-alkyl- and n-fluoroalkylphosphonic acids. Langmuir 2003, 19, 7904–7910. [Google Scholar] [CrossRef]
- Fiurasek, P.; Reven, L. Phosphonic and sulfonic acid-functionalized gold nanoparticles: A solid-state NMR study. Langmuir 2007, 23, 2857–2866. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Z.; Zhou, S.; Wu, L. Self-assembled monolayers on magnesium alloy surfaces from carboxylate ions. Appl. Surf. Sci. 2006, 252, 3818–3827. [Google Scholar] [CrossRef]
- Shustak, G.; Domb, A.J.; Mandler, D. Preparation and characterization of n-alkanoic acid self-assembled monolayers adsorbed on 316L stainless steel. Langmuir 2004, 20, 7499–7506. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Castner, D.G.; Ratner, B.D. Multitechnique surface characterization of derivatization efficiencies for hydroxyl-terminated self-assembled monolayers. Langmuir 1998, 14, 3545–3550. [Google Scholar] [CrossRef]
- Allara, D.L.; Nuzzo, R.G. Spontaneously organized molecular assemblies. 2. Quantitative infrared spectroscopic determination of equilibrium structures of solution-adsorbed n-alkanoic acids on an oxidized aluminum surface. Langmuir 1985, 1, 52–66. [Google Scholar] [CrossRef]
- Snyder, R.G.; Strauss, H.L.; Elliger, C.A. Carbon-hydrogen stretching modes and the structure of n-alkyl chains. 1. Long, disordered chains. J. Phys. Chem. 1982, 86, 5145–5150. [Google Scholar] [CrossRef]
- Allara, D.L.; Nuzzo, R.G. Spontaneously organized molecular assemblies. 1. Formation, dynamics, and physical properties of n-alkanoic acids adsorbed from solution on an oxidized aluminum surface. Langmuir 1985, 1, 45–52. [Google Scholar] [CrossRef]
- Ito, E.; Konno, K.; Noh, J.; Kanai, K.; Ouchi, Y.; Seki, K.; Hara, M. Chain length dependence of adsorption structure of COOH-terminated alkanethiol SAMs on Au(111). Appl. Surf. Sci. 2005, 244, 584–587. [Google Scholar] [CrossRef]
- Spori, D.M.; Venkataraman, N.V.; Tosatti, S.G.P.; Durmaz, F.; Spencer, N.D.; Zürcher, S. Influence of alkyl chain length on phosphate self-assembled monolayers. Langmuir 2007, 23, 8053–8060. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, R.; Raman, A.; Gawalt, E.S. An approach to differentiating between multi- and monolayers using MALDI-TOF MS. Surf. Interface Anal. 2007, 39, 593–600. [Google Scholar] [CrossRef]
- Ploux, L.; Beckendorff, S.; Nardin, M.; Neunlist, S. Quantitative and morphological analysis of biofilm formation on self-assembled monolayers. Colloid Surf. B 2007, 57, 174–181. [Google Scholar] [CrossRef]
- Raman, A.; Gawalt, E.S. Self-assembled monolayers of alkanoic acids on the native oxide surface of SS316L by solution deposition. Langmuir 2007, 23, 2284–2288. [Google Scholar] [CrossRef]
- Sittig, C.; Textor, M.; Spencer, N.D.; Wieland, M.; Vallotton, P.H. Surface characterization of implant materials. J. Mater. Sci. Mater. Med. 1999, 10, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, R.; Gawalt, E.S. Study of the formation of self-assembled monolayers on nitinol. Langmuir 2007, 23, 10123–10130. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Buckholtz, G.A.; Gawalt, E.S. Effect of Alkyl Chain Length on Carboxylic Acid SAMs on Ti-6Al-4V. Materials 2012, 5, 1206-1218. https://doi.org/10.3390/ma5071206
Buckholtz GA, Gawalt ES. Effect of Alkyl Chain Length on Carboxylic Acid SAMs on Ti-6Al-4V. Materials. 2012; 5(7):1206-1218. https://doi.org/10.3390/ma5071206
Chicago/Turabian StyleBuckholtz, Gavin A., and Ellen S. Gawalt. 2012. "Effect of Alkyl Chain Length on Carboxylic Acid SAMs on Ti-6Al-4V" Materials 5, no. 7: 1206-1218. https://doi.org/10.3390/ma5071206




