Nanocrystalline Akaganeite as Adsorbent for Surfactant Removal from Aqueous Solutions
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
| Class | Head group | Main applications |
|---|---|---|
| Anionic | –COO−Na | Soaps |
| –SO3−Na | Synthetic detergent | |
| –OSO3−Na | Detergents, personal care products | |
| –OPO3−Na | Corrosion inhibitors, emulsifiers | |
| –(OCH2CH2) nOSO3−Na | Liquid detergents, toiletries, emulsifiers | |
| Cationic | –N(CH3)3+Cl− | Bitumen emulsions |
| >N(CH3)2+Cl− | Fabric and hair conditioners | |
| Zwitterionic | –N+(CH3)2CH2COO+ | Shampoos, cosmetics |
| –N+(CH3)2CH2SO3+ | ||
| Nonionic | –(OCH2CH2) nOH | Detergents, emulsifiers |
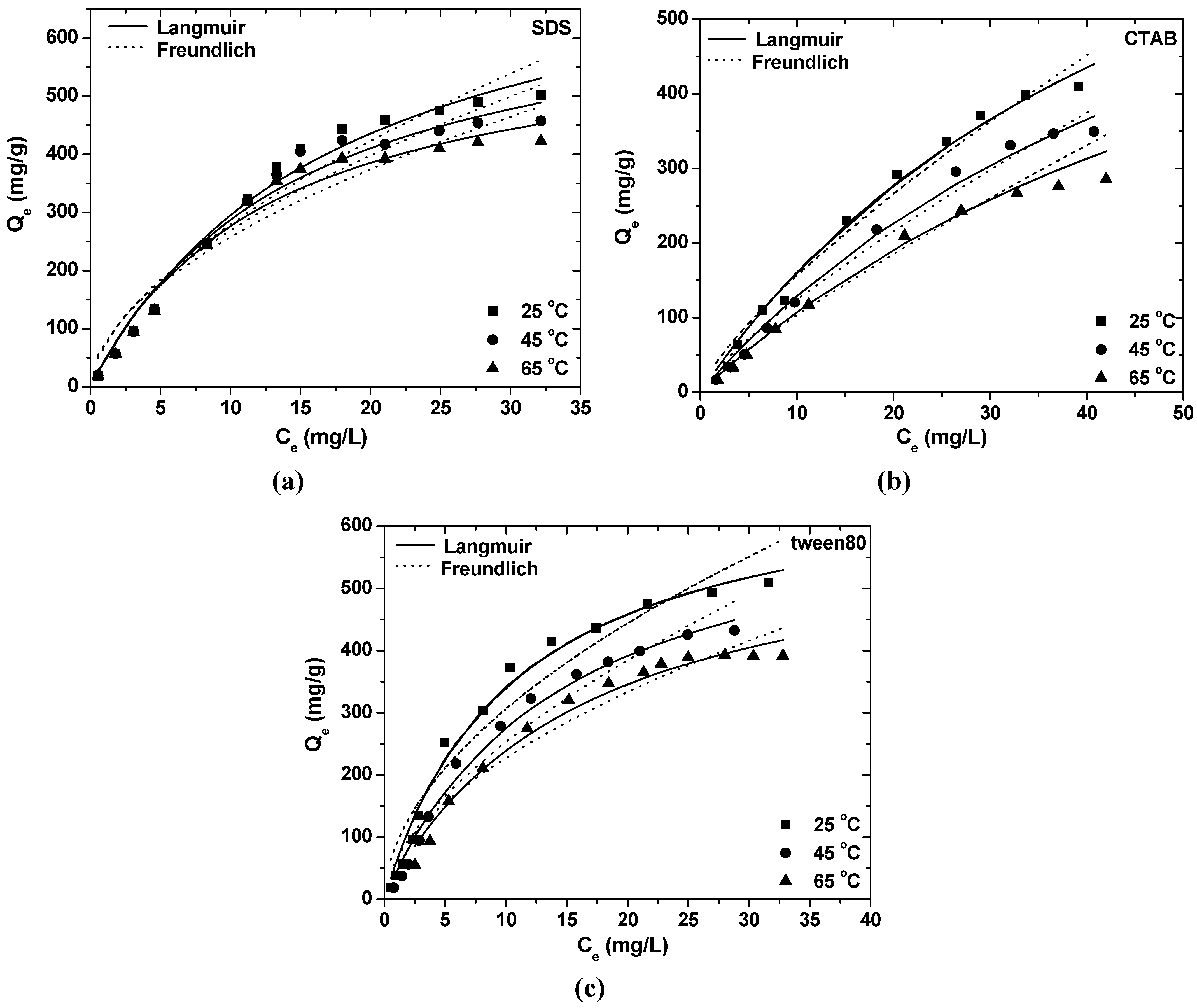
| Surfactant | θ (°C) | Langmuir constants | Freundlich constants | ||||
|---|---|---|---|---|---|---|---|
| R2 (–) | Qmax (mg/g) | KL (L/mg) | R2 (–) | KF (mg1−(1/n) L1/n g−1) | n (–) | ||
| SDS | 25 | 0.983 | 823.96 | 0.056 | 0.950 | 70.74 | 1.67 |
| 45 | 0.976 | 710.49 | 0.069 | 0.933 | 74.31 | 1.78 | |
| 65 | 0.975 | 631.06 | 0.079 | 0.925 | 75.45 | 1.87 | |
| CTAB | 25 | 0.992 | 1007.93 | 0.019 | 0.981 | 27.25 | 1.32 |
| 45 | 0.992 | 935.21 | 0.016 | 0.983 | 19.93 | 1.26 | |
| 65 | 0.996 | 867.44 | 0.014 | 0.991 | 15.09 | 1.19 | |
| tween80 | 25 | 0.989 | 699.03 | 0.096 | 0.945 | 89.86 | 1.87 |
| 45 | 0.989 | 671.38 | 0.070 | 0.958 | 64.71 | 1.66 | |
| 65 | 0.981 | 613.01 | 0.065 | 0.939 | 63.45 | 1.83 | |
| Adsorbent | θ (°C) | lnK0 (–) | ΔG0 (kJ/mol) | ΔH0 (kJ/mo)l | ΔS0 (kJ/mol K) | ΔHx (kJ/mol) |
|---|---|---|---|---|---|---|
| SDS | 25 | 3.839 | –9.511 | –7.170 | 0.0079 | –7.05 |
| 45 | 3.681 | –9.732 | ||||
| 65 | 3.497 | –9.828 | ||||
| CTAB | 25 | 2.919 | –7.234 | –9.564 | 0.0081 | –9.68 |
| 45 | 2.597 | –6.868 | ||||
| 65 | 2.459 | –6.912 | ||||
| tween80 | 25 | 4.983 | –12.346 | –8.048 | 0.0366 | –7.00 |
| 45 | 4.160 | –10.994 | ||||
| 65 | 3.872 | –10.882 |
| Adsorbent | Surfactant | Qmax (mg/g) | Qmax (mg/g m2) | Reference |
|---|---|---|---|---|
| akaganeite | SDS | 823.96 | 2.50 | this study |
| sand | SDS | 1.31 | – | [39] |
| Ca-montmorillonite | SDS | 7.00 | 0.92 | [40] |
| activated carbon | SDS | 271 | 0.32 | [41] |
| carbon | SDS | 55.68 | 0.58 | [42] |
| paper fiber | SDS | 0.30 | 0.20 | [42] |
| akaganeite | CTAB | 1007.93 | 3.05 | this study |
| perlite | CTAB | 38.40 | 16.70 | [43] |
| powdered activated carbon | CTAB | 400.90 | 0.86 | [44] |
| silica gel waste | CTAB | 73.85 | 0.28 | [45] |
| akaganeite | tween80 | 699.03 | 2.12 | this study |
4. Conclusions
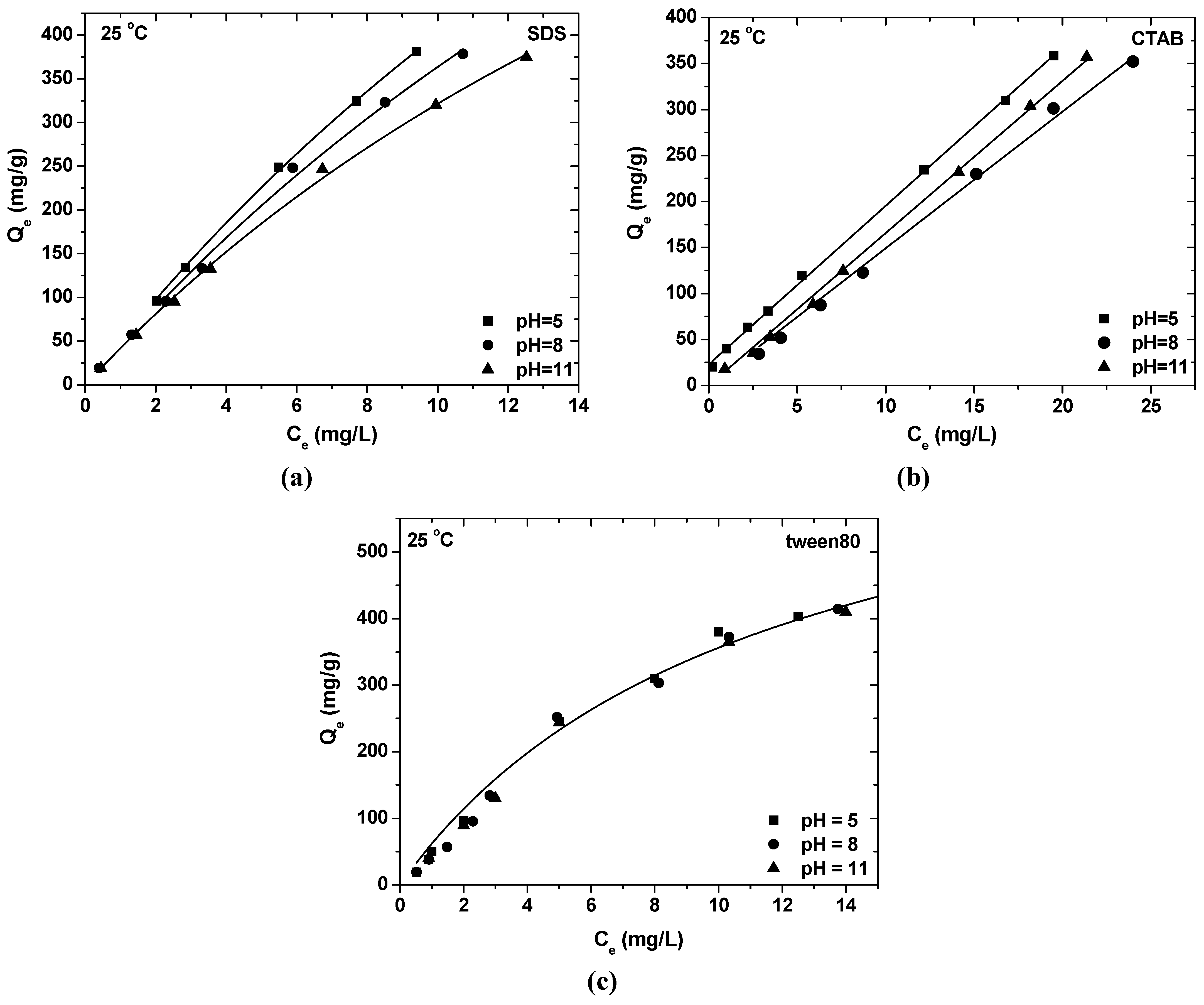
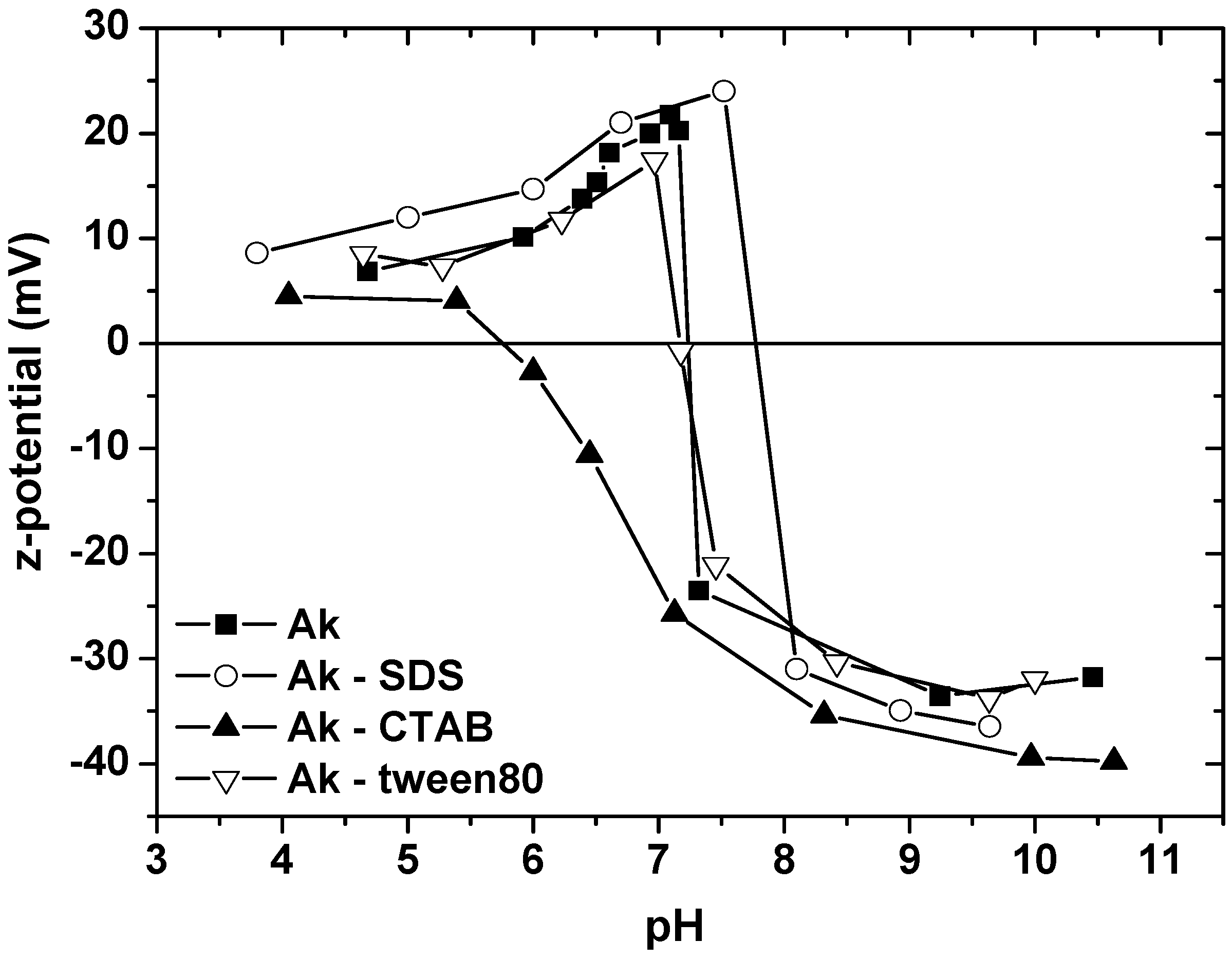
- The maximum SDS adsorption occurs at the lowest pH value, due to the fact that in this pH range, the surface of Ak is positively charged and electrostatically attracts the negatively charged –SO42− surfactant headgroups. The opposite is observed in the case of CTAB, where the highest pH enforces the adsorption. At this pH range, the surface of akaganeite is negatively charged and electrostatically attracts the positively charged –NH4+ surfactant headgroups. In the case of tween80, the change of pH value has no reflection on surfactant sorption onto Ak due to the absence of polar groups of the surfactant.
- Equilibrium data could be described by Freundlich and Langmuir isotherms. The maximum adsorption capacity at 25 °C and pH = 8 was found to be 823.96 mg/g for SDS, 1007.93 mg/g for CTAB and 699.03 mg/g for tween80.
- The negative values of ΔG0 for all surfactants suggest that the process is spontaneous with the high preference of surfactants for Ak. The negative values of ΔH0 for all surfactants (SDS, −7.170 kJ/mol; CTAB, −9.564 kJ/mol; tween80, −8.048 kJ/mol) suggest the exothermic nature of the process. The positive values of ΔS0 (SDS, 0.0079 kJ/mol; CTAB, 0.0081 kJ/mol; tween80, 0.0366 kJ/mol) indicated an increased randomness. The negative values of ΔHx (SDS, −7.05 kJ/mol; CTAB, −9.68 kJ/mol; tween80, −7.00 kJ/mol) suggest also the exothermic nature of the process.
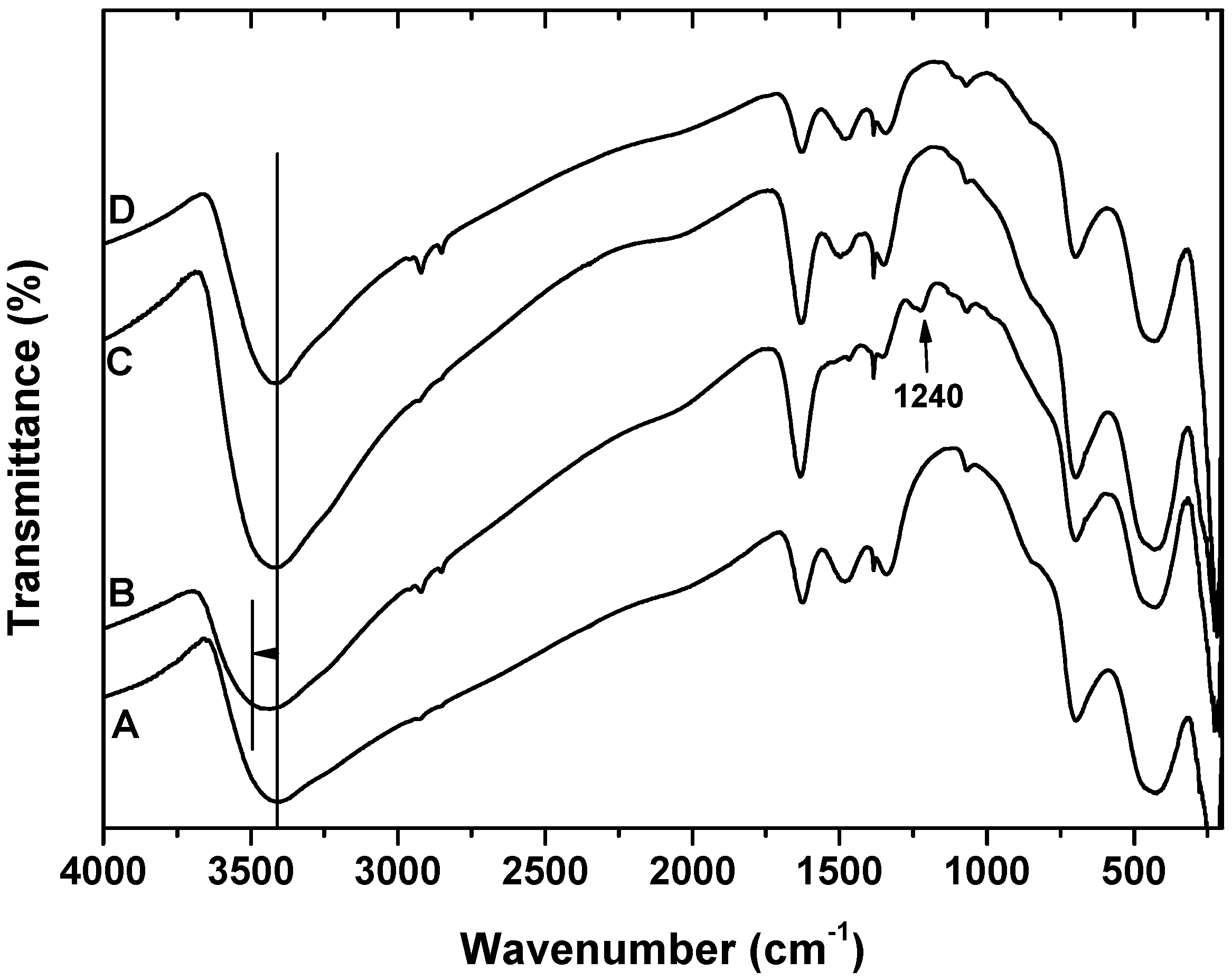
- FTIR measurements established that surfactants are adsorbed on the surface of akaganeite, replacing adsorbed water.
Acknowledgments
References
- Majewska-Nowak, K.; Kowalska, I.; Kabsch-Korbutowicz, M. Ultrafiltration of SDS solutions using polymeric membranes. Desalination 2005, 184, 415–422. [Google Scholar] [CrossRef]
- Adak, A.; Bandyopadhyay, M.; Pal, A. Removal of anionic surfactant from wastewater by alumina: A case study. Colloids Surf. A 2005, 254, 165–171. [Google Scholar] [CrossRef]
- Kyzas, G.Z. A decolorization technique with spent “Greek coffee” grounds as zero-cost adsorbents for industrial textile wastewaters. Materials 2012, 5, 2069–2087. [Google Scholar] [CrossRef]
- Kyzas, G.Z. Commercial coffee wastes as materials for adsorption of heavy metals from aqueous solutions. Materials 2012, 5, 1826–1840. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Vassiliou, A.A.; Lazaridis, N.K. Treatment of real effluents from dyeing reactor: Experimental and modeling approach by adsorption onto chitosan. Chem. Eng. J. 2011, 168, 577–585. [Google Scholar] [CrossRef]
- Dirilgen, N.; Ince, N. Inhibition effect of the anionic surfactant SDS on duckweed, Lemna minor with considerations of growth and accumulation. Chemosphere 1995, 31, 4185–4196. [Google Scholar] [CrossRef]
- Pettersson, A.; Adamsson, M.; Dave, G. Toxicity and detoxification of Swedish detergents and softener products. Chemosphere 2000, 41, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Önder, E.; Koparal, A.S.; Öğütveren, U.B. An alternative method for the removal of surfactants from water: Electrochemical coagulation. Sep. Purif. Technol. 2007, 52, 527–532. [Google Scholar] [CrossRef]
- Basar, C.A.; Karagunduz, A.; Cakici, A.; Keskinler, B. Removal of surfactants by powdered activated carbon and microfiltration. Water Res. 2004, 38, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Pavan, P.C.; Crepaldi, E.L.; Valim, J.B. Sorption of anionic surfactants on layered double hydroxides. J. Colloid Interface Sci. 2000, 229, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Delgado, R.A.; Cotoruelo, L.M.; Rodriguez, J.J. Adsorption of anionic surfactant mixtures by polymeric resins. Sep. Sci. Technol. 1992, 27, 1065–1076. [Google Scholar] [CrossRef]
- Alkan, M.; Karadaş, M.; Doğan, M.; Demirbaş, Ö. Adsorption of CTAB onto perlite samples from aqueous solutions. J. Colloid Interface Sci. 2005, 291, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.M. On the removal of cationic surfactants from dilute streams by granular charcoal. Water Res. 2006, 40, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Zareen, U. Sand sorption process for the removal of sodium dodecyl sulfate (anionic surfactant) from water. J. Hazard. Mater. 2006, 133, 269–275. [Google Scholar] [CrossRef]
- Ariapad, A.; Zanjanchi, M.A.; Arvand, M. Efficient removal of anionic surfactant using partial template-containing MCM-41. Desalination 2012, 284, 142–149. [Google Scholar] [CrossRef]
- Pal, A.; Pan, S.; Saha, S. Synergistically improved adsorption of anionic surfactant and crystal violet on chitosan hydrogel beads. Chem. Eng. J. 2013, 217, 426–434. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Bakoyannakis, D.N.; Zouboulis, A.I.; Matis, K.A. Sorption of As(V) ions by akaganéite-type nanocrystals. Chemosphere 2003, 50, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Deliyanni, E.A.; Peleka, E.N.; Lazaridis, N.K. Comparative study of phosphates removal from aqueous solutions by nanocrystalline akaganéite and hybrid surfactant-akaganéite. Sep. Purif. Technol. 2007, 52, 478–486. [Google Scholar] [CrossRef]
- Lazaridis, N.K.; Bakoyannakis, D.N.; Deliyanni, E.A. Chromium(VI) sorptive removal from aqueous solutions by nanocrystalline akaganèite. Chemosphere 2005, 58, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Deliyanni, E.A.; Matis, K.A. Sorption of Cd ions onto akaganéite-type nanocrystals. Sep. Purif. Technol. 2005, 45, 96–102. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Peleka, E.N.; Matis, K.A. Removal of zinc ion from water by sorption onto iron-based nanoadsorbent. J. Hazard. Mater. 2007, 141, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Deliyanni, E.A.; Bakoyannakis, D.N.; Zouboulis, A.I.; Peleka, E. Removal of arsenic and cadmium by akaganeite fixed-beds. Sep. Sci. Technol. 2003, 38, 3967–3981. [Google Scholar] [CrossRef]
- Ståhl, K.; Nielsen, K.; Jiang, J.; Lebech, B.; Hanson, J.C.; Norby, P.; van Lanschot, J. On the akaganéite crystal structure, phase transformations and possible role in post-excavational corrosion of iron artifacts. Corros. Sci. 2003, 45, 2563–2575. [Google Scholar] [CrossRef]
- Mackay, A.L. β-ferric oxyhydroxide. Mineral. Mag. 1960, 32, 545–557. [Google Scholar] [CrossRef]
- Mackay, A.L. β-ferric oxyhydroxide—Akaganeite. Mineral. Mag. 1962, 34, 270–279. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Lazaridis, N.K.; Matis, K.A. Arsenates sorption by nanocrystalline hybrid surfactant-akaganéite. Sep. Sci. Technol. 2012, 47, 2331–2339. [Google Scholar]
- Deliyanni, E.A.; Peleka, E.N.; Matis, K.A. Effect of cationic surfactant on the adsorption of arsenites onto akaganeite nanocrystals. Sep. Sci. Technol. 2007, 42, 993–1012. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Nalbandian, L.; Matis, K.A. Adsorptive removal of arsenites by a nanocrystalline hybrid surfactant-akaganeite sorbent. J. Colloid Interface Sci. 2006, 302, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Deliyanni, E.A.; Peleka, E.N.; Matis, K.A. Modeling the sorption of metal ions from aqueous solution by iron-based adsorbents. J. Hazard. Mater. 2009, 172, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Deliyanni, E.A.; Bakoyannakis, D.N.; Zouboulis, A.I.; Matis, K.A.; Nalbandian, L. Akaganéite-type β-FeO(OH) nanocrystals: Preparation and characterization. Microporous Mesoporous Mater. 2001, 42, 49–57. [Google Scholar] [CrossRef]
- ASTM Standard D3049. In Standard Test Method for Synthetic Anionic Ingredient by Cationic Titration; ASTM International: West Conshohocken, PA, USA, 2009.
- ISO 2271:1989. In Surface Active Agents—Detergents—Determination of Anionic-Active Matter, Direct Two-Phase Titration Procedure; International Organization for Standardization: Geneva, Switzerland, 1989.
- Cross, J. Nonionic Surfactants: Chemical Analysis; Marcel Dekker: New York, NY, USA, 1987. [Google Scholar]
- Clint, J.H. Nature of Surfactants. Surfactant Aggregation; Chapman and Hall: New York, NY, USA, 1992. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Corkill, J.M.; Goodman, J.F.; Tate, J.R. Adsorption of non-ionic surface-active agents at the graphon/solution interface. Trans. Faraday Soc. 1966, 62, 979–986. [Google Scholar] [CrossRef]
- Smith, J.M.; van Ness, H.C. Introduction to Chemical Engineering Thermodynamics, 4th ed.; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 3973–3993. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compound, 3rd ed.; John Wiley: New York, NY, USA, 1978. [Google Scholar]
- Khan, Y.; Durrani, S.K.; Siddique, M.; Mehmood, M. Hydrothermal synthesis of alpha Fe2O3 nanoparticles capped by Tween-80. Mater. Lett. 2011, 65, 2224–2227. [Google Scholar] [CrossRef]
- Sritapunya, T.; Jairakdee, S.; Kornprapakul, T.; Somabutr, S.; Siemanond, K.; Bunyakiat, K.; Kitiyanan, B.; Scamehorn, J.F.; Grady, B.P.; Chavadej, S. Adsorption of surfactants on carbon black and paper fiber in the presence of calcium ions. Colloids Surf. A 2011, 389, 206–212. [Google Scholar] [CrossRef]
- De Keizer, A.; Lyklema, J. Adsorption of tetraalkylammonium ions at the silver iodide-electrolyte interface. J. Colloid Interface Sci. 1980, 75, 171–184. [Google Scholar]
- Clunie, J.S.; Ingram, B.T. Adsorption of nonionic surfactants. In Adsorption from Solutions at the Solid/Liquid Interface; Academic Press: New York, NY, USA, 1983; pp. 105–152. [Google Scholar]
- Koner, S.; Pal, A.; Adak, A. Utilization of silica gel waste for adsorption of cationic surfactant and adsolubilization of organics from textile wastewater: A case study. Desalination 2011, 276, 142–147. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kyzas, G.Z.; Peleka, E.N.; Deliyanni, E.A. Nanocrystalline Akaganeite as Adsorbent for Surfactant Removal from Aqueous Solutions. Materials 2013, 6, 184-197. https://doi.org/10.3390/ma6010184
Kyzas GZ, Peleka EN, Deliyanni EA. Nanocrystalline Akaganeite as Adsorbent for Surfactant Removal from Aqueous Solutions. Materials. 2013; 6(1):184-197. https://doi.org/10.3390/ma6010184
Chicago/Turabian StyleKyzas, George Z., Efrosyni N. Peleka, and Eleni A. Deliyanni. 2013. "Nanocrystalline Akaganeite as Adsorbent for Surfactant Removal from Aqueous Solutions" Materials 6, no. 1: 184-197. https://doi.org/10.3390/ma6010184







