Photocatalytic Oxidation of Low-Level Airborne 2-Propanol and Trichloroethylene over Titania Irradiated with Bulb-Type Light-Emitting Diodes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison of Baking Temperatures for Photocatalytic Decomposition Efficiencies (PDEs)
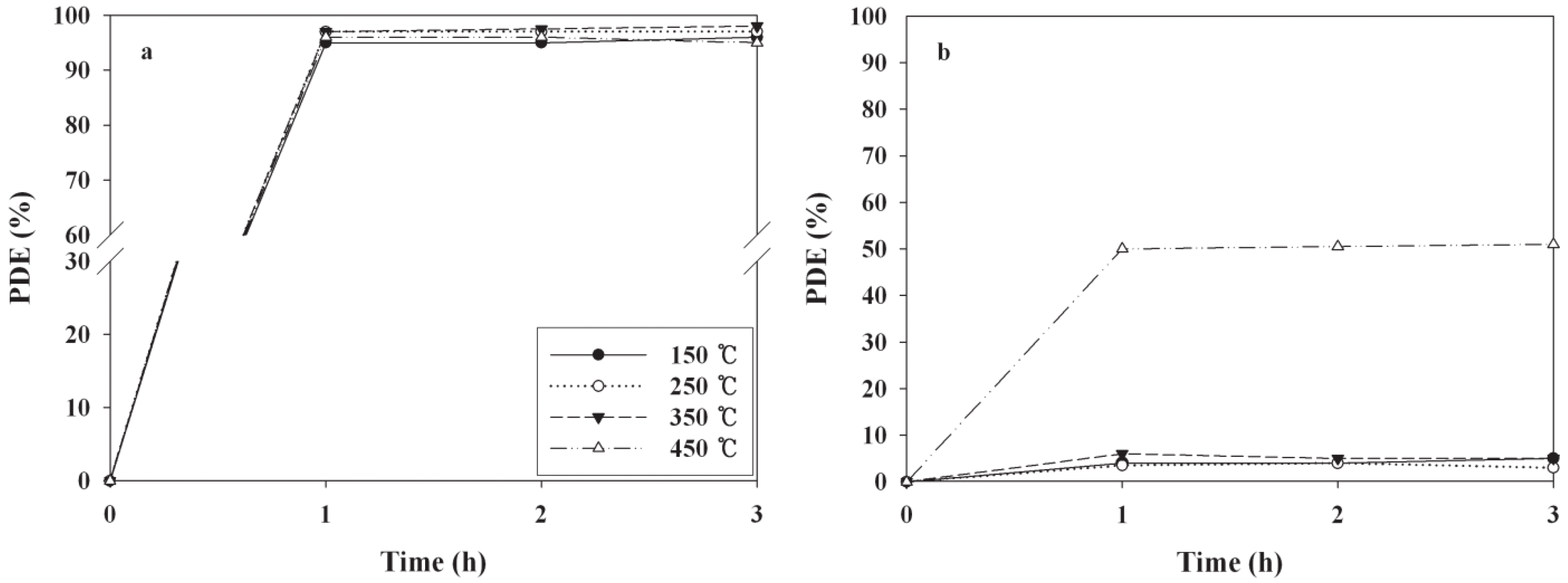
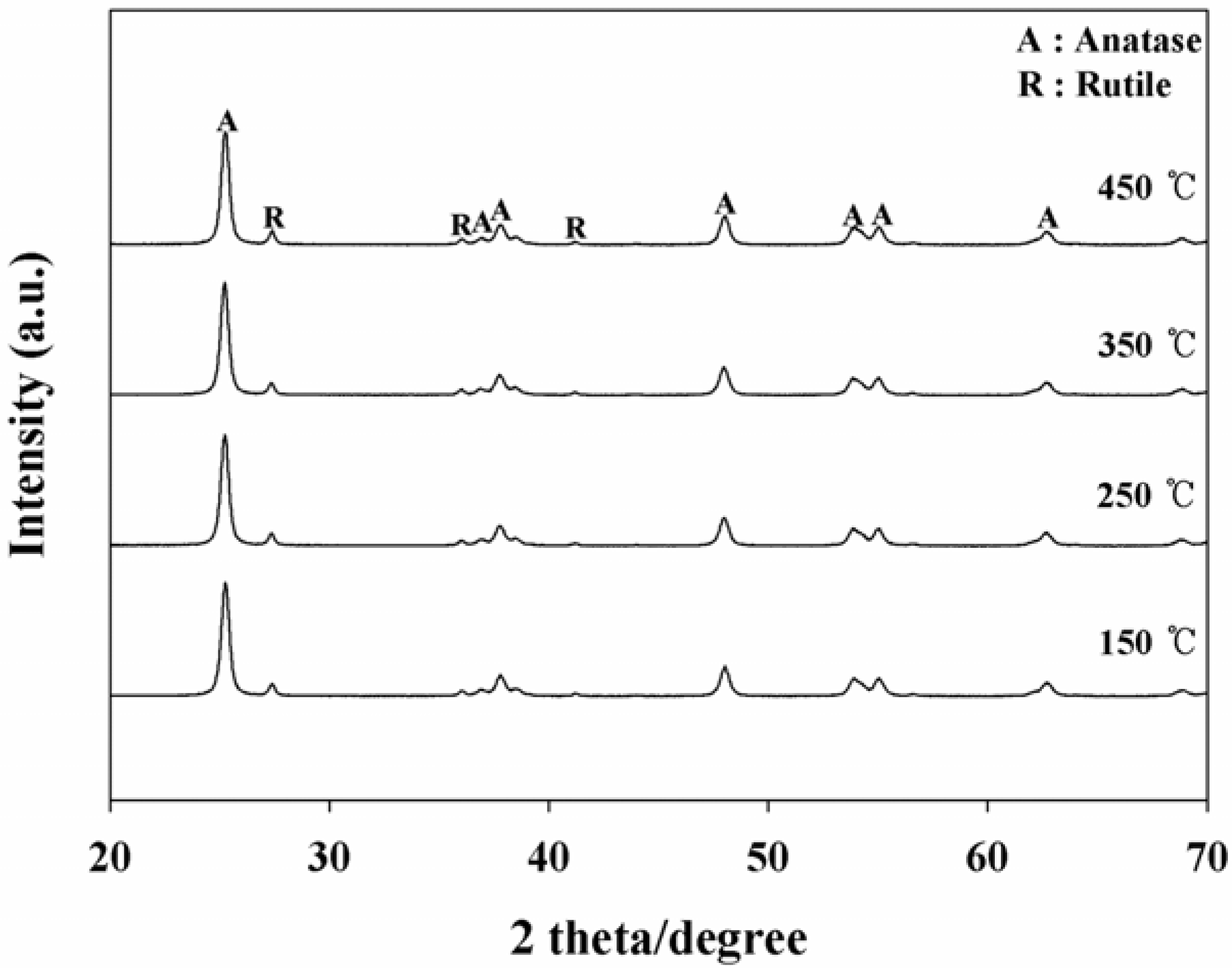
| Baking temperature (°C) | Ratio of anatase/rutile |
|---|---|
| 150 | 8.8 |
| 250 | 8.5 |
| 350 | 8.8 |
| 450 | 7.7 |
2.2. Comparison of Light Sources for PDEs
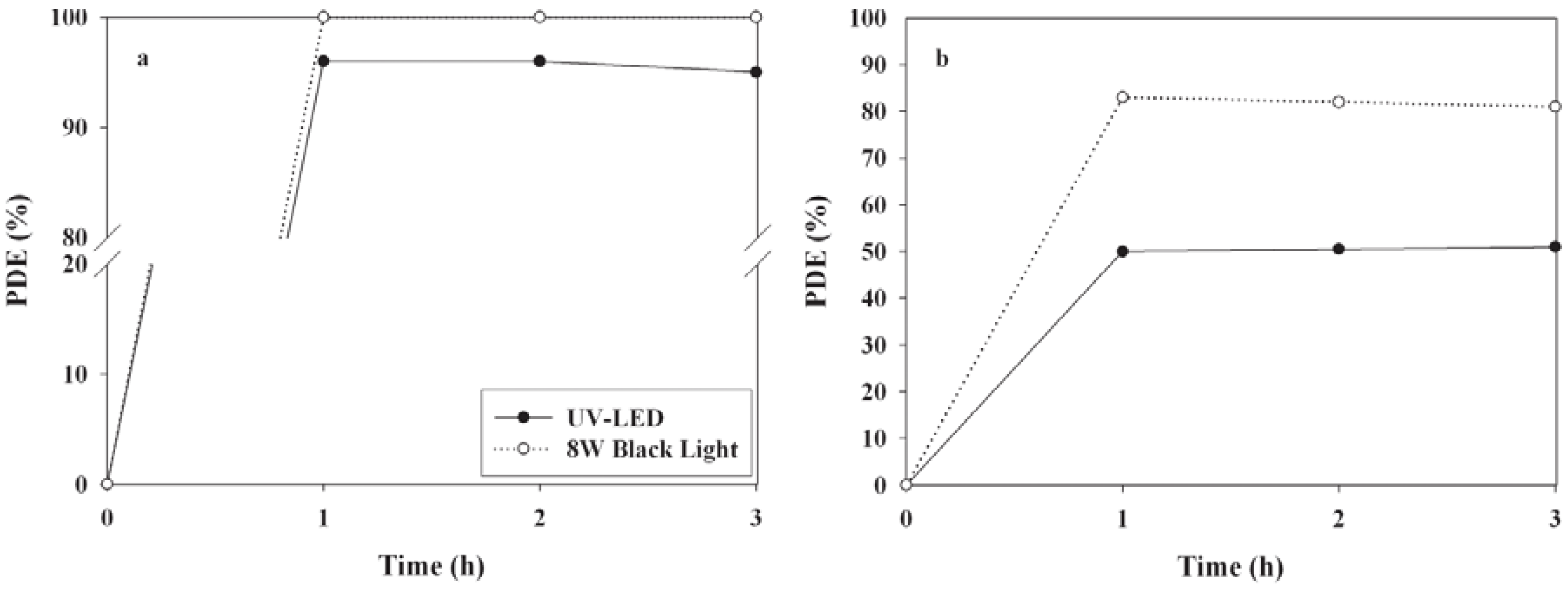
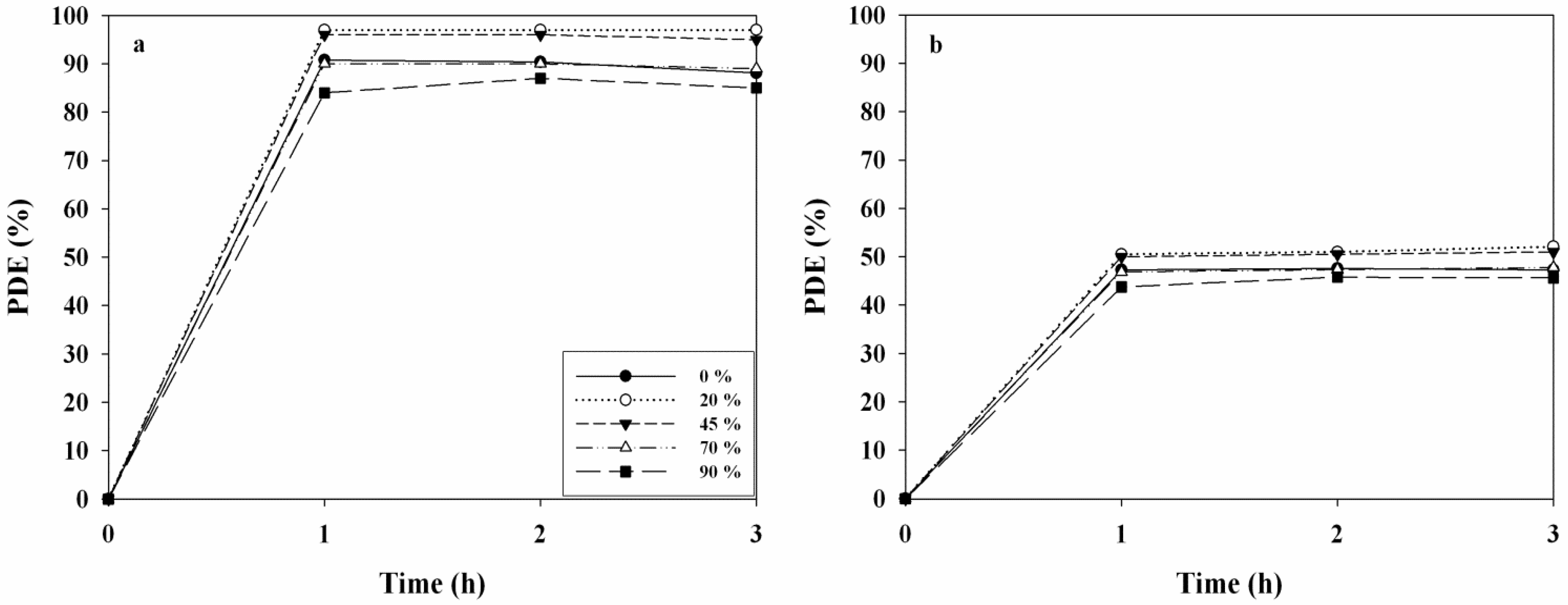
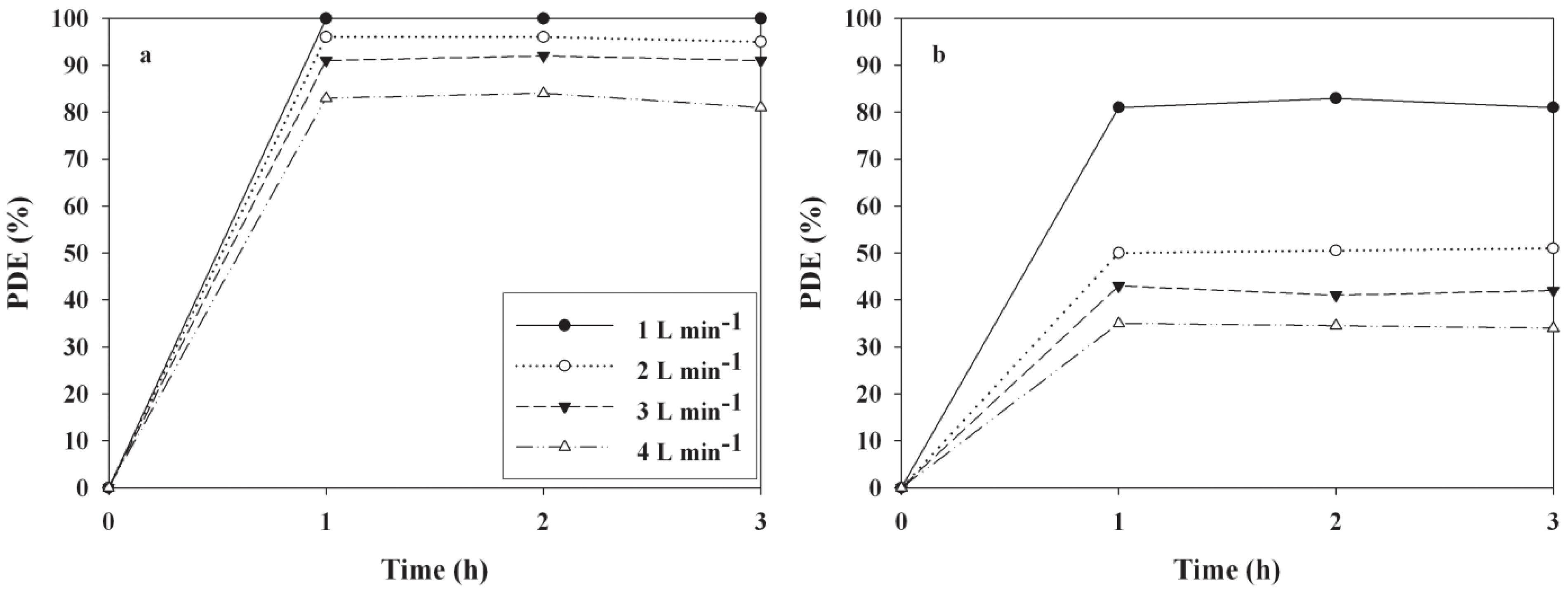
2.3. Effects of Operational Conditions on PDEs
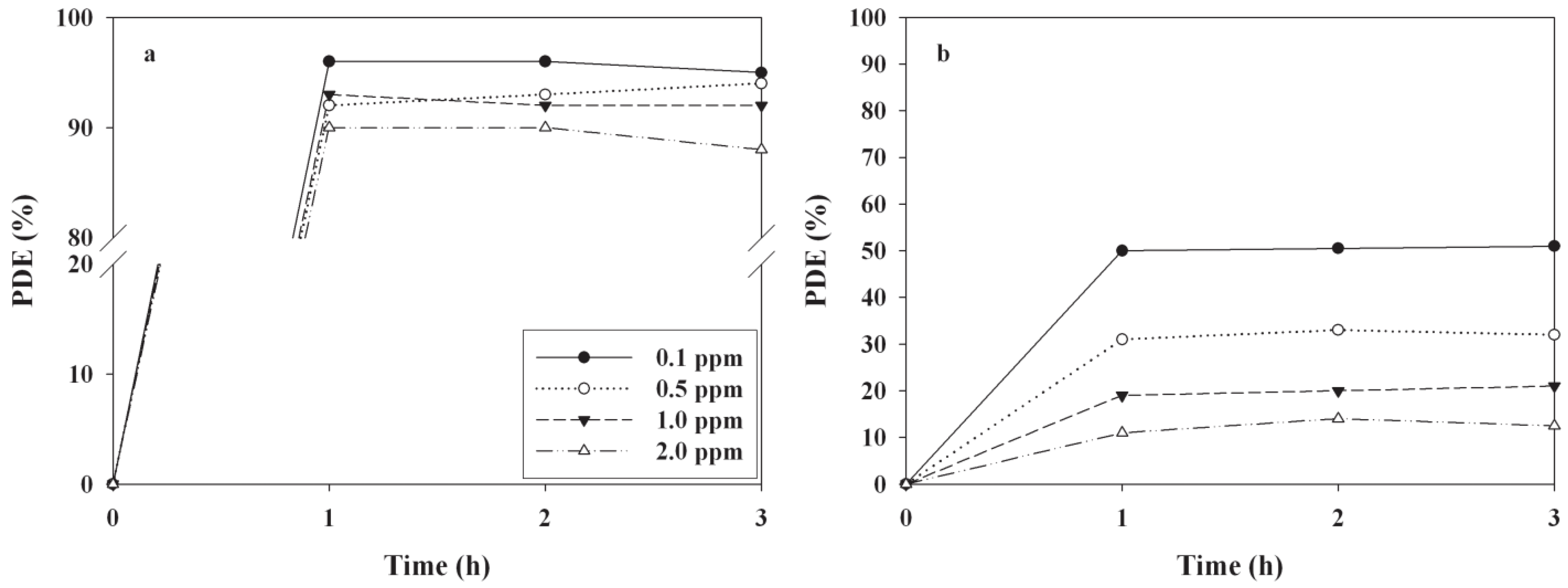
| Compound | IC (ppm) | |||
|---|---|---|---|---|
| 0.1 | 0.5 | 1.0 | 2.0 | |
| 2-Propanol | 1.2 × 10−3 | 4.1 × 10−3 | 5.7 × 10−3 | 8.7 × 10−3 |
| TCE | 1.0 × 10−3 | 2.1 × 10−3 | 3.3 × 10−3 | 5.2 × 10−3 |
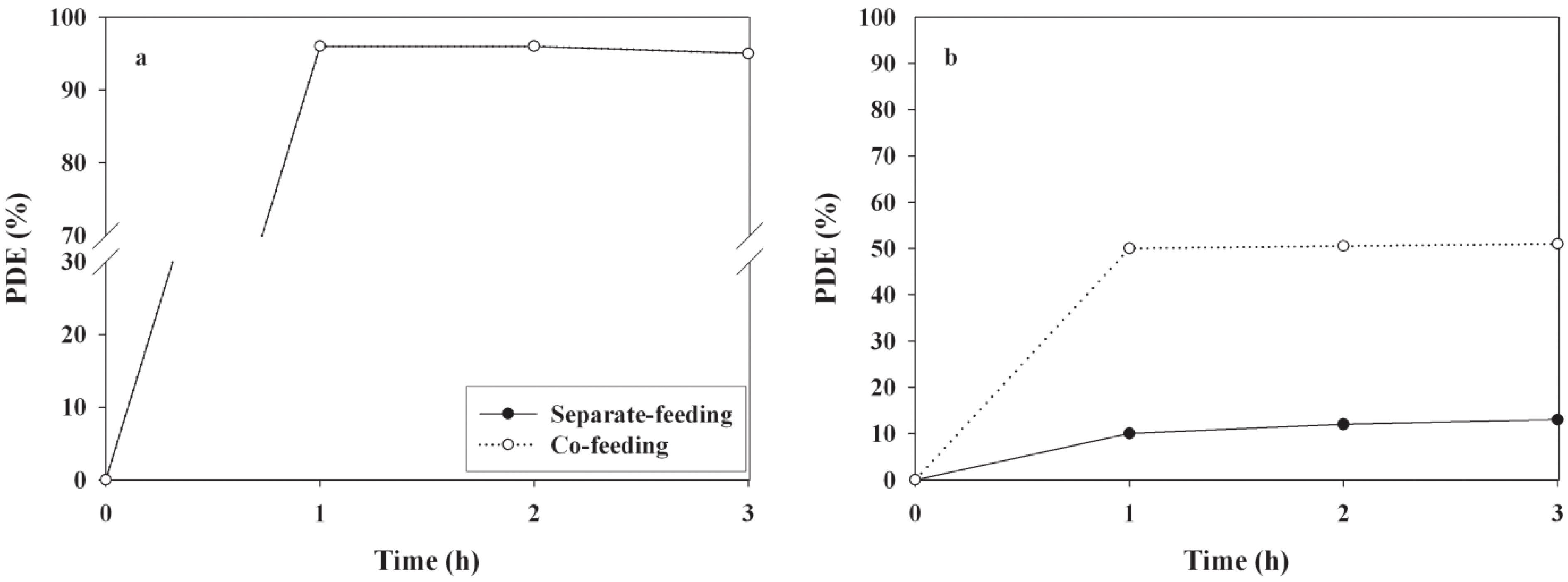
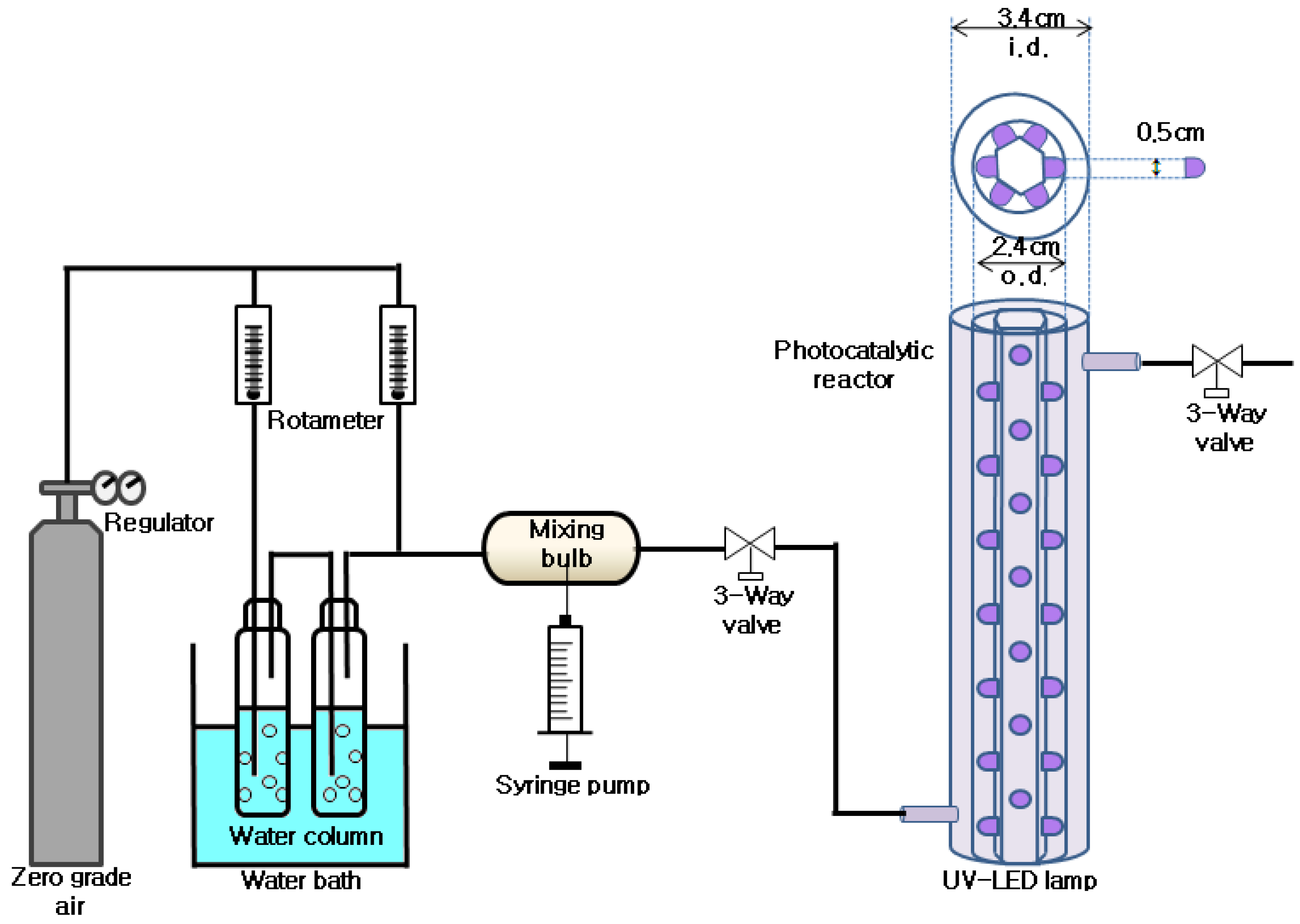
3. Experimental Section
4. Conclusions
Acknowledgements
References
- Takeda, M.; Saijo, Y.; Yuasa, M.; Kanazawa, A.; Araki, A.; Kishi, R. Relationship between sick building syndrome and indoor environmental factors in newly built Japanese dwellings. Int. Arch. Occup. Environ. Health 2009, 82, 583–93. [Google Scholar] [CrossRef] [PubMed]
- Araki, A.; Kawai, T.; Eitaki, Y.; Kanazawa, A.; Morimoto, K.; Nakayama, K.; Shibata, E.; Tanaka, M.; Takigawa, T.; Yoshimura, T.; Chikara, H.; Saijo, Y.; Kishi, R. Relationship between selected indoor volatile organic compounds, so-called microbial VOC, and the prevalence of mucous membrane symptoms in single family homes. Sci. Total Environ. 2010, 408, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, J.; Zhang, L.; Turpinb, B.J.; Weisel, C.P.; Morandi, M.T.; Stock, T.H.; Colome, S.; Korn, L.R. Estimating contributions of indoor and outdoor sources to indoor carbonyl concentrations in three urban areas of the United States. Atmos. Environ. 2006, 40, 2202–2214. [Google Scholar] [CrossRef]
- Guo, H. Source apportionment of volatile organic compounds in Hong Kong homes. Build. Environ. 2011, 46, 2280–2286. [Google Scholar] [CrossRef]
- Jia, C.; Batterman, S.; Godwin, C. VOCs in industrial, urban and suburban neighborhoods—Part 2: Factors affecting indoor and outdoor concentrations. Atmos. Environ. 2008, 42, 2101–2116. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Monographs on the Evaluation of the Carcinogenic Risks of Chemicals to Man; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Matsumoto, H.; Shimizu, M.; Sato, H. The contaminant removal efficiency of an air cleaner using the adsorption/desorption effect. Build. Environ. 2009, 44, 1371–1377. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Fundamentals and misconceptions in photocatalysis. J. Photochem. Photochem. A 2010, 216, 85–93. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C 2008, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Henderson, M.A.; Deskins, N.A.; Zehr, R.T.; Dupuis, M. Generation of organic radicals during photocatalytic reactions on TiO2. J. Catal. 2011, 279, 205–212. [Google Scholar] [CrossRef]
- Reyes, C.; Fernández, J.; Freer, J.; Mondaca, M.A.; Zaror, C.; Malato, S.; Mansilla, H.D. Degradation and inactivation of tetracycline by TiO2 photocatalysis. J. Photochem. Photobiol. A 2006, 184, 141–146. [Google Scholar] [CrossRef]
- Son, H.-S.; Ko, G.; Zoh, K.-D. Kinetics and mechanism of photolysis and TiO2 photocatalysis of triclosan. J. Hazard. Mater. 2009, 166, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Kannan, A. Photocatalytic degradation of phenol in a rotating annular reactor. Chem. Eng. Sci. 2010, 65, 2727–2740. [Google Scholar] [CrossRef]
- Chevremont, A.-C.; Farnet, A.-M.; Coulomb, B.; Boudenne, J.-L. Effect of coupled UV-A and UV-C LEDs on both microbiological and chemical pollution of urban wastewaters. Sci. Total Environ. 2012, 426, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Shie, J.L.; Lee, C.H.; Chiou, C.S.; Chang, C.T.; Chang, C.C.; Chang, C.Y. Photodegaradation kinetics of formaldehyde using light sources of UVA, UVC and UVLED in the presence of composed silver titanium oxide photocatalyst. J. Hazard. Mater. 2008, 155, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.P.; Sui, R.; Langford, C.H.; Achari, G.; Berlinguette, C.P. A comparison of several nanoscale photocatalysts in the degradation of a common pollutants using LEDs and conventional UV light. Water Res. 2009, 43, 4499–4506. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.-K.; Park, K.-H. Heterogeneous photocatalysis of aromatic and chlorinated volatile organic compounds (VOCs) for non-occupational indoor air application. Chemosphere 2004, 57, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Quincy, N.; Vera, M.L.; Choi, H.; Li Puma, G.; Dionysiou, D.D.; Litter, M.I.; Destaillats, H. Effect of key parameters on the photocatalytic oxidation of toluene at low concentrations in air under 254 + 185 nm UV irradiation. Appl. Catal. B 2010, 95, 312–319. [Google Scholar] [CrossRef]
- Puddu, V.; Choi, H.; Dionysiou, D.D.; Li Puma, G. TiO2 photocatalyst for indoor air remediation: influence of crystallinity, crystal phase, and UV radiation intensity on trichloroethylene degradation. Appl. Catal. B 2010, 94, 211–218. [Google Scholar] [CrossRef]
- Znad, H.; Kawase, Y. Synthesis and characterization of S-doped Degussa P25 with application in decolorization of orange II dye as a model substrate. J. Mol. Catal. A 2009, 314, 55–62. [Google Scholar] [CrossRef]
- Nosaka, Y.; Matsushita, M.; Nishino, J.; Nosaka, A.Y. Nitrogen-enhanced titanium dioxide photocatalysts for visible response prepared by using organic compounds. Sci. Technol. Adv. Mater. 2005, 6, 143–148. [Google Scholar] [CrossRef]
- Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photochem. Photobiol. C 2012, 13, 224–245. [Google Scholar] [CrossRef] [Green Version]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Pengyi, Z.; Fuyan, L.; Gang, Y.; Qing, C.; Wanpeng, Z. A comparative study on decomposition of gaseous toluene by O3/UV, TiO2/UV and O3/TiO2/UV. J. Photochem. Photobiol. A 2003, 156, 189–194. [Google Scholar] [CrossRef]
- Zhao, W.; Dai, J.; Liu, F.; Bao, J.; Wang, Y.; Yang, Y.; Yang, Y.; Zhao, D. Photocatalytic oxidation of indoor toluene: Process risk analysis and influence of relative humidity, photocatalysts, and VUV irradiation. Sci. Total Environ. 2012, 438, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K.; Yang, S.H.; Shin, S.H.; Yang, S.B. Utilization of fin-installed annular reactors coated with visible light- or ultraviolet-driven photocatalysts for removal of gas-phase monocyclic aromatic compounds. Environ. Eng. Sci. 2011, 28, 43–51. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; van Langenhove, H. Heterogeneous photocatalysis as an advanced oxidation process for the abatement of chlorinated, monocyclic aromatic and sulfurous volatile organic compounds in air: State of the art. Crit. Rev. Environ. Sci. Technol. 2007, 37, 489–538. [Google Scholar] [CrossRef]
- Li Pumaa, G.; Salvado-Estivill, I.; Obee, T.N.; Hay, S.O. Kinetics rate model of the photocatalytic oxidation of trichloroethylene in air over TiO2 thin films. Sep. Purif. Technol. 2009, 67, 226–232. [Google Scholar] [CrossRef]
- Queffeulou, A.; Geron, L.; Archambeau, C.; Gall, H.L.; Marquaire, P.M.; Zahraa, O. Kinetic study of acetaldehyde photocatalytic oxidation with a thin film of TiO2 coated on stainless steel and CFD modeling approach. Ind. Eng. Chem. Res. 2010, 49, 6890–6897. [Google Scholar] [CrossRef]
- Palau, J.; Penya-Roja, J.M.; Gabaldon, C.; Alvarez-Hornos, F.J.; Sempere, F.; Martinez-Soria, V. UV photocatalytic oxidation of paint solvent compounds in air using an annular TiO2-supported reactor. J. Chem. Technol. Biotechnol. 2011, 86, 273–281. [Google Scholar] [CrossRef]
- Lee, D.M.; Yun, H.J.; Yu, S.; Yun, S.J.; Lee, S.Y.; Kang, S.H.; Yi, J. Design of an efficient photocatalytic reactor for the decomposition of gaseous organic contaminants in air. Chem. Eng. J. 2012, 187, 203–209. [Google Scholar] [CrossRef]
- Vildozo, D.; Portela, R.; Ferronato, C.; Chovelon, J.-M. Photocatalytic oxidation of 2-propanol/toluene binary mixtures at indoor air concentration levels. Appl. Catal. B 2011, 107, 347–354. [Google Scholar] [CrossRef]
- Sauer, M.L.; Hale, M.A.; Ollis, D.F. Heterogeneous photocatalytic oxidation of dilute toluene-chlorocarbon mixtures in air. J. Photochem. Photobiol. A 1995, 88, 169–178. [Google Scholar] [CrossRef]
- Young, C.; Lim, T.M.; Chiang, K.; Scott, J.; Amal, R. Photocatalytic oxidation of toluene and trichloroethylene in the gas-phase by metallised (Pt, Ag) titanium dioxide. Appl. Catal. 2008, 78, 1–10. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jo, W.-K. Photocatalytic Oxidation of Low-Level Airborne 2-Propanol and Trichloroethylene over Titania Irradiated with Bulb-Type Light-Emitting Diodes. Materials 2013, 6, 265-278. https://doi.org/10.3390/ma6010265
Jo W-K. Photocatalytic Oxidation of Low-Level Airborne 2-Propanol and Trichloroethylene over Titania Irradiated with Bulb-Type Light-Emitting Diodes. Materials. 2013; 6(1):265-278. https://doi.org/10.3390/ma6010265
Chicago/Turabian StyleJo, Wan-Kuen. 2013. "Photocatalytic Oxidation of Low-Level Airborne 2-Propanol and Trichloroethylene over Titania Irradiated with Bulb-Type Light-Emitting Diodes" Materials 6, no. 1: 265-278. https://doi.org/10.3390/ma6010265




