A Hybrid Methacrylate-Sodium Carboxymethylcellulose Interpolyelectrolyte Complex: Rheometry and in Silico Disposition for Controlled Drug Release
Abstract
:1. Introduction
2. Results and Discussion
2.1. Basic Viscosity (η) of the Native Polymers and IPEC
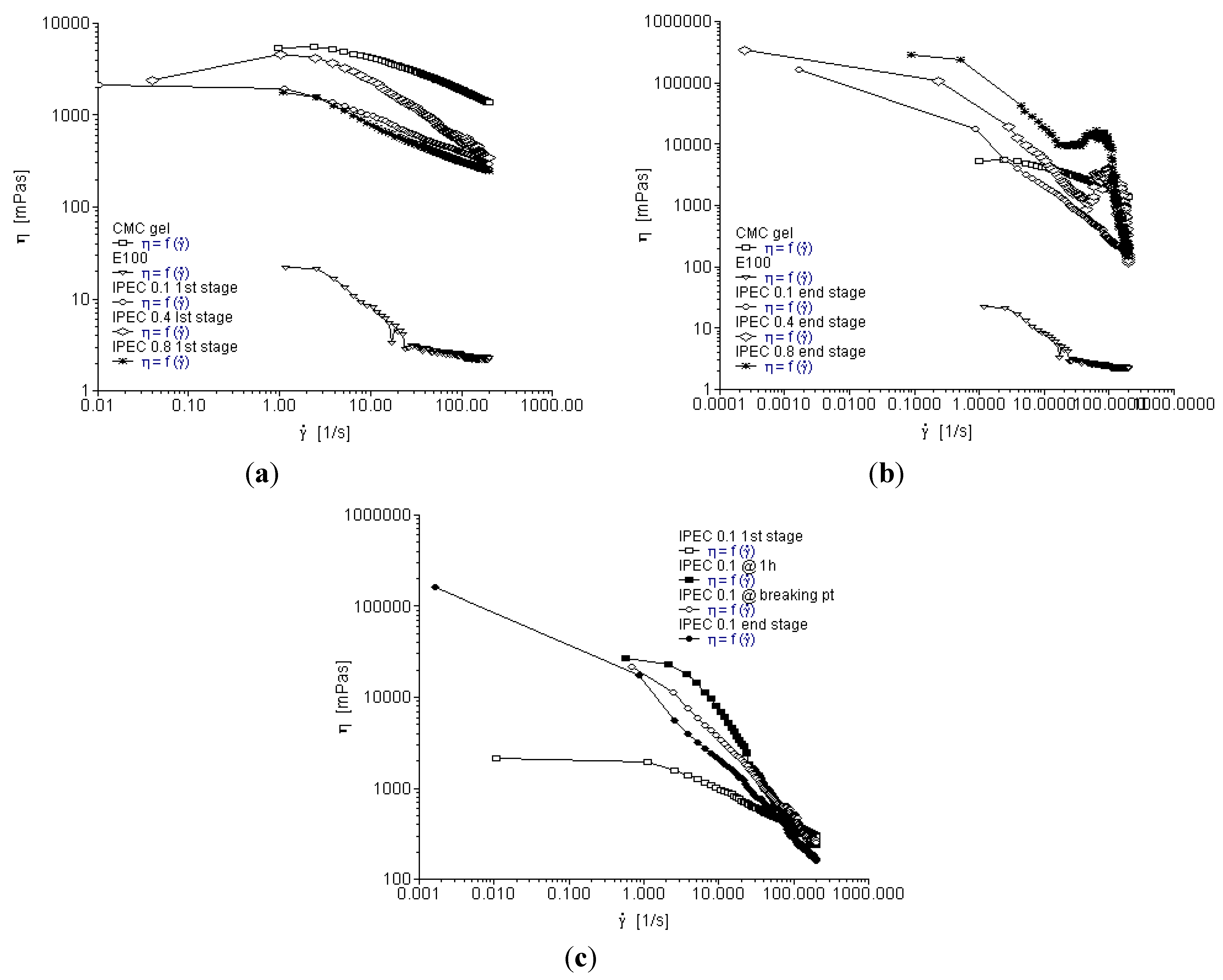

2.2. Yield Stress Analyses
| Sample | Yield value (Pa) | Shear | Time (s) |
|---|---|---|---|
| NaCMC | 0.2911 | 0.02802 | 31.47 |
| E100 | 0.07212 | 0.9648 | 32.17 |
| IPEC1 0.1 N initial stage | 0.2482 | 0.01442 | 31.22 |
| IPEC 0.1 N @ 1h | 0.2356 | 0.001318 | 30.98 |
| IPEC 0.1 N breaking pt | 41.42 | 0.9988 | 72.35 |
| IPEC 0.1 N final stage | 53.31 | 0.5160 | 83.77 |
| IPEC 0.4 N initial stage | 0.2904 | 0.006955 | 31.13 |
| IPEC 0.4 N final stage | 0.2311 | 0.0001735 | 30.67 |
| IPEC 0.8 N initial stage | 0.1923 | 0.01345 | 30.66 |
| IPEC 0.8 N final stage | 0.3329 | 0.0001819 | 31.03 |

2.3. Oscillation Stress Sweep
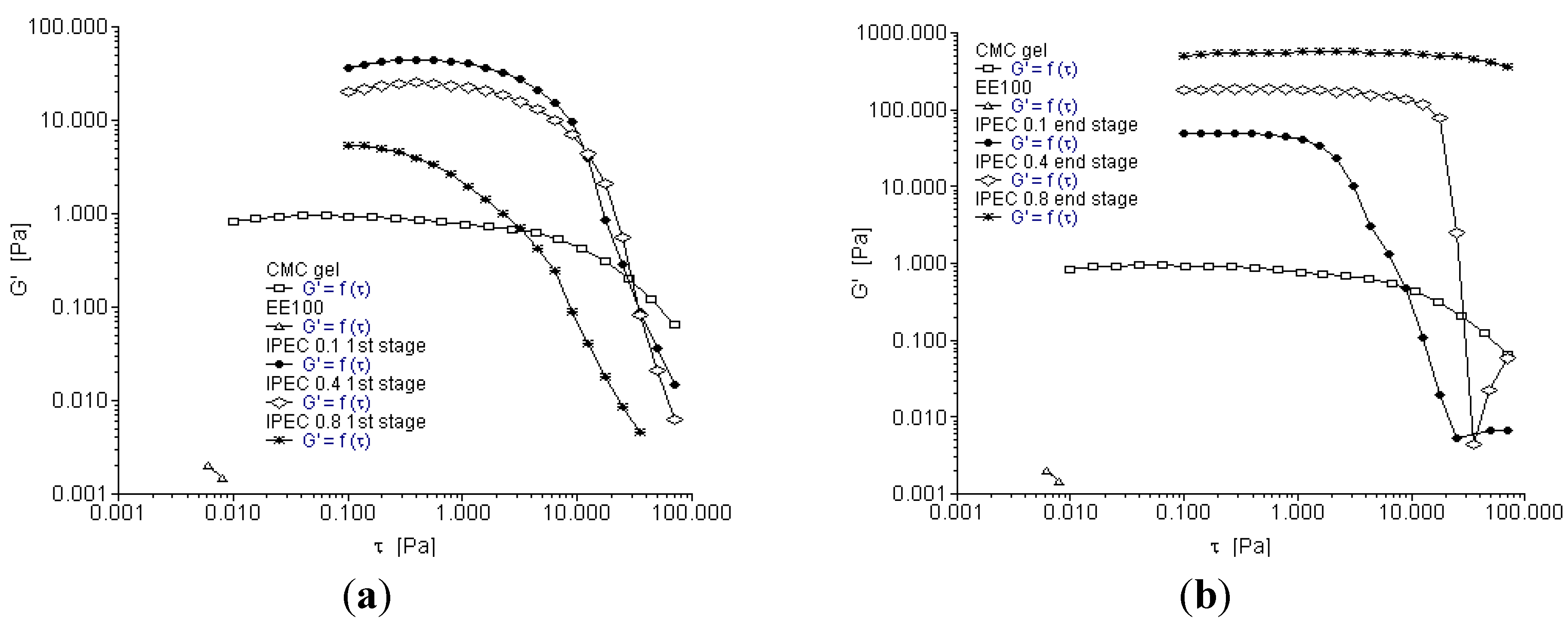
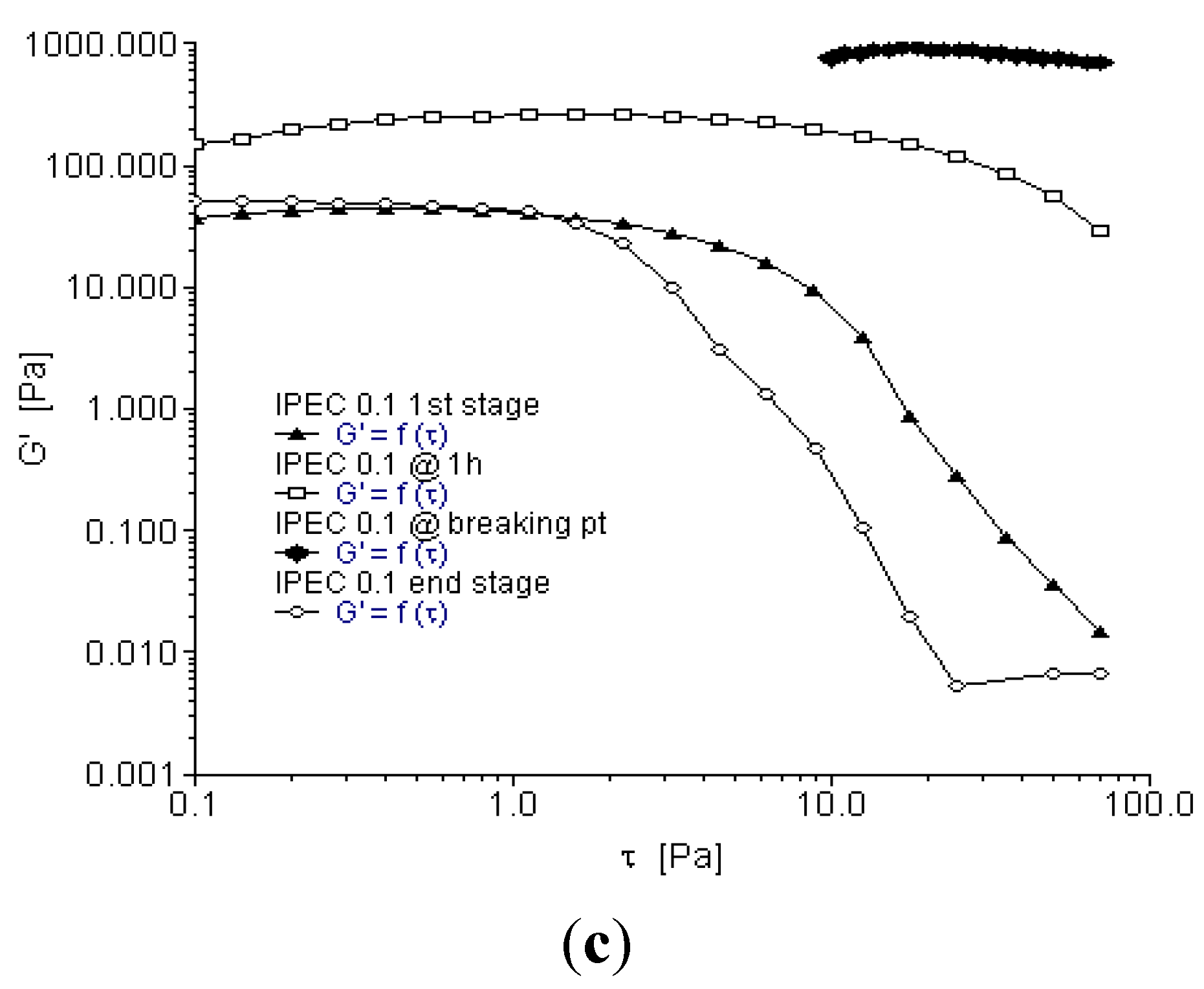
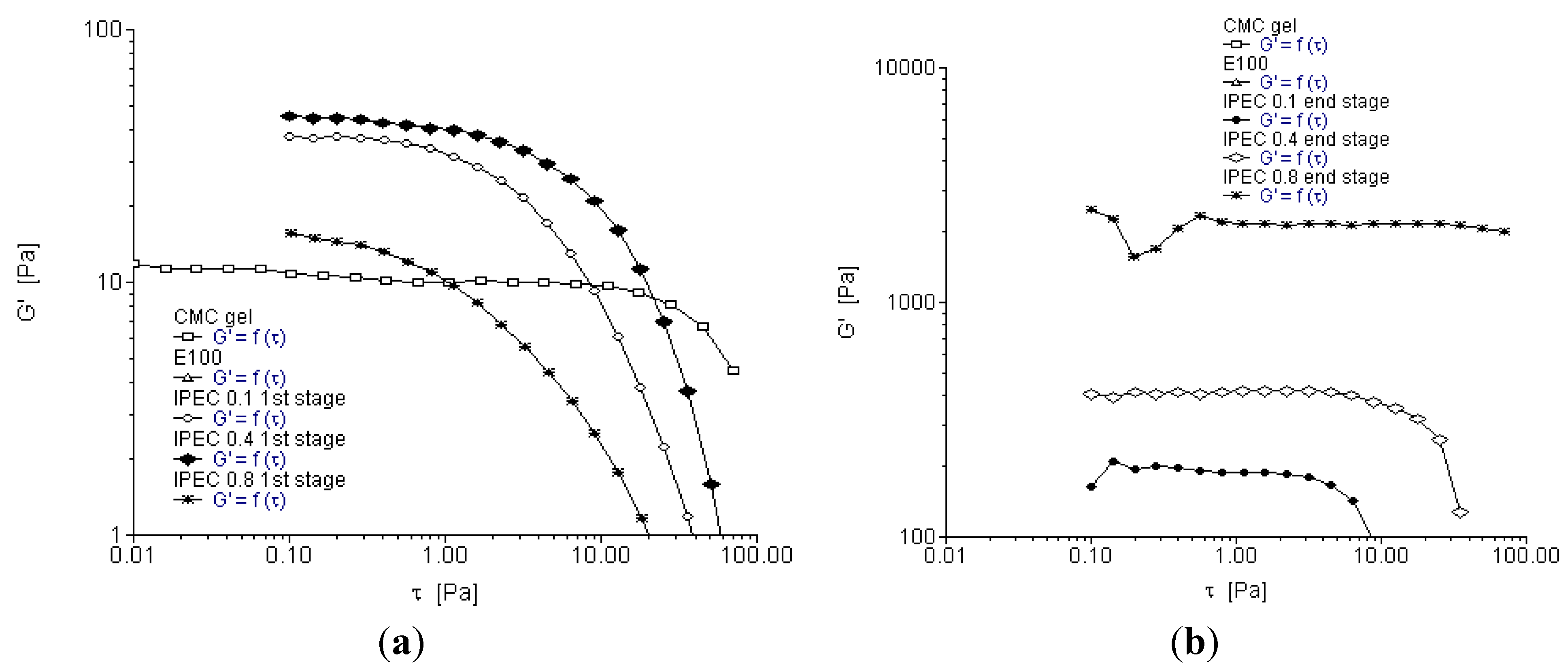
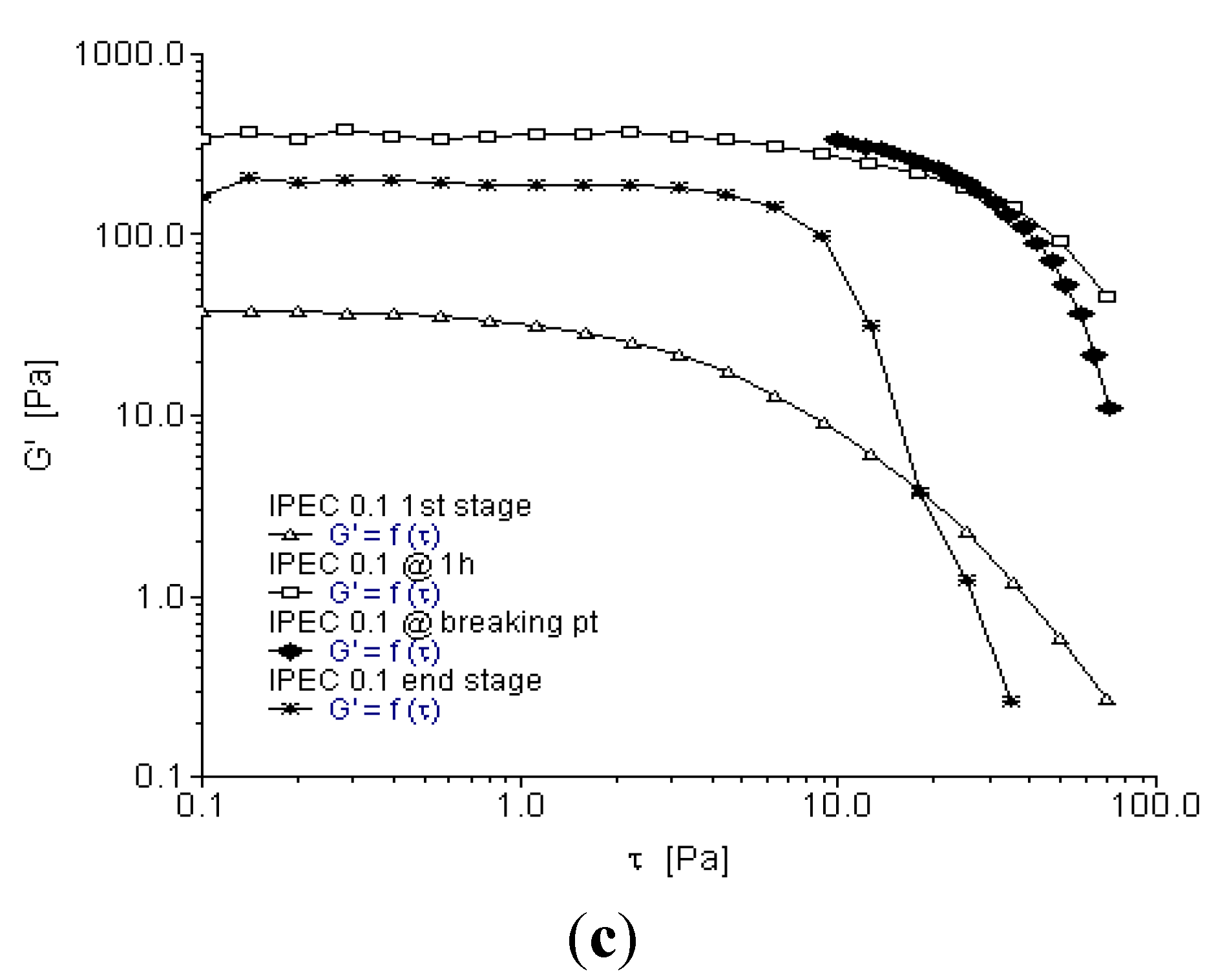
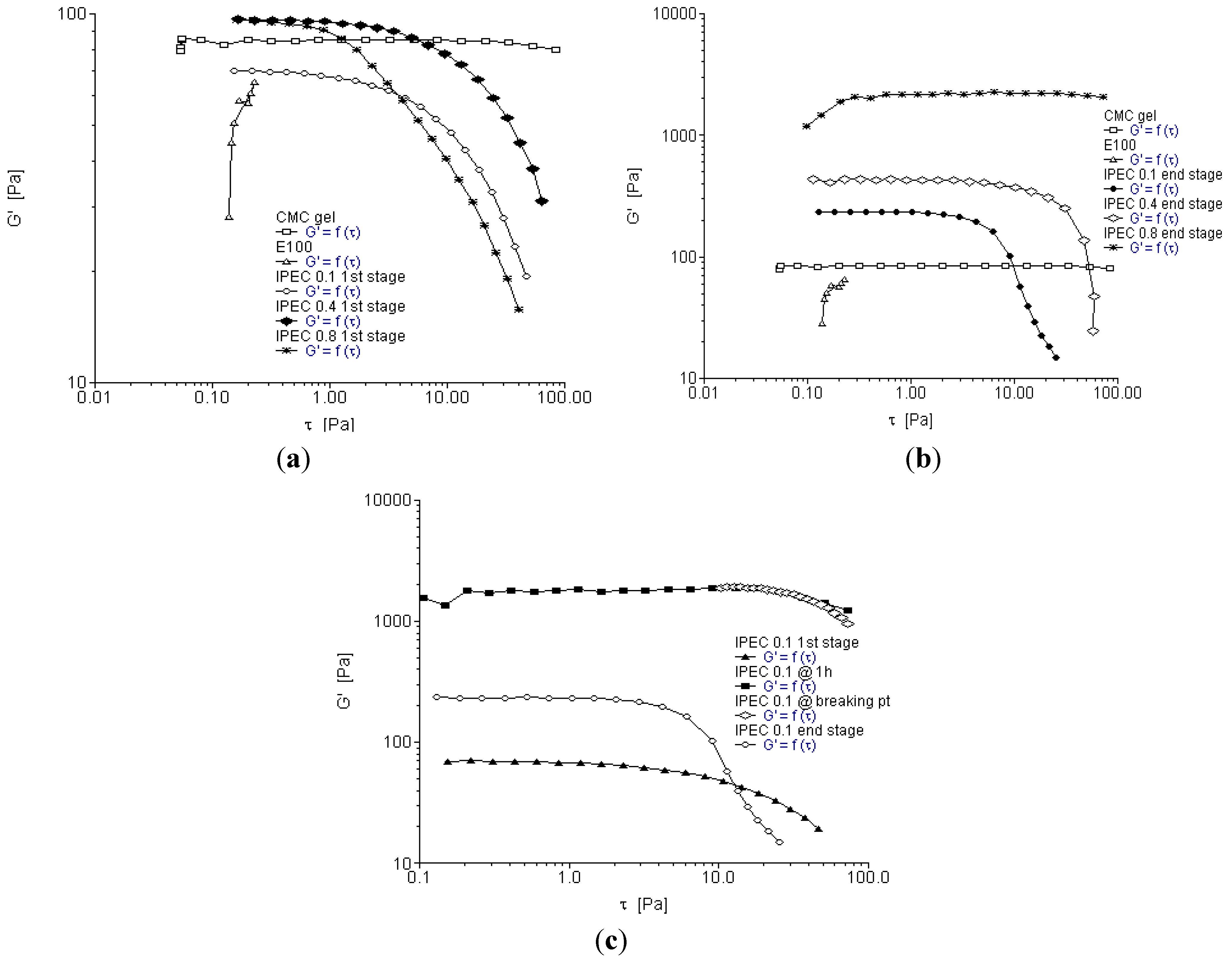
2.4. Oscillation Frequency Sweep


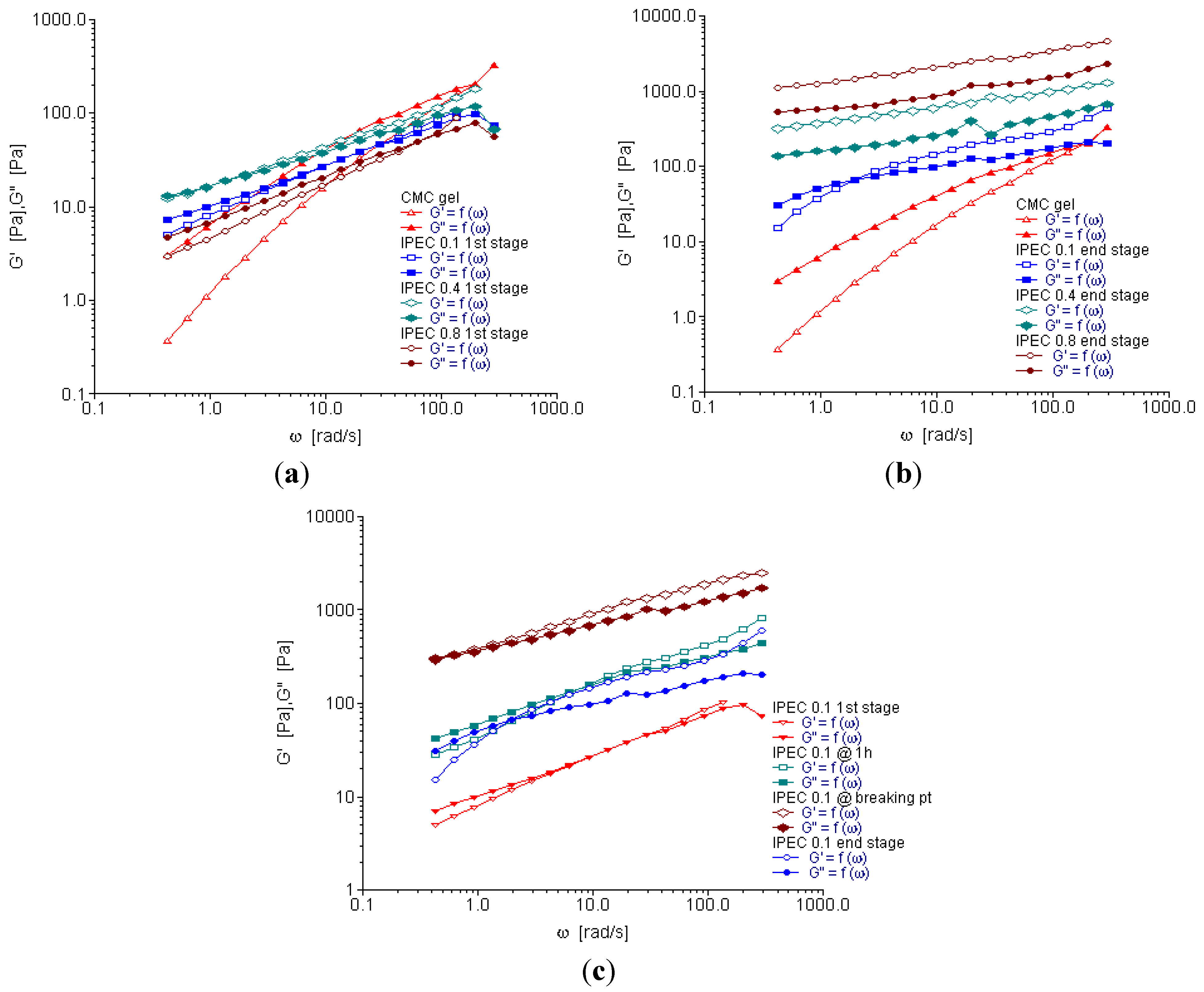
2.5. Creep and Recovery Test
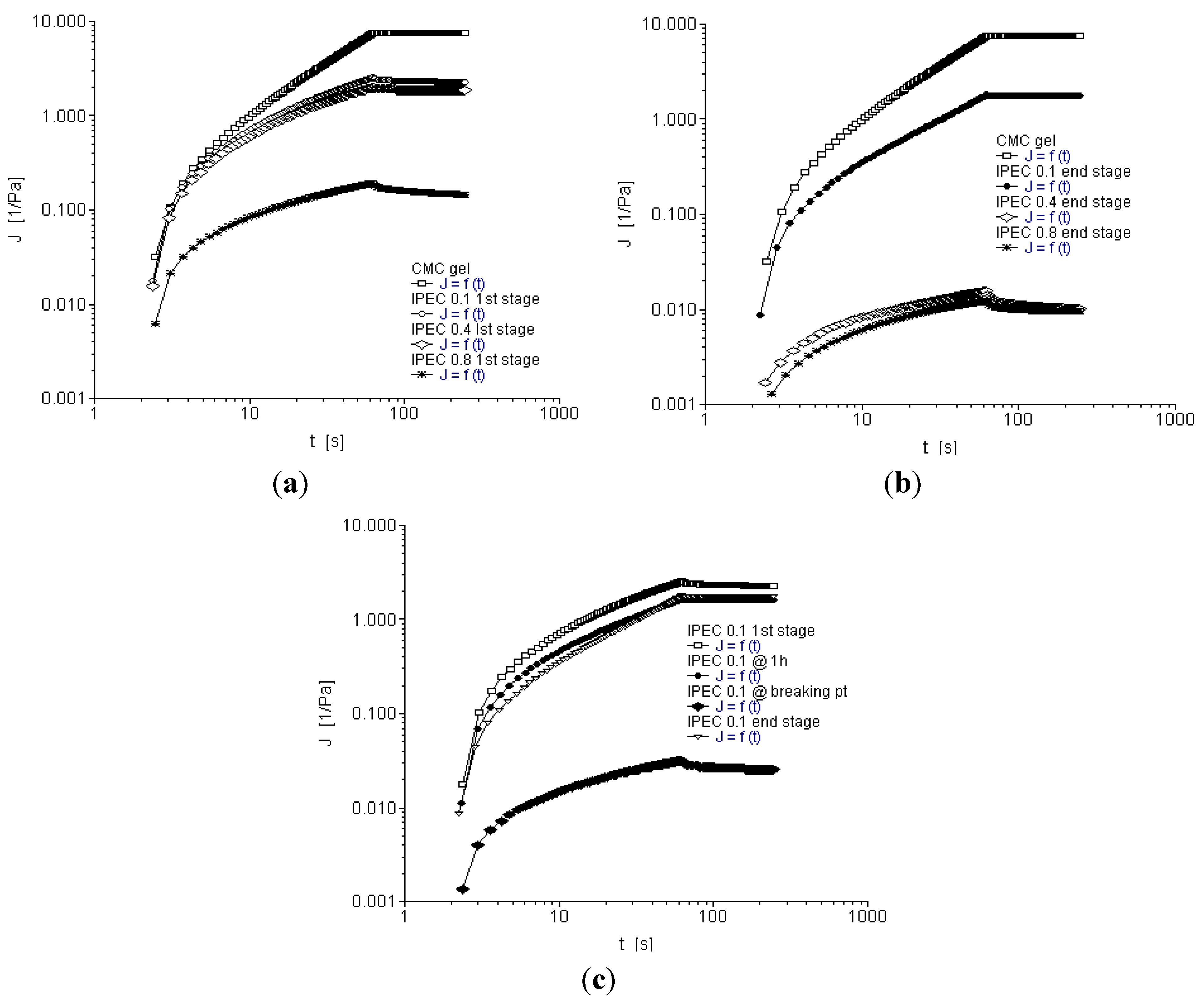
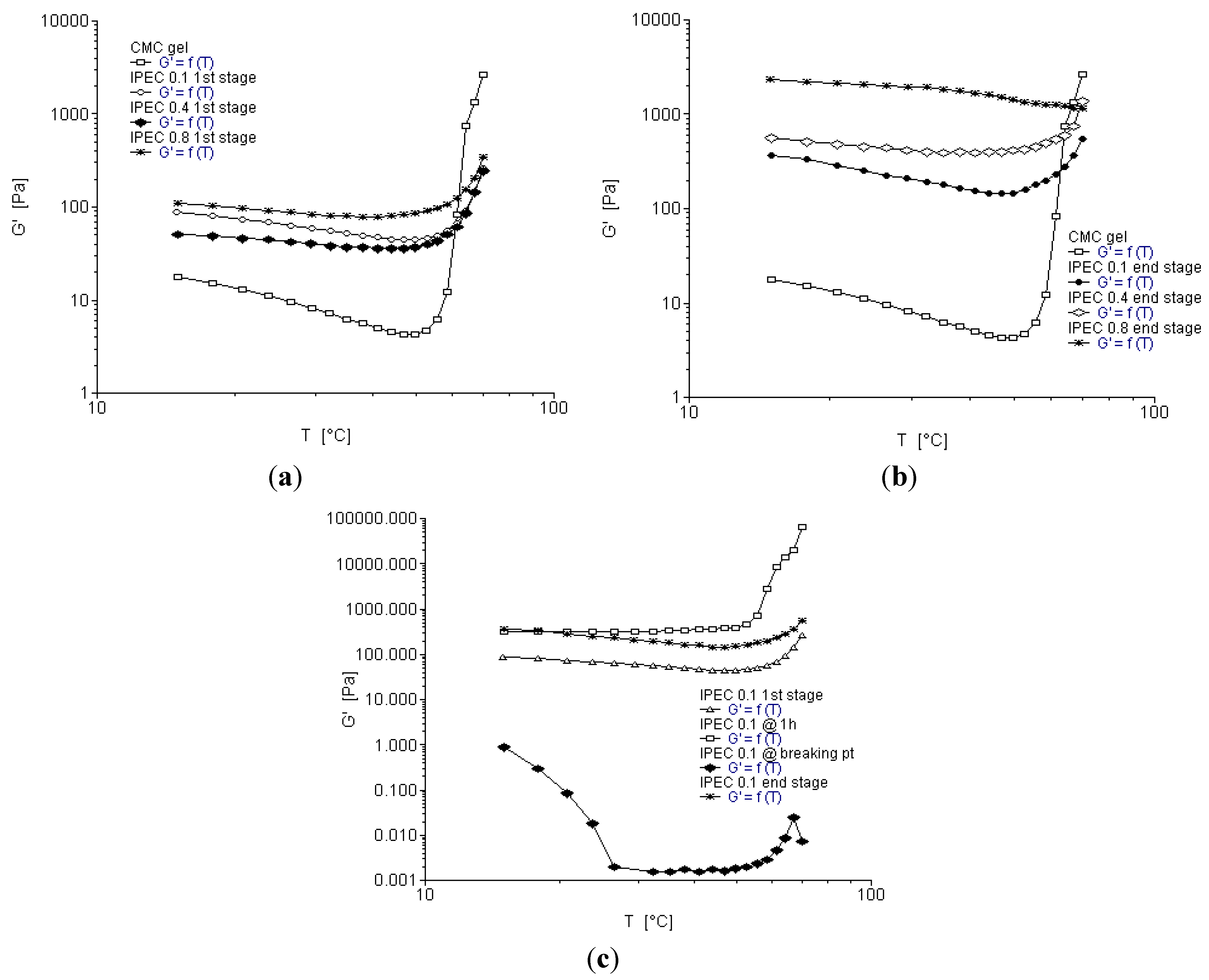
2.6. Temperature Ramp
2.7. Molecular Mechanics Assisted Model Building and Energy Refinements
2.7.1. MMER Analysis
2.7.2. Elucidation of Step-by-Step Synthetic Profile of IPEC
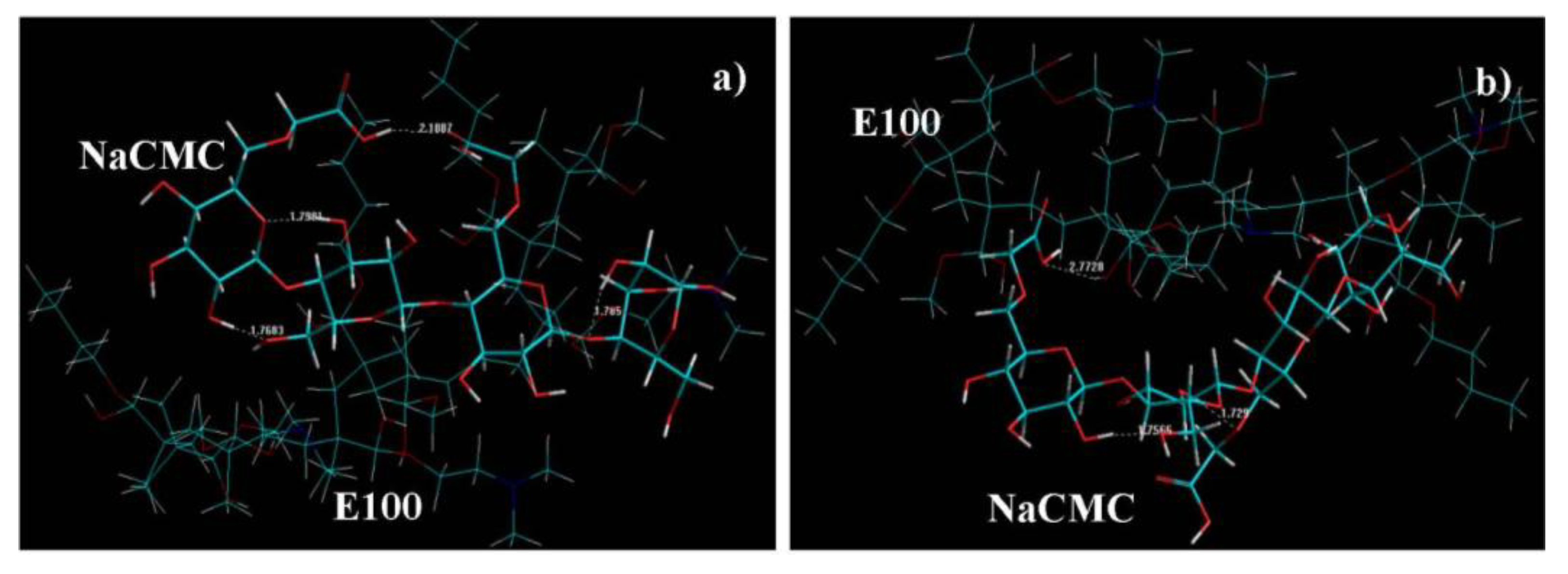
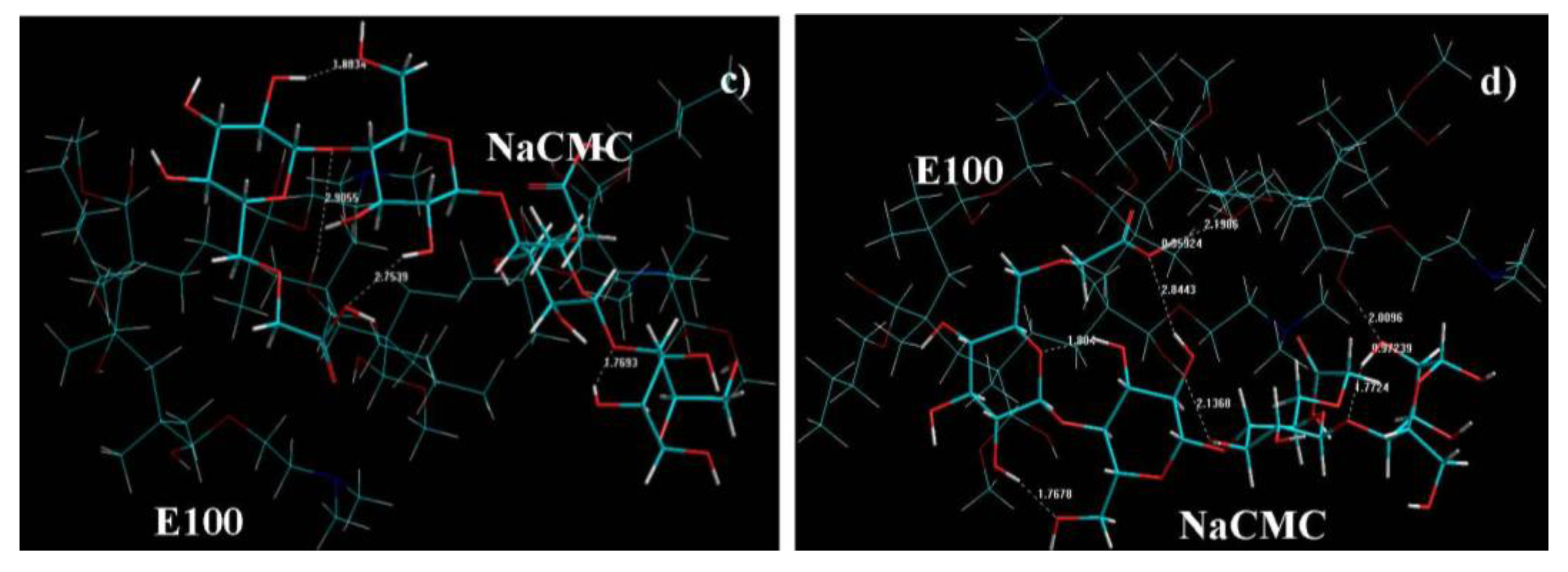
- At the initial stage (where the E100 was added to NaCMC), numerous intramolecular bonds in NaCMC were observed with no intermolecular bonding between E100-NaCMC (Figure 9a). This is confirmed by the initial formation of a gel phase by NaCMC in the reaction medium observed at the onset of synthesis.
- At 1h into synthesis, it was observed that NaCMC intramolecular bonds reduced in number and bond-length also decreased along with formation of an intermolecular bond with E100 depicting the first evidence of formation of an IPEC. This is corroborated experimentally by the increase in elastic modulus at one hour into synthesis (Figure 3c, Figure 4c and Figure 5c). The formation of intermolecular bonding enable crosslinking between E100 and NaCMC leading to increased elastic modulus.
- At breaking point, again the intramolecular bond of NaCMC started rebuilding with a slight increase in the length of the intermolecular bonds. This supports observations during synthesis and rheological studies. A thickening of the reaction medium, increase in elastic modulus and shear stress occurred at this stage which was generated by the entanglements of NaCMC coils due to rebuilding of intramolecular bonds and then gradual increase in intermolecular bonds between E100 and NaCMC.
- The formation of final product displayed a homogenous IPEC, which was accompanied with the formation of numerous intra- and inter-molecular bonds depicting the formation of a well interconnected polymeric matrix. Additionally, the bond length also seemed to be playing a part as the average bond length decreased to <2Å. The formation of numerous bonds yielded a polymeric matrix with increased resistance to flow as observed with increased yield values in comparison to the individual polymers.
| Name | ΔETotal 1 | ΔEBond | ΔEAngle | ΔEDihedral | ΔEVdw | ΔEH-bond | ΔEElec |
|---|---|---|---|---|---|---|---|
| EIPEC-II | −18.072 | 0.087 | 1.012 | 0.133 | −19.103 | −0.742 | 0.541 |
| EIPEC-III | −21.429 | −0.436 | 1.197 | −2.757 | −20.535 | −2.498 | 3.600 |
| EIPEC-IV | −24.753 | −0.859 | −1.096 | −0.558 | −25.576 | −2.152 | 5.488 |
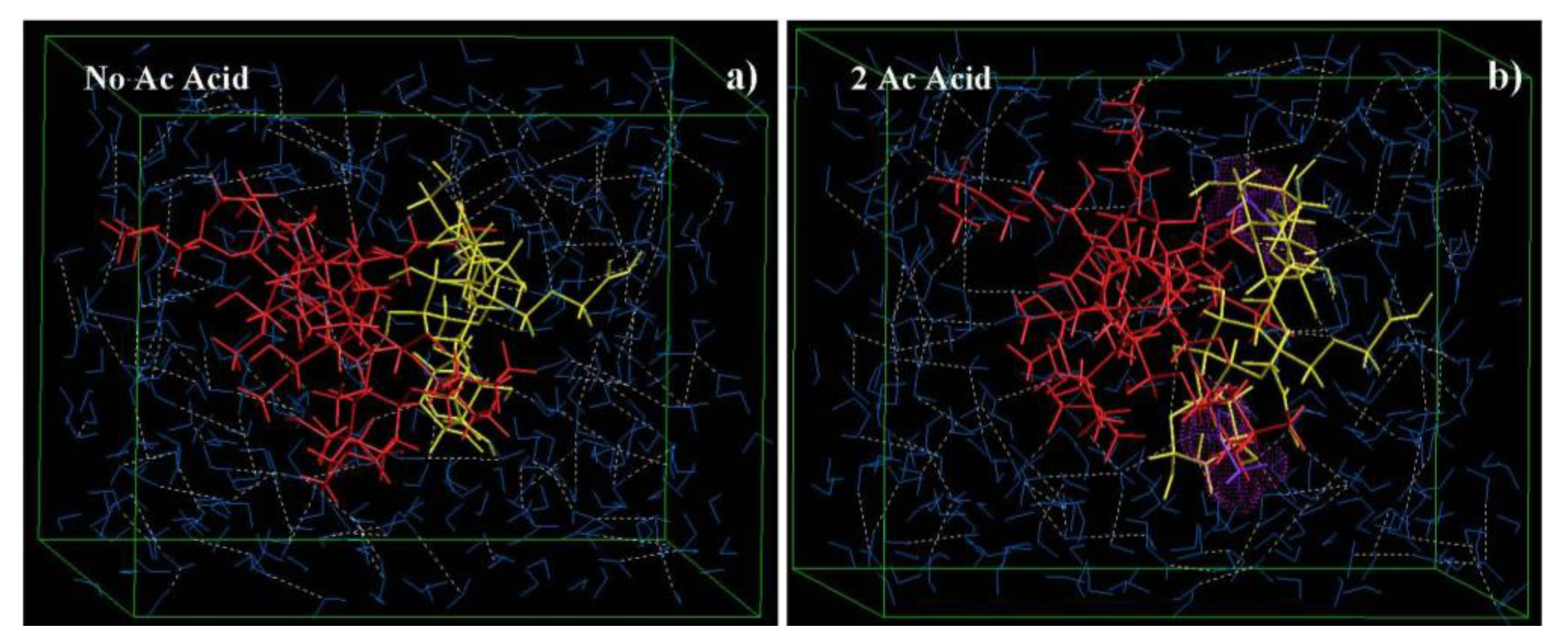
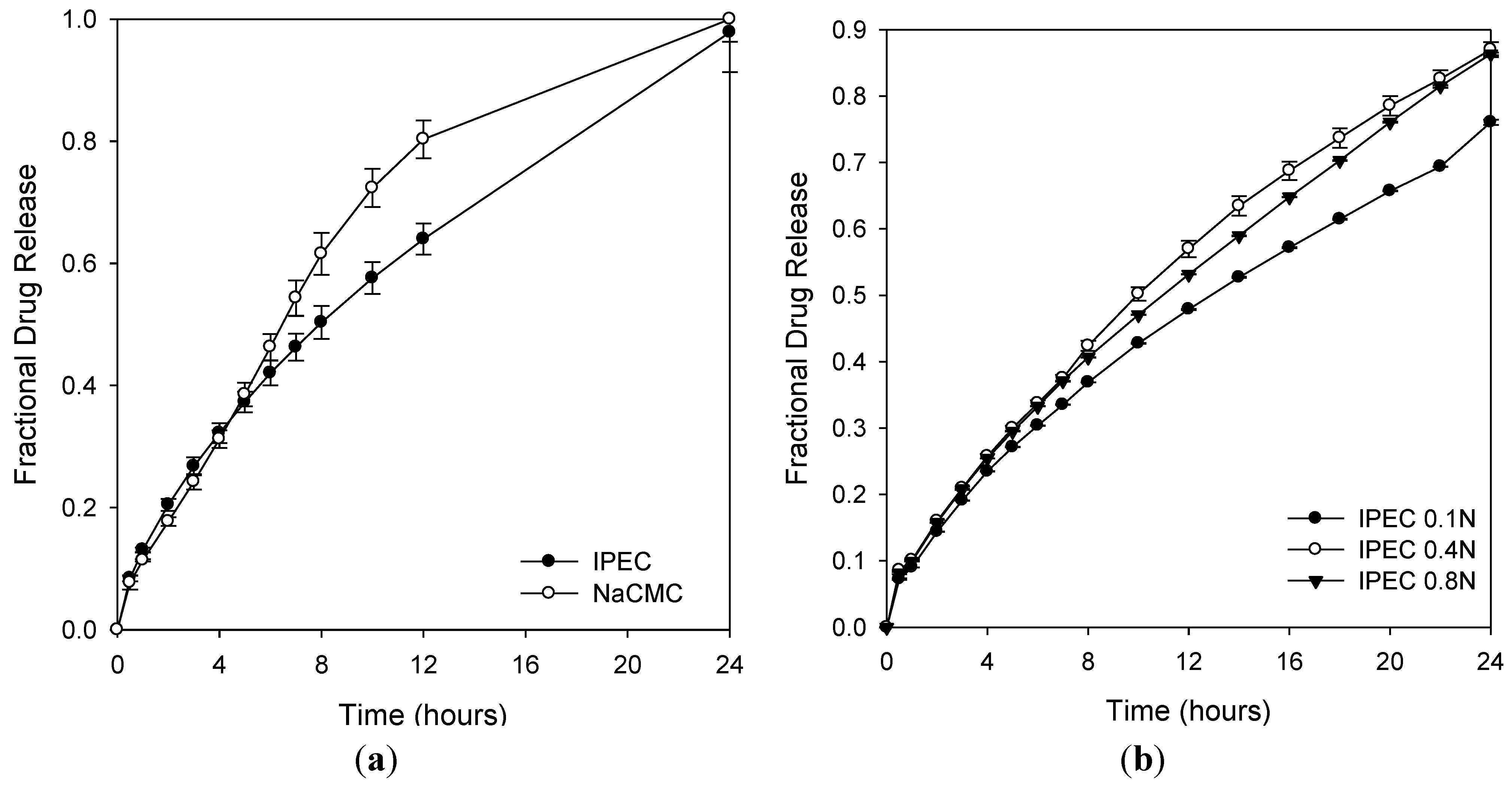
2.7.3. Effect of Normality of Acetic Acid on the Reactional Profile of IPEC

2.8. Drug Release Profiles

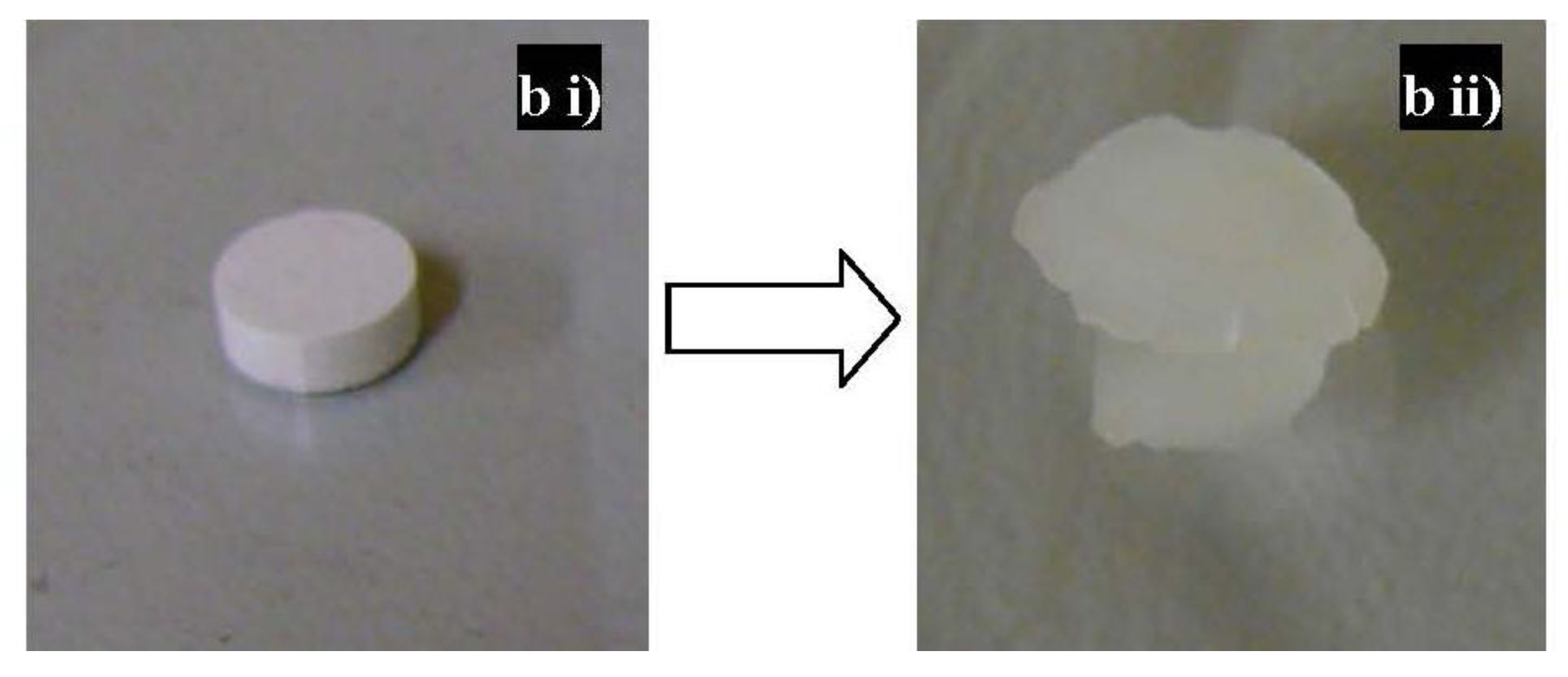
3. Experimental Section
3.1. Materials
3.2. Equipment
3.3. Synthesis of the Interpolyelectrolyte Complex
3.4. Rheological Studies
3.4.1. Basic Viscosity
3.4.2. Yield Stress Test
3.4.3. Oscillatory Stress Sweep
3.4.4. Oscillatory Frequency Sweep
3.4.5. Creep and Recovery Test
3.4.6. Temperature Ramp
3.4.7. Static Lattice Atomistic Simulations (SLAS)
3.4.8. Drug Release Studies
4. Conclusions
Conflicts of Interest
References
- Lipatov, Y.S.; Shumsky, V.F.; Getmanchuk, I.P.; Gorbatenko, A.N. Rheology of polymer blends. Rheol. Acta 1982, 21, 270–279. [Google Scholar] [CrossRef]
- Pénzes, T.; Csóka, I.; Erős, I. Rheological analysis of the structural properties effecting the percutaneous absorption and stability in pharmaceutical organogels. Rheol. Acta 2004, 43, 457–463. [Google Scholar] [CrossRef]
- Lee, K.Y.; Bouhadir, K.H.; Mooney, D.J. Controlled degradation of hydrogels using multi-functional cross-linking molecules. Biomaterials 2004, 25, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Bertoni, M.; Caramella, C. Influence of medium on dissolution-erosion behaviour of na carboxymethylcellulose and on viscoelastic properties of gels. Int. J. Pharm. 1995, 117, 41–48. [Google Scholar] [CrossRef]
- Michailova, V.; Titeva, S.; Kotsilkova, R.; Krusteva, E.; Minkov, E. Influence of aqueous medium on viscoelastic properties of carboxymethylcellulose sodium, hydroxypropyimethyl cellulose, and thermally pre-gelatinized starch gels. Colloids Surf. Physicochem. Eng. Asp. 1999, 149, 515–520. [Google Scholar] [CrossRef]
- Santoveña, A.; Alvarez-Lorenzo, C.; Llabrés, M.; Concheiro, A.; Fariña, J.B. HGH Release from directly compressed hgh-plga biodegradable implantable tablets: Influence of physicomechanical factors. Eur. Polym. J. 2009, 45, 2830–2838. [Google Scholar] [CrossRef]
- Ghosh, K.; Shu, X.Z.; Mou, R.; Lombardi, J.; Prestwich, G.D.; Rafailovich, M.H.; Clark, R.A.F. Rheological characterization of in situ cross-linkable hyaluronan hydrogels. Biomacromolecules 2005, 6, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Chen, X.; Chen, W. Rheological characterization of in situ crosslinkable hydrogels formulated from oxidized dextran and n-carboxyethyl chitosan. Biomacromolecules 2007, 8, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.C.W.; Morishima, Y.; Winnik, F.M. A rheological evaluation of the interactions in water between a cationic cellulose ether and sodium poly(2-acrylamido-2-methylpropanesulfonates). Macromolecules 2001, 34, 9117–9124. [Google Scholar] [CrossRef]
- Abdelrahim, K.A.; Ramaswamy, H.S. High temperature/pressure rheology of carboxymethyl cellulose (CMC). Food Res. Int. 1995, 28, 285–290. [Google Scholar] [CrossRef]
- Benchabane, A.; Bekkour, K. Rheological properties of carboxymethyl cellulose (cmc) solutions. Colloid Polym. Sci. 2008, 286, 1173–1180. [Google Scholar] [CrossRef]
- Edali, M.; Esmail, M.N.; Vatistas, G.H. Rheological properties of high concentrations of carboxymethyl cellulose solutions. J. Appl. Polym. Sci. 2001, 79, 1787–1801. [Google Scholar] [CrossRef]
- Elliot, J.; Ganz, A. Some rheological properties of sodium carboxymethylcellulose solutions and gels. Rheol. Acta 1974, 13, 670–674. [Google Scholar] [CrossRef]
- Kulicke, W.; Kull, A.H.; Kull, W.; Thielking, H.; Engelhardt, J.; Pannek, J. Characterization of aqueous carboxymethylcellulose solutions in terms of their molecular structure and its influence on rheological behaviour. Polymer 1996, 37, 2723–2731. [Google Scholar] [CrossRef]
- Yaşar, F.; Toğrul, H.; Arslan, N. Flow properties of cellulose and carboxymethyl cellulose from orange peel. J. Food Eng. 2007, 81, 187–199. [Google Scholar] [CrossRef]
- Florjancic, U.; Zupancic, A.; Zumer, M. Rheological characterization of aqueous polysaccharide mixtures undergoing shear. Chem. Biochem. Eng. Q. 2002, 16, 105–118. [Google Scholar]
- Mustafin, R.I.; Van den Mooter, G.; Kemenova, V.A. Eudragit E modified by inclusion into an interpolyelectrolyte complex. Pharm. Chem. J. 2005, 39, 39–42. [Google Scholar] [CrossRef]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J. Controll. Release 2009, 136, 88–98. [Google Scholar] [CrossRef]
- Dapía, S.; Tovar, C.A.; Santos, V.; Parajó, J.C. Rheological behaviour of carboxymethylcellulose manufactured from TCF-bleached milox pulps. Food Hydrocoll. 2005, 19, 313–320. [Google Scholar] [CrossRef]
- Nakason, C.; Kaewsakul, W.; Kaesaman, A. Thermoplastic natural rubbers based on blending of ethylene-vinyl acetate copolymer with different types of natural rubber. J. Elastom. Plast. 2012, 44, 89–111. [Google Scholar] [CrossRef]
- Ghannam, M.T.; Esmail, M.N. Rheological properties of carboxymethyl cellulose. J. Appl. Polym. Sci. 1997, 64, 289–301. [Google Scholar] [CrossRef]
- Rodríguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Rheological evaluation of the interactions between cationic celluloses and carbopol 974P in water. Biomacromolecules 2001, 2, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.B.; Rosen, M.R. Rheology Modifiers Handbook: Practical Use and Application; William Andrew Publishing: New York, NY, USA, 2000. [Google Scholar]
- Rudraraju, V.S.; Wyandt, C.M. Rheological characterization of microcrystalline cellulose/sodium carboxymethyl cellulose hydrogels using a controlled stress rheometer: Part I. Int. J. Pharm. 2005, 292, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.A.; Walters, K. The Yield stress myth? Rheol. Acta 1985, 24, 323–326. [Google Scholar] [CrossRef]
- Barnes, H.A. A Handbook of Elementary Rheology; The University of Wales Institute of Non-Newtonian Fluid Mechanics: Wales, UK, 2000. [Google Scholar]
- Moura, M.J.; Figueiredo, M.M.; Gil, M.H. Rheological study of genipin cross-linked chitosan hydrogels. Biomacromolecules 2007, 8, 3823–3829. [Google Scholar] [PubMed]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hawk, J.L.; Loveless, D.M.; Jeon, S.L.; Craig, S.L. Mechanism of shear thickening in reversibly cross-linked supramolecular polymer networks. Macromolecules 2010, 43, 3556–3565. [Google Scholar] [CrossRef] [PubMed]
- Nisato, G.; Schosseler, F.; Candau, S.J. Swelling equilibrium properties of partially charged gels: The effect of salt on the shear modulus. Polym. Gels Netw. 1996, 4, 481–498. [Google Scholar] [CrossRef]
- Choudhary, S.; White, J.; Stoppel, W.; Roberts, S.; Bhatia, S. Gelation behavior of polysaccharide-based interpenetrating polymer network (IPN) hydrogels. Rheol. Acta 2011, 50, 39–52. [Google Scholar] [CrossRef]
- Sullivan, J.P.; Friedmann, T.; Baca, A. Stress relaxation and thermal evolution of film properties in amorphous carbon. J. Electron. Mater. 1997, 26, 1021–1029. [Google Scholar] [CrossRef]
- Adalja, S.B.; Otaigbe, J.U. Creep and recovery behavior of novel organic-inorganic polymer hybrids. Polym. Compos. 2002, 23, 171–181. [Google Scholar] [CrossRef]
- Bokias, G.; Mylonas, Y.; Staikos, G.; Bumbu, G.G.; Vasile, C. Synthesis and aqueous solution properties of novel thermoresponsive graft copolymers based on a carboxymethylcellulose backbone. Macromolecules 2001, 34, 4958–4964. [Google Scholar] [CrossRef]
- Cancela, M.A.; Álvarez, E.; Maceiras, R. Effects of temperature and concentration on carboxymethylcellulose with sucrose rheology. J. Food Eng. 2005, 71, 419–424. [Google Scholar] [CrossRef]
- Lin, C.; Ko, S. Effects of temperature and concentration on the steady shear properties of aqueous solutions of carbopol and CMC. Int. Commun. Heat Mass Transf. 1995, 22, 157–166. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, W. Viscosity properties of sodium carboxymethylcellulose solutions. Cellulose 2007, 14, 409–417. [Google Scholar] [CrossRef]
- Warhurst, D.; Craig, J.; Adagu, I.; Meyer, D.; Lee, S. The relationship of physico-chemical properties and structure to the differential antiplasmodial activity of the cinchona alkaloids. Malar. J. 2003, 2. [Google Scholar] [CrossRef]
- Pearlman, D.A.; Case, D.A.; Caldwell, J.W.; Ross, W.S.; Cheatham, T.E.; DeBolt, S.; Ferguson, D.; Seibel, G.; Kollman, P. AMBER, a package of computer programs for applying molecular mechanics, normal mode analysis, molecular dynamics and free energy calculations to simulate the structural and energetic properties of molecules. Comput. Phys. Commun. 1995, 91, 1–41. [Google Scholar] [CrossRef]
- Yu, B.Y.; Chung, J.W.; Kwak, S. Reduced migration from flexible poly(vinyl chloride) of a plasticizer containing beta-cyclodextrin derivative. Environ. Sci. Technol. 2008, 42, 7522–7527. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Pillay, V.; Choonara, Y.E.; Modi, G.; Naidoo, D.; Du Toit, L.C. In silico theoretical molecular modeling for Alzheimer’s disease: the nicotine-curcumin paradigm in neuroprotection and neurotherapy. Int. J. Mol. Sci. 2011, 12, 694–724. [Google Scholar] [CrossRef] [PubMed]
- Choonara, Y.E.; Pillay, V.; Ndesendo, V.M.K.; du Toit, L.C.; Kumar, P.; Khan, R.A.; Murphy, C.S.; Jarvis, D. Polymeric emulsion and crosslink-mediated synthesis of super-stable nanoparticles as sustained-release anti-tuberculosis drug carriers. Colloids Surf. B 2011, 87, 243–254. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ngwuluka, N.C.; Choonara, Y.E.; Kumar, P.; Modi, G.; Toit, L.C.d.; Pillay, V. A Hybrid Methacrylate-Sodium Carboxymethylcellulose Interpolyelectrolyte Complex: Rheometry and in Silico Disposition for Controlled Drug Release. Materials 2013, 6, 4284-4308. https://doi.org/10.3390/ma6104284
Ngwuluka NC, Choonara YE, Kumar P, Modi G, Toit LCd, Pillay V. A Hybrid Methacrylate-Sodium Carboxymethylcellulose Interpolyelectrolyte Complex: Rheometry and in Silico Disposition for Controlled Drug Release. Materials. 2013; 6(10):4284-4308. https://doi.org/10.3390/ma6104284
Chicago/Turabian StyleNgwuluka, Ndidi Chinyelu, Yahya Essop Choonara, Pradeep Kumar, Girish Modi, Lisa Claire du Toit, and Viness Pillay. 2013. "A Hybrid Methacrylate-Sodium Carboxymethylcellulose Interpolyelectrolyte Complex: Rheometry and in Silico Disposition for Controlled Drug Release" Materials 6, no. 10: 4284-4308. https://doi.org/10.3390/ma6104284





