1. Introduction
Tellurite glasses are a relatively new vitreous material and appropriate candidates for new optical materials due to their excellent properties, such as high refractive index, high dielectric constants, a wide band infrared transmittance and substantial third order non-linear optical susceptibility. Their low melting temperatures and non-hygroscopic nature, which limit applications of phosphate and borate glasses, make them of much current interest [
1,
2]. It has been established that the basic structure of these tellurite glasses is a TeO
4 trigonal bipyramid (tbp) with a lone pair of electrons in one of its equatorial sites [
2].
The binary zinc oxide-tellurite glasses have been systematically studied where their densities increase with substitution of up to 40 mol% ZnO into the TeO
2 network system. However, their molar volume decreases [
3]. The addition of ZnO into the binary tellurite glass network reveals opposite behaviors to that of ZnCl
2 [
4], which are closely related to the structural changes.
The linear increase of the elastic modulus of ZnCl
2-TeO
2 glasses is associated with the force constants (f) for a constant mean ring size of each of the atoms involved [
2]. The elastic moduli of ZnCl
2-TeO
2 glasses also increase due to cross-links for force constants of vitreous tellurite networks. The change in the force constant of 10% can be obtained by altering one Te-O bond into a Te-Cl bond and one Zn-Cl bond into a ZnO bond. This is most likely the cause of the higher modulus and hence a reduction in the size of rings. However there are no coordination number changes in substituting Cl atoms with O atoms in ZnCl
2-TeO
2 glasses.
The sound velocities at a frequency of 10 MHz and the elastic moduli for a Na
2O-ZnO-B
2O
3 glass system as a function of ZnO concentration have been studied recently [
5]. Both sound velocities and elastic moduli were found to increase with the addition of ZnO content. Poisson’s ratio and Debye temperature were also found to increase with ZnO concentration. The results indicate that the Zn
2+ ions are likely to occupy network forming positions in this glass system.
Meanwhile, both longitudinal and shear ultrasonic velocities, as well as their elastic moduli decreased with increasing ZnF
2 mol% content in the TeO
2-WO
3-ZnF
2 glasses [
6]. In this case, the addition of ZnF
2 to the tellurite glass matrix weakens the structure by opening the network where the formation of non-bridging oxygen (NBO) is most influenced by the halide ions effect.
The objective the current work is to study the effect of AlF3 on the density and the elastic properties of zinc tellurite glass systems. The short-term significance of this work is to establish a baseline for the elastic properties of vitreous zinc tellurite with the addition of AlF3 into the glass network.
2. Results and Discussion
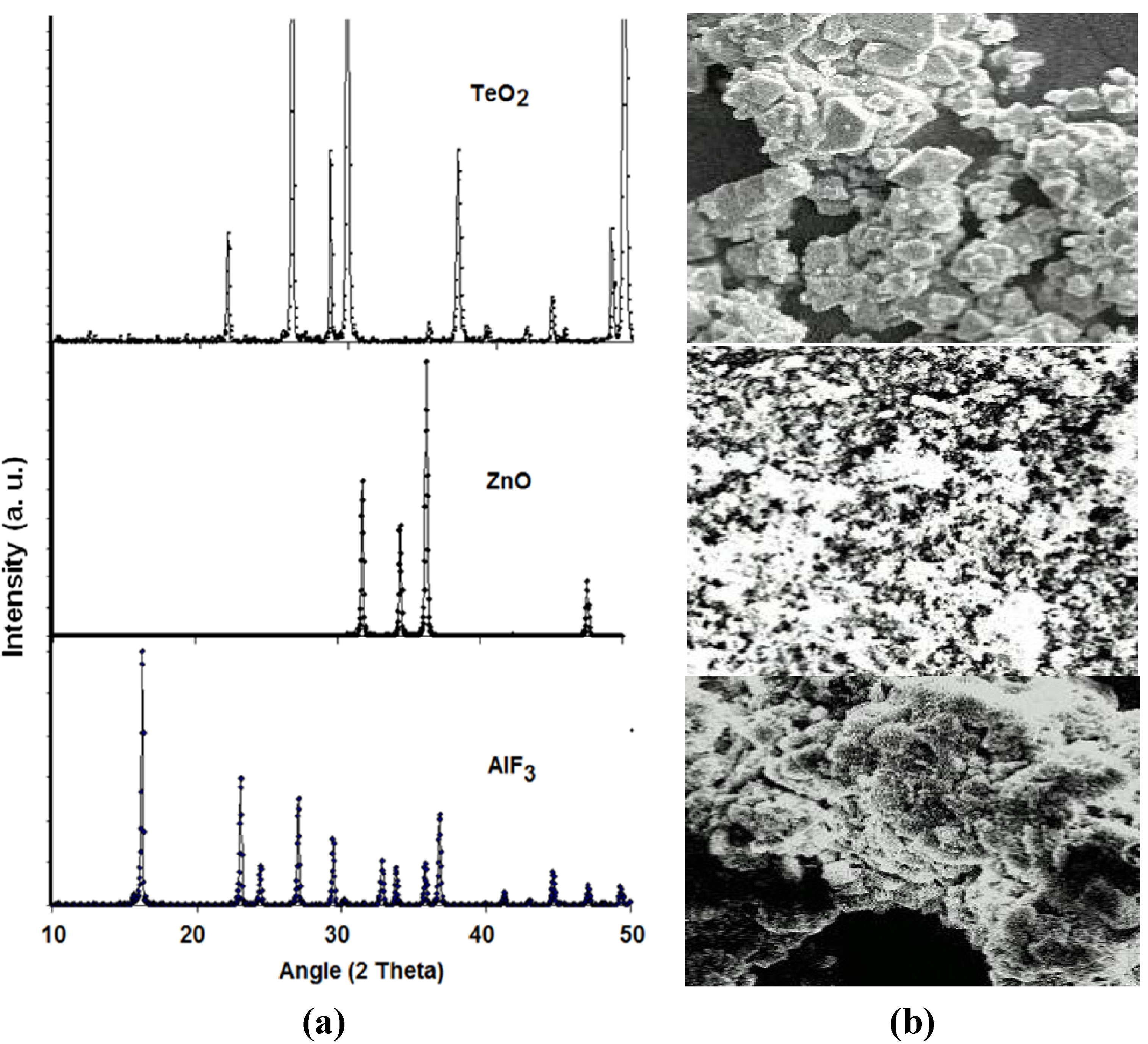
Figure 1 shows the X-ray diffraction (XRD) patterns of (the chemical compositions) and the corresponding scanning electron microscope (SEM) photographs of crystalline tellurite (TeO2), zinc oxide (ZnO) and aluminum fluoride (AlF3) while the XRD patterns of zinc oxyfluoro tellurite glass samples obtained are shown in
Figure 2. As depicted in
Figure 2, all the zinc oxyfluorotellurite glass series were found to show no discrete or continuous sharp peaks but a broad halo at around 2
26°–30°, which reflected the characteristic amorphous glass structure. This indicates the absence of a long-range atomic arrangement and the periodicity of the three dimensional network in the quenched material [
7,
8].
Figure 1.
(a) The X-ray diffraction (XRD) pattern; and (b) the corresponding scanning electron microscope (SEM) photographs of chemical powders of TeO2 (Technical Grade), ZnO (99.9%) and AlF3 (97.0%).
Figure 1.
(a) The X-ray diffraction (XRD) pattern; and (b) the corresponding scanning electron microscope (SEM) photographs of chemical powders of TeO2 (Technical Grade), ZnO (99.9%) and AlF3 (97.0%).
Figure 2.
The XRD patterns of ternary zinc oxyfluoro tellurite glass series.
Figure 2.
The XRD patterns of ternary zinc oxyfluoro tellurite glass series.
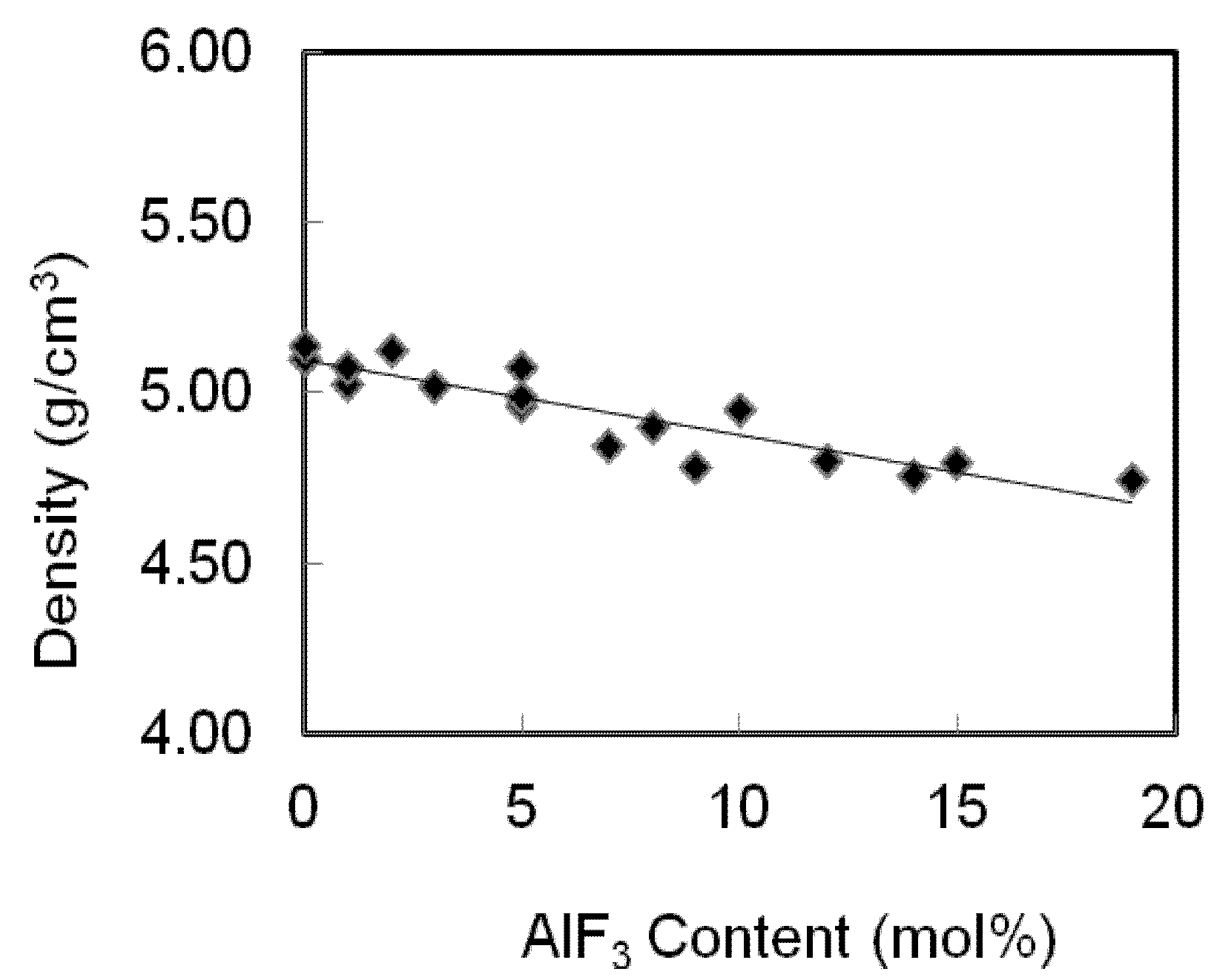
The densities and molar volumes for an AlF
3 zinc tellurite glass system are presented in
Table 1 and shown in
Figure 3. The densities of TeO
2-ZnO-AlF
3 samples decrease as the AlF
3 was added to substitute the ZnO content. These results are in reasonable agreement with the statement of Mallawany’s that the halogen substitution lowered the density [
9]. Even the addition of ZnCl into the oxychloride glass system (TeO
2-ZnO-ZnCl) also lowered the densities of the TeO
2-ZnO glass system [
10].
Figure 3.
Variation of density of AlF3-ZnO-TeO2 glasses. The line is drawn to guide the eye.
Figure 3.
Variation of density of AlF3-ZnO-TeO2 glasses. The line is drawn to guide the eye.
Table 1.
Glass composition, density, molar volume, molecular weight, longitudinal and shear ultrasonic wave velocities of (AlF3)x-(ZnO)y-(TeO2)z glasses. The pure TeO2 glass is included for comparison.
Table 1.
Glass composition, density, molar volume, molecular weight, longitudinal and shear ultrasonic wave velocities of (AlF3)x-(ZnO)y-(TeO2)z glasses. The pure TeO2 glass is included for comparison.
| Glass sample | Composition (mol%) | Density (kg·m−3) | Molar volume (cm3/mol) | Molar weight (g/mol) | Vl | Vs |
|---|
| AlF3 x | ZnOy | TeO2z | (m s−1) |
|---|
| Pure | 0 | 0 | 100 | 4806 | 33.21 | 159.61 | 3435 |
| A1 | 0 | 10 | 90 | 5098 | 29.77 | 151.77 | 3324 |
| A2 | 1 | 9 | 90 | 5023 | 30.22 | 151.80 | 3316 |
| A3 | 3 | 7 | 90 | 5018 | 30.26 | 151.84 | 3364 |
| A4 | 5 | 5 | 90 | 4963 | 30.61 | 151.92 | 3393 |
| A5 | 7 | 3 | 90 | 4846 | 31.36 | 151.97 | 3424 |
| A6 | 9 | 1 | 90 | 4779 | 31.81 | 152.02 | 3435 |
| B1 | 0 | 15 | 85 | 5102 | 28.98 | 147.86 | 3307 |
| B2 | 1 | 14 | 85 | 5075 | 29.14 | 147.89 | 3334 |
| B3 | 5 | 10 | 85 | 4990 | 29.66 | 148.00 | 3409 |
| B4 | 8 | 7 | 85 | 4898 | 30.23 | 148.07 | 3480 |
| B5 | 12 | 3 | 85 | 4799 | 30.88 | 148.19 | 3486 |
| B6 | 14 | 1 | 85 | 4756 | 31.65 | 150.53 | 3488 |
| C1 | 0 | 20 | 80 | 5136 | 28.03 | 143.96 | 3296 |
| C2 | 2 | 18 | 80 | 5124 | 28.1 | 143.98 | 3353 |
| C3 | 5 | 15 | 80 | 5074 | 28.4 | 144.10 | 3398 |
| C4 | 10 | 10 | 80 | 4950 | 28.33 | 140.23 | 3471 |
| C5 | 15 | 5 | 80 | 4792 | 28.4 | 136.09 | 3528 |
| C6 | 19 | 1 | 80 | 4743 | 30.45 | 144.42 | 3542 |
The densities decrease gradually with the addition of AlF
3 content. In this case, as more AlF
3 content are added into the zinc tellurite glass system, two Zn-F single bonds will replace the Zn=O double bonds, hence the structure of the glasses becomes loose, which results in a decrease in density. Fluorine plays an important role in the glass matrix; replacing the O
2− ions causes the compositional dependence of the density and molar volume [
11]. The molecular weight of TeO
2 is 159.599 which is greater than AlF
3 (83.977) and ZnO (81.408). Whereas the atomic weight of individual constituent atom Te (127.6) > Zn (65.39) > Al (26.9815) > F (18.998) > O (15.9999), the atomic radius of each of the constituent atoms (in ppm) is Al (143) > Te (140) > Zn (134) > O (66) > F (64).
The slight increase in molar volumes is due to the rearrangement of the lattice and a decrease in the porosity of the glass. The increase in molar volumes for all series in ternary glass systems as shown
Table 1 is related to a decrease in the bond length or inter-atomic spacing between the atoms. The radius of Te
2+ (0.097 nm) is much greater than that of Zn
2+ (0.074 nm) and since the radius of Al
3+ is 0.0535 nm which also has a smaller radius than Zn
2+ attributed to this situation.
The molar volumes for AlF3-ZnO-TeO2 results show slightly lower values than that of the pure tellurite glasses. The much higher reduction in the molar volume for the sample containing fluorine is attributed to the decrease in viscosity due to the breaking of the Te-O-Te bond to form two Te-F bonds, which increase the efficiency of the crystallization process [
12].
The addition of AlF3 that consists of Al3+ and F will modify the glass structure by creating NBOs in the oxyflorotellurite glass system. The NBOs created were believed to alter the glass structure in a way that packing of the molecule becomes denser as more network modifier ions (in this case Al3+), attempt to occupy the interstices within the network.
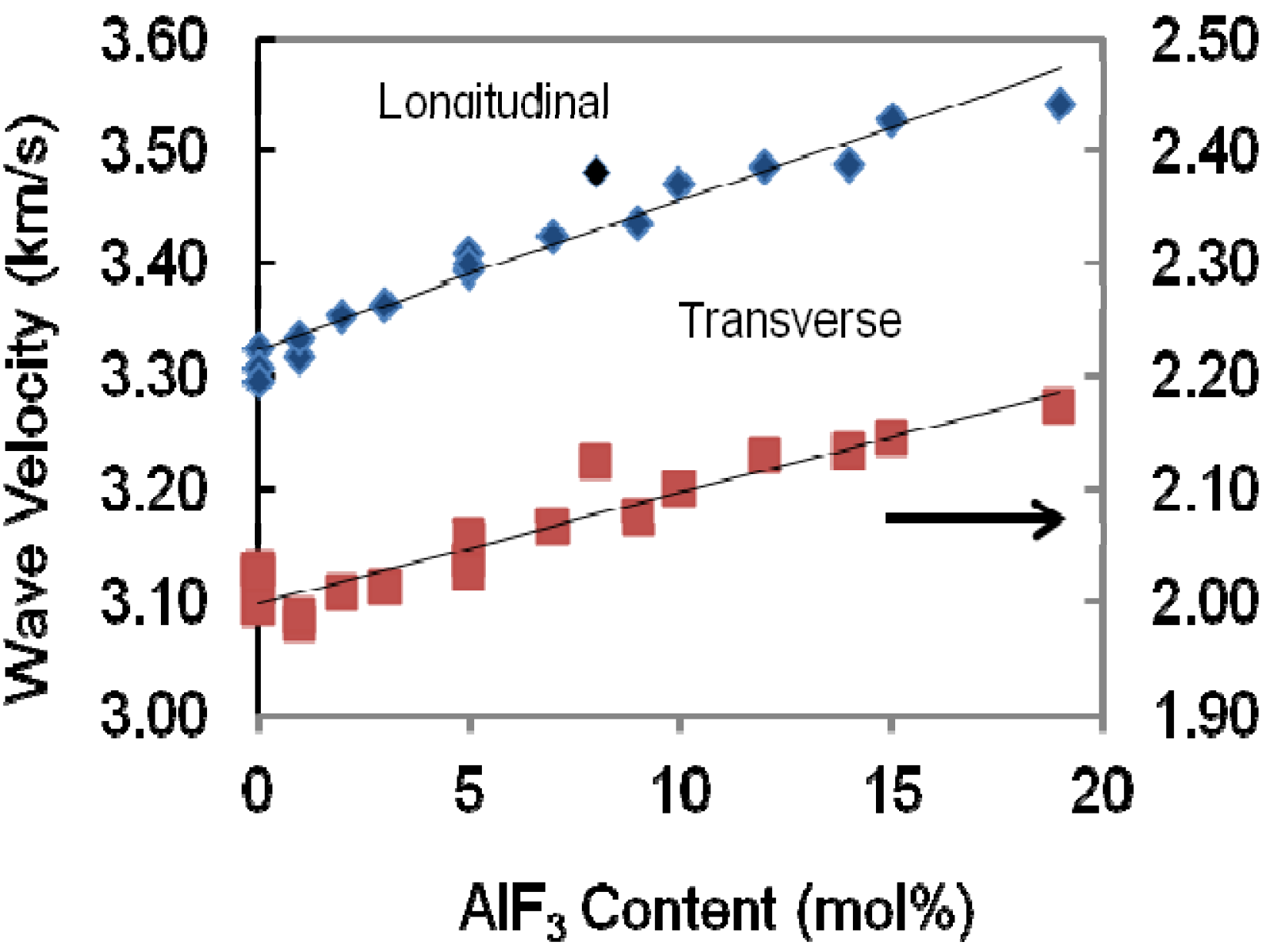
The variation of both longitudinal and shear wave velocities that propagated in the present bulk samples depends on the structural change of the glass network. The longitudinal and shear ultrasonic velocities in ternary glass TeO
2-ZnO-AlF
3 are depicted in
Figure 4 and presented in
Table 1 for different mole fractions of AlF
3 content. It can be seen that in the glasses studied, both longitudinal and shear ultrasonic velocities increase when more AlF
3 is added into the ZnO-TeO
2 glass system.
Figure 4.
Variation of longitudinal and transverse wave velocities propagated in the AlF3-ZnO-TeO2 glasses.
Figure 4.
Variation of longitudinal and transverse wave velocities propagated in the AlF3-ZnO-TeO2 glasses.
The percentage increase of velocity can be seen in
Table 2. The percentage of decrement in velocity increases with the increasing substitution of AlF
3 content. Substituting more AlF
3 content to ZnO content means that the difference between maximum velocity and minimum velocity is higher for both longitudinal and transverse or shear velocity. The addition of AlF
3 content increase both wave velocities propagated in glass, and hence the elastic stiffness is also increased [
13] as shown in
Figure 5.
Table 2.
The elastic moduli, Young’s modulus (E), longitudinal modulus (L), shear modulus (G), bulk modulus (K) and Poisson’s ratio () of ternary zinc tellurite glass.
Table 2.
The elastic moduli, Young’s modulus (E), longitudinal modulus (L), shear modulus (G), bulk modulus (K) and Poisson’s ratio () of ternary zinc tellurite glass.
| Glass sample | Elastic moduli (GPa) | | d = 4G/K |
|---|
| L | G | E | K | H |
|---|
| Pure | 56.71 | 21.50 | 51.37 | 28.04 | 4.38 | 0.19 | 3.07 |
| A1 | 56.33 | 21.01 | 50.53 | 28.32 | 4.17 | 0.20 | 2.97 |
| A2 | 55.23 | 19.67 | 48.13 | 29.00 | 3.63 | 0.22 | 2.71 |
| A3 | 56.79 | 20.33 | 49.66 | 29.67 | 3.78 | 0.22 | 2.74 |
| A4 | 57.14 | 20.61 | 50.21 | 29.65 | 3.88 | 0.22 | 2.78 |
| A5 | 56.81 | 20.72 | 50.27 | 29.18 | 3.97 | 0.21 | 2.84 |
| A6 | 56.39 | 20.58 | 49.91 | 28.95 | 3.94 | 0.21 | 2.84 |
| B1 | 55.80 | 20.90 | 50.18 | 27.93 | 4.17 | 0.20 | 2.99 |
| B2 | 56.41 | 20.04 | 49.07 | 29.70 | 3.68 | 0.22 | 2.70 |
| B3 | 57.99 | 21.11 | 51.25 | 29.84 | 4.03 | 0.21 | 2.83 |
| B4 | 59.32 | 22.08 | 53.14 | 29.88 | 4.36 | 0.20 | 2.96 |
| B5 | 58.32 | 21.75 | 52.32 | 29.32 | 4.31 | 0.20 | 2.97 |
| B6 | 57.86 | 21.66 | 52.02 | 28.98 | 4.32 | 0.20 | 2.99 |
| C1 | 55.80 | 20.44 | 49.51 | 28.54 | 3.94 | 0.21 | 2.86 |
| C2 | 57.61 | 20.68 | 50.46 | 30.03 | 3.86 | 0.22 | 2.75 |
| C3 | 58.59 | 20.83 | 50.99 | 30.82 | 3.83 | 0.22 | 2.70 |
| C4 | 59.64 | 21.83 | 52.88 | 30.53 | 4.20 | 0.21 | 2.86 |
| C5 | 59.64 | 22.05 | 53.21 | 30.25 | 4.31 | 0.21 | 2.92 |
| C6 | 59.50 | 22.42 | 53.70 | 29.62 | 4.52 | 0.20 | 3.03 |
Figure 5.
Variation of elastic moduli in the AlF3-ZnO-TeO2 glasses. The line is drawn to guide the eye.
Figure 5.
Variation of elastic moduli in the AlF3-ZnO-TeO2 glasses. The line is drawn to guide the eye.
As a small quantity of AlF
3 is added into the TeO
2-ZnO glass network, a breaking of Te-O-Te takes place. The conversion of this linkage results in depolymerization of the network leading to the formation of Te-
axO
eq-Te bridges with the appearance of non-bridging oxygen (NBO) [
14]. Therefore, the continuous breaking of Te-O-Te linkages with the addition of AlF
3 content leads to the loose packing of the glass network and hence, a decrease in density (
Figure 3). The observed results can be further substantiated by revealing the composition dependent on ultrasonic parameters.
It is inferred that a decrease in Te coordination number (N) has resulted with the increase in the modifier content [
15]. Further, a decrease in the coordination number results in a slight decrease in the mean Te-O bond length (R) [
16]. Thus, it is inferred that the observed continuous increase in sound velocity in the present glasses is due to the change in coordination number with the substitution of AlF
3.
The introduction of fluorine into TeO
2-based glass system results in a reduction of Te-O-Te linkages due to a gradual transformation of trigonal bipyramid TeO
4 (tbp) through TeO
3+1 to trigonal pyramid TeO
3, decreasing the connectivity of the tellurite glass former network [
17]. This behavior is strengthened by the higher concentration of F
− ions.
Elastic properties (longitudinal, transverse/shear, bulk and Young’s moduli, Debye temperature and Poisson’s ratio) of the present glasses have been determined from the measured ultrasonic velocities and densities.
Table 2 gives experimental values of the elastic moduli: Young’s modulus (E), longitudinal modulus (L), shear modulus (G), bulk modulus (K) and Poisson’s ratio (
).
Similarly, all the elastic moduli show a monotonic increase as that of velocities, with an incremental increase of AlF3 into the ZnO-TeO2 glass system for every series. For series A, the Young’s modulus increases from 48.13 to 49.91 GPa. This pattern is applied to the rest of the glass series. The additional increase of AlF3 results in higher network rigidity, which in turns results in an increase of the longitudinal and shear modulus. The increase in shear modulus, G, and bulk modulus, K, is due to the stronger tendency of the change in the coordination number with increasing AlF3 content.
In ternary glasses both the degree of cross-linking and the relative proportions of different types of bonds may be changed with composition. The existence of AlF3 and the nature of TeO2 not only cause an increase in the elastic moduli, but also an increase in Poisson’s ratio.
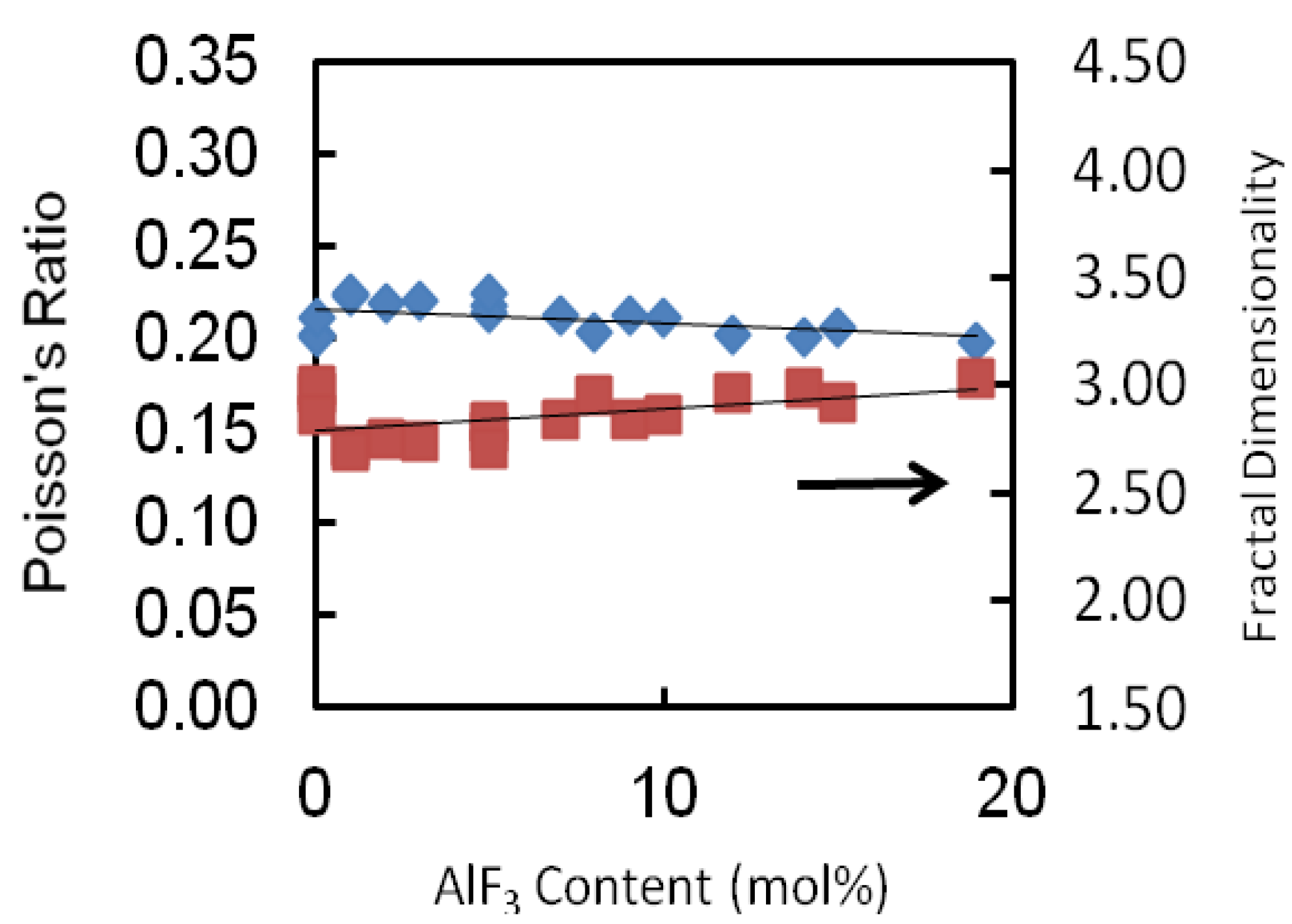
The values of Poisson’s ratio of each glass series is tabulated in
Table 2 and depicted in
Figure 6. The Poisson’s ratio shows a monotonic decrease as the AlF
3 content increases for all glass series. For this glass series as an example, the Poisson’s ratio increases from 0.223 to 0.213. The values of the Poisson’s ratio are of the order 0.2 which reveals a high cross-link density [
18,
19]. For all series of glass, the transformation of cross-linkage is negligibly small (changes of about 0.02), almost remaining constant.
Figure 6.
Poisson ratio and fractal dimensionality of AlF3-ZnO-TeO2 glasses. The line is drawn to guide the eye.
Figure 6.
Poisson ratio and fractal dimensionality of AlF3-ZnO-TeO2 glasses. The line is drawn to guide the eye.
Table 2 collects the calculated values of microhardness. It can be seen that the microhardness of the studied glass samples, except for Series A, increases with the addition of AlF
3 content. As an example, for Series B, the microhardness increases from 4.17 to 4.32 GPa. The rest of glass samples show the same trend. It also indicates a strengthening in the glass structure which may be due to the introduction of stronger ionic bonds in the glass network [
19,
20].
The shear to bulk modulus ratio of oxyfluorotellurite glasses at room temperature is given in
Table 2. The
G/
K tends to decrease with increasing AlF
3 content: consistent with the loss or weakening of cross-link between TeO
2 chains. In this study, the fractal dimensionality, (
d) of these glasses ranges between 2.70 and 3.03. These suggest an intermediate connectivity in a three-dimensional disordered network, which implies a marked degree of cross-linkage between TeO
2 chains, as would be anticipated for modifier cations whose valency is greater than unity, or increased branching of the network of TeO
2 chains facilitated by an increase in the number of the end and branching units incorporated into the basic tellurite network.
All the current experimental data were analyzed using Microsoft Excel, by fitting regression curves, with the results of the regression coefficients presented in
Table 3. The regression coefficients obtained from each curve is shown in
Figure 3,
Figure 4,
Figure 5 and
Figure 6. In
Table 3,
stands for the variables shown in the first column and
is the ZnO concentration. As can be seen in previous figures, for most of the variables, a linear curve (
=
+
) gives the best fit. Except for bulk modulus, Poisson’s ratio and fractal dimensionality, d, the R
2 values lie between 0.527 to 0.9384.
Table 3.
Linear regression analysis of the variables ( stands for the variables shown in the first column and is the ZnO concentration. As can be seen in previous figures, for most of the variables, a linear curve ( = + ) for various properties of glass (density; longitudinal and shear ultrasonic velocities, vl, vs; elastic moduli, L, G, K, E; Poisson’s ratio, ; and fractal dimensionality, d).
Table 3.
Linear regression analysis of the variables ( stands for the variables shown in the first column and is the ZnO concentration. As can be seen in previous figures, for most of the variables, a linear curve ( = + ) for various properties of glass (density; longitudinal and shear ultrasonic velocities, vl, vs; elastic moduli, L, G, K, E; Poisson’s ratio, ; and fractal dimensionality, d).
| Variables () | | | R2 | % change |
|---|
| Density, | −0.022 | 5.095 | 0.832 | −8.286 |
| Longitudinal Wave Velocity, VL | 0.013 | 3.323 | 0.938 | +7.464 |
| Transverse Wave, VS | 0.010 | 1.998 | 0.894 | +9.853 |
| Longitudinal Modulus, L | 0.189 | 56.294 | 0.578 | +7.984 |
| Shear Modulus, G | 0.1079 | 20.348 | 0.651 | +13.98 |
| Young’s Modulus, E | 0.225 | 49.52 | 0.668 | +11.573 |
| Bulk Modulus, K | 0.046 | 29.163 | 0.120 | +10.347 |
| Poisson’s Ratio, | −0.001 | 0.217 | 0.275 | −9.998 |
| Fractal Dimensionality, d | 0.01 | 2.794 | 0.275 | +12.222 |
The overall results from
Table 3 show that the addition of AlF
3 less than 19 mol% into the zinc tellurite glass system causes a small effect (less than 12%) on their physical properties as well as their elastic properties. However, it is difficult to completely separate the effects of increasing the AlF
3 content and decreasing the ZnO content from the observed changes in glass properties.
3. Experimental Procedure
All glass samples were prepared by rapid melting quenching method. The ternary system consists of (TeO
2)
x (ZnO)
y (AlF
3)
z with x, y and z being the mole fractions. The aluminum fluoride, AlF
3, and zinc oxide, ZnO, act as a glass modifier, while tellurite, TeO
2 is a glass former.
Table 1 shows the initial composition (mol%): density, molar volume, molecular weight, longitudinal and shear ultrasonic wave velocities of (AlF3)
x-(ZnO)
y-(TeO2)
z glasses.
All the glass samples were prepared from commercial powders by mixing the specific weights of batches using tellurium (IV) oxide, TeO
2 (Technical grade, Alfa Aesar, Ward Hill, MA, USA), zinc oxide, ZnO (99.99%, Assay, Alfa Aesar, Ward Hill, MA, USA) and aluminum fluoride, AlF
3 (97%, Assay, Alfa Aesar, Ward Hill, MA, USA). Those starting materials were weighed using an electronic balance to obtain 20 g batches and mixed in the alumina crucible. Their initial glass compositions are presented in
Table 1.
For the premelting process, a lidded alumina crucible which contained well mixed 20 g batches were then preheated in a preliminary furnace to 400 °C for 30 minutes. This process was carried out to evaporate water vapor and remove gases in the mixture and to allow the tellurite to decompose and react with other batch constituents before the melting process occurs [
21]. The mixture of starting materials was then transferred into a second electric furnace and kept at 750 °C–800 °C for 1 hour. During the process, the crucible was slightly shaken using several times using a metal holder to ensure homogeneity and proper mixing. All the melting processes were performed using an electric furnace manufactured by Lindberg, Thermolyne and Bole.
After the melting process, each melt was rapidly quenched into a cylindrical stainless steel split mould which had been preheated to 400 °C for the glass casting process. The samples formed a glass rod of 2.0 cm height and 1.1 cm diameter. The reason to maintain the mould at this temperature is to relieve the mechanical stress in the glass sample. Then, the mould halves were released to prevent cracking [
19]. Each glass sample was then annealed at 350 °C for about an hour before the furnace was switched off. The glass samples were allowed to cool down
in situ to room temperature for a day.
All the ternary glasses were free of bubbles, of exceptional quality and yellow in color. These samples were then cut into the required dimension using an Isomet Low Speed Saw machine (Buehler). The thickness of the glass sample was taken five times for each measurement. The glass samples were then ground using various grades of sand paper. The grades used were 150, 300, 600, 1000, 1200, 2000 and 2700. This was done to obtain parallel, smooth and clear surfaces for each glass sample. There were three series of zinc oxyfluoride tellurite glass system, namely A, B and C, where each series consisted of six samples as shown in
Table 1.
The densities (ρ) of the glasses were determined by the Archimedes method with acetone as buoyant liquid [
22]. All the glass samples’ weights were measured with a digital balance (±0.0001 g accuracy). Their molar volume was calculated from the molecular weight (
M) and density (ρ). The accuracy in the measurement of the density is ±0.01 g·cm
−3 and the relative error is ±0.05%.
The chemically estimated elemental composition values present in the glass samples with the Atomic Absorption Studies (Perkin-Elmer, Model 1372, Waltham, MA, USA) were found to be slightly smaller than the corresponding elemental nominal composition values (before the glass formation), which we considered to be due to evaporation losses and uncertainties in the chemical analysis.
All the glass samples were checked by X-ray diffraction for their amorphous nature using an X’Pert Pro Panalytical PW 3040 MPD X-ray powder diffractometer by employing Cr-Ka radiation.
For the measurements of ultrasonic velocity in each glass sample, the samples were shaped into a circular disc of 12 mm diameter and 10–12 mm thickness. The opposite faces of the disc shaped glass samples were highly polished using ultra fine lapping papers to achieve a good surface finish with plane parallelism having an accuracy of ±5 micron.
Ultrasonic velocity measurements were carried out at a frequency of 10 MHz using x-cut and y-cut quartz transducers. A pulse superposition technique was employed using Ultrasonic Data Acquisition System (MATEC 8020, Matec Instruments, Northborough, MA, USA) [
23]. Burnt honey was used as a bonding material between the glass samples and transducers. By measuring the thickness of the sample (
d), longitudinal (
Vl) and transverse (
Vt) wave velocities were calculated using the relationship,
V = 2
d/
t [
24]. The absolute accuracy in the measurement of the velocity is ±5 ms
−1 and the relative error is ±0.1%. Glasses are isotropic and have only two independent elastic constants,
L and
G, which can be obtained from their longitudinal and shear sound wave velocities and densities. The various elastic properties of the glasses were calculated using the following standard relations [
25]: